Abstract
Physical inactivity is an important contributor to obesity and fat accumulation in tissues. Critical complications of obesity include type II diabetes and nonalcoholic fatty liver disease (NAFLD). Exercise has been reported to exert ameliorating effects on obesity and NAFLD. However, the underlying mechanism is not fully understood. We showed the increase of microRNA-451 (miR-451) and decrease of macrophage migration inhibitory factor (MIF) in liver, after swim training in high fat diet (HFD) mice. MIF expression was regulated by miR-451. HFD intake caused the increase of body weights in WT and MIF knockout (KO) mice. In addition, HFD-induced liver anomalies were associated with Akt activation and autophagy suppression, which were reversed by MIF KO. In hepatocytes from HFD WT and MIF KO mice, autophagy was inhibited by exogenous rmMIF through Akt activation. Meanwhile, miR-451 antagonized the regulation of MIF on Akt signaling and autophagy. Taken together, these results indicate that MIF was decreased in liver of HFD mice due to physical exercise, and might prevent hepatic steatosis by suppressing Akt signaling and promoting autophagy.
Keywords: NAFLD, swimming, miR-451, macrophage migration inhibitory factor, high fat diet
Introduction
Obesity is a global pandemic, leading to the increase in the prevalence of obesity-related systematic disorders including nonalcoholic fatty liver disease (NAFLD) [1]. NAFLD is characterized by extra fat accumulation (>5%) in liver with potential risks for scarring (cirrhosis) over time and may even lead to liver cancer or liver failure [2]. Studies have revealed an alarming rate in the increase of obesity and metabolic syndrome in western countries [3]. According to the National Health and Nutrition Examination surveys of 2009-2010, obesity rates were 35.5% and 35.8% in men and women respectively [4,5]. Physical inactivity is one major cause of obesity with other serious complications, including cardio vascular diseases, high blood pressure, osteoporosis, lipid disorders, depression and anxiety. According to world health organization (WHO), approximately 2 million deaths are attributed to physical inactivity per year, forcing the organization to issue a warning against sedentary lifestyle, which has risen to among the highest causes of death and disability [1].
Exercise, including aerobics, swimming, jogging and cycling, has been shown to exert ameliorating effects on obesity and NAFLD [6]. However, the precise molecular mechanism has not been fully understood. Diet and lifestyle modification have been proven to be an effective strategy to attenuate NAFLD in greater than 90% of patients, because they can lead to weight loss of 10% or more [6]. Recently, efforts have been made to elucidate the molecular mechanism of these ameliorating effects [7,8]. Migration inhibitory factor (MIF) is a putative factor in regulating monocyte mobility [9]. Since the first report on MIF in the mid-1960s, the role of MIF has gone beyond a monocyte motility-regulating cytokine to a pleiotropic regulator of a vast array of cellular and biological processes [10]. Multiple clinical studies have pointed to the utility of MIF as a biomarker for inflammatory diseases, including systemic infections and sepsis, autoimmune diseases, cancer, and metabolic disorders such as type 2 diabetes and obesity [11]. Recent evidences indicated that MIF plays an important role in obesity-induced complications, including NAFLD. However, direct link between swimming and MIF has not been established and how MIF governs the pathogenesis of NAFLD is remains largely unclear. Therefore, to foster efficient clinical care of patients with NAFLD, further research is needed to clarify the correlation between exercise and MIF and the underpinning mechanism of this process.
Autophagy is an intracellular pathway that targets and transports cellular components such as organelles and proteins to lysosomes for degradation. The autophagosome fuses to a lysosome resulting in the degradation of the contents of the autophagosome by the hydrolytic enzymes of the lysosome [12]. Autophagy is generally activated by conditions of nutrient deprivation but has also been associated with physiological and pathological processes such as development, differentiation, neurodegenerative diseases, stress, infection, and cancer [13]. Akt, as one essential member of PI3K/Akt pathway, plays various biological roles via managing substrate phosphorylation, such as the regulation of cell proliferation and death [14]. In addition, some researchers have found that autophagy is closely linked with Akt signaling [14,15]. For instance, autophagy induced by advanced glycation end products could be reduced via activating Akt pathway [15]. Moreover, MIF has close relationships with Akt activation in many diseases or cells, such as breast cancer, and obesity-associated cardiac anomalies [16,17].
Herein, the purpose of the study is to explore the functions of swimming on MIF in obesity-induced NAFLD and the action of MIF on Akt signaling and autophagy that is a catabolic process that results in the autophagosomic-lysosomal degradation of bulk cytoplasmic contents, abnormal protein aggregates, and excess or damaged organelles [14]. We found that MIF was upregulated in mice on HFD and swim training decreased MIF expression. MIF expression was regulated by miR-451, a microRNA (miRNA) that was previously reported to be linked to NAFLD [15]. Akt suppression and autophagy activation were also observed in mice with swim training.
Materials and methods
Blood sample collection
All experiments were conducted in accordance to protocols approved by the Ethics Committee of the Affiliated First Hospital of Dalian Medical University, and was carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from all study subjects prior to enrollment. Blood samples from normal subjects (n=20) and patients with NAFLD (n=20) were collected from the Affiliated First Hospital of Dalian Medical University, February 2012 to February, 2014. NAFLD Subjects with serious liver or kidney diseases, malignancy and acute heart failure were excluded. In normal control, we excluded the people with a history of alcoholic liver disease, chronic viral hepatitis, autoimmune hepatitis, drug-induced liver disease, primary biliary cirrhosis, biliary obstruction, hemochoursomatosis, Wilson’s disease, α-1 antitrypsin and other deficiency-associated liver disease. The information of these subjects was shown in Table 1.
Table 1.
The information of normal subjects and NAFLD patients
| Variables | Normal (n=20) | NAFLD (n=20) |
|---|---|---|
| Age (years) | 45 ± 15 | 47.5 ± 14.3 |
| Sex ratio (M/F) | 12/8 | 9/11 |
| ALT (IU/L) | 14.5 ± 6.7 | 20.5 ± 15.2 |
| AST (IU/L) | 15.5 ± 3.8 | 28.5 ± 9.5 |
| Triglycerides (mg/dl) | 100.4 ± 31.2 | 152 ± 28.5 |
| HDL-cholesterol (mg/dl) | 45.2 ± 8.1 | 35.2 ± 9.1 |
| Glucose (mg/dl) | 92.1 ± 4.2 | 97.8 ± 7.2 |
Animal experiments
All animal procedures in this study were approved by the Animal Care and Use Committee at the Dalian Medical University and were carried out in compliance to the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health. In brief, six-week-old adult male C57/BL6 (wild type, WT) and MIF KO mice, were fed with a high fat diet (45% and 35% of total calorie from fat and carbohydrate, respectively, #D12451) for 20 weeks. Mice were housed in a climate-controlled environment (22.8 ± 2.0°C, 45%-50% humidity) with a 12-hour-light/dark cycle with free access to tap water and diet. At the 10th week of the dietary regimen, each group was divided into sedentary (Sed) or swim training (Swim) group. Thus, the final groups consisted of Wide type-sedentary (WT-Sed), Wide type-sedentary (WT-swim), MIF knockout-sedentary (KO-Sed), and MIF knockout-sedentary (KO-swim). Swim training was performed according to a previously published protocol [16]. Briefly, exercise procedures include 10 weeks of training, with a progressive adjustment of exercise duration (6 min/day until 60 min/day, 5 times/week) without increase of weight in the tail. For the exercise training, a swimming apparatus was especially planned for mice measuring 90 cm length, 30 cm width and 30 cm height, and divided into 12 lanes (surface area of 15 cm × 15 cm per lane). The temperature of water was maintained between 30 and 32°C.
Body mass measurement and serum analysis
The body mass was measured weekly (Monday, 12 a.m.). Fresh diet was provided daily and any remaining diet from the previous day was discarded. After blood collection, serum was acquired by centrifugation (120 g for 15 min) at room temperature. Serum total cholesterol (TC) and triacylglycerol (TG) were measured by the colorimetric enzymatic method using corresponding kits (Bioclin, Quibasa, Belo Horizonte, MG, Brazil).
Liver stereology
Several fragments from all parts of the liver were prepared, included in Paraplast Plus (Sigma-Aldrich, St. Louis, MO, USA), sectioned at 3 mm and stained with hematoxylin-eosin. Five random microscopic fields were analyzed per animal using video-microscopy (Leica DMRBE microscope with planachromatic objectives, Leica, Wetzlar, Germany) and a 36-point test-system (PT). The volume density (Vv) of NAFLD was estimated by point-counting on fat droplets in hepatocytes: Vv[steatosis] = PP[steatosis]/PT, where PP is the number of points that hit the steatosis [21].
Luciferase reporter assays
To validate the binding between miR-451 and the 3’ untranslated region (UTR) of the MIF, luciferase reporter assays were performed. Wild-type and mutant renilla luciferase constructs for MIF 3’UTR were kindly provided by Dr. Eva Bandres (University of Navarra, Pamplona, Spain). Wild-type renilla-MIF-3’UTR constructs contained the following oligonucleotide sequences: MIF-3’UTR-F: 5’-CTAGAGCCCACCCCAACCTTCTGGTGGGGAGA AATAAACGGTTTAGAGACTGC-3’, and MIF-3’ UTR-R: 5’-GGCCGCA GTCTCTAAACCGTTTATTTCTCCCCACCAGAAGGTTGGGGTGGGCT-3’, while the mutated constructs contained: MIF-3’ UTR-Mut-F: 5’-CTAGAGCCCACCCCAACCTTCTGGTGGGGAGAAATAGGTACTGAAGAGACTGC-3’, and MIF-3’ UTR-Mut-R: 5’-GGCCGCAGTCTCTTCAGTACCTATTTCTCCCCACCAGAAGGTTGGGGTGGGCT-3’. HEK293T cells were co-transfected with the Renilla-MIF 3’UTR vector (0.2 μg), Renilla-MIF-3’UTR-mutant, or a control vector containing renilla luciferase (0.05 μg; pGL3-Promoter; Promega Corp., Madison, WI, USA) plus either pre-miR-451 mimic oligonucleotide (451 mimic) or non-targeting (NT) mimic controls (30 nM each) using Lipofectamine 2000. All cells were cultured in 24-well plates for 24 h at 37°C after which both Renilla (reporter) and firefly (control for transfection efficiency) luciferase activity was determined using the Dual Luciferase reporter assay system following the protocol supplied by Promega.
Western blot analysis
Western blot was performed using standard procedures. Polyclonal rabbit antibodies against Akt, phosphorylated Akt (pAkt) at Ser473, LC3B, and GAPDH (1:1,000; Rabbit; Cell Signaling Technology, Danvers, MA, USA); MIF (1:1,000; Rabbit; Santa Cruz Biotechnology, Santa Cruz, CA, USA); and p62 (1:1,000; Guinea Pig; Enzo Life Sciences, Plymouth Meeting, PA, USA) were examined by standard western immunoblotting. Membranes probed respective antibodies and GAPDH served as the loading control.
RT-PCR
RNA was isolated from livers using Trizol reagent (Life Technologies, Rockville, MD). Synthesis of cDNA was performed using an Advantage RT for PCR kit (Clontech, Palo Alto, CA). Real-time reverse transcriptase PCR (RT-PCR) was conducted with primers from Invitrogen (Carlsbad, CA), and Taqman probes from Applied Biosystems (Foster City, CA). Target mRNA level was normalized to 18s rRNA, using the formula: Target/18s = 2Ct (18s) ± Ct (target).
Primary hepatocyte culture
Primary hepatocytes were isolated by collagenase digestion. Briefly, mice were anesthetized and the portal vein was cannulated with a 22-gauge i.v. catheter. The liver was perfused with Hank’s buffer solution (Invitrogen) containing 0.5 mM EGTA and 0.05 M HEPES (pH 7.4) maintained at 37°C at a rate of 5 mL/min for 5 min. Then, the collagenase-containing solution, Hyclone Medium 199/EBSS (Thermo Scientific, Logan, UT, USA) with 0.05% collagenase Type IA (Sigma), was used for the perfusion for 5 min (5 ml/min). The liver was transferred to 10 mm dishes with 15 ml DMEM containing 0.05% collagenase, followed by dissociation into single cells. Ten milliliters of DMEM supplemented with 10% heat-inactivated FBS were added to the cells to reduce collagenase activity. Cells were then passed through a 70 mm pore size strainer (BD, San Jose, CA, USA) and centrifuged at 120 g (Percoll gradient centrifugation) for three times. The cell yield was counted using a hemocytometer and the viability of the cells was assessed using Trypan blue exclusion test.
Cell transfection
Hepatocyte cells were cultured in plates for 24 h before transfection. The miRNA-mimics and inhibitors were respectively mixed with Lipofectamine 3000 (Thermo Fisher Scientific), incubated at room temperature for 15 min, following added to the plates. Finally, transfected cells were obtained according to the manufacturer’s instructions.
Data analysis
Data was expressed as mean ± SD. Statistical significance (P < 0.05) was estimated by one-way analysis of variation (ANOVA) followed by a Tukey’s test for post hoc analysis. All statistics were performed with GraphPad Prism 4.0 software (GraphPad, San Diego, CA, USA).
Result
Swimming and MIF knockout attenuate body weight increased in mice on high-fat diet
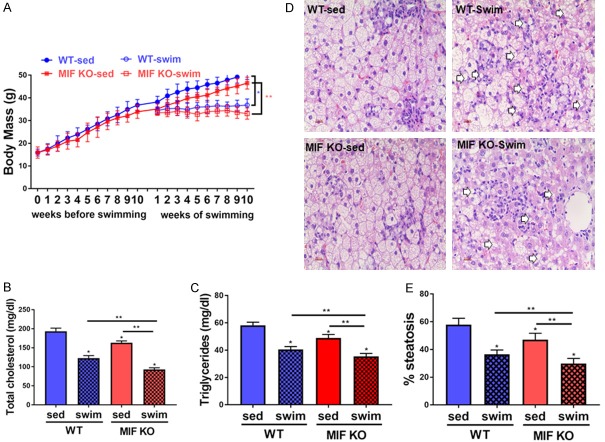
We first tested the effects of swimming and MIF knockout (KO) on the obesity of mice. It was found that body mass of wild-type (WT) group and MIF KO group was greatly increased due to high-fat diet (HFD) prior to exercise training, while MIF KO group had relative slower increase rate of body weight. The difference continued until the start of the training. The mice with swim training showed reduction of the body mass since the first week of training (after the adjustment period) (Figure 1A). Consistent with the trend of body mass, TC and TG were higher of WT mice than those of MIF KO mice (P < 0.05, one-way ANOVA and post hoc test of Tukey). Further, swimming induced an approximately 40% reduction in TC and approximately 20% reduction in TG compared to those sedentary mice on HFD (HFD-Sed group) (P < 0.05, one-way ANOVA and post hoc test of Tukey) (Figure 1B and 1C).
Figure 1.
Biometry and lipid metabolism. A. Body mass evolution. High fat diets were administered for 20 weeks and swim training for 10 weeks (n=10 for each group; *P < 0.05 between WT sed and WT swim, **P < 0.05 between MIF KO sed and MIF KO swim). B and C. Total cholesterol and triglycerides (mg/dl) in all those groups (n=10 for each group; *P < 0.05 vs. WT sed, **P < 0.05 vs. MIF KO swim). D and E. Volume density hepatic steatosis. Values are means ± standard error of the mean (n=10 for each group; *P < 0.05 vs. WT sed, **P < 0.05 vs. MIF KO swim). WT sed, Wild type sedentary group; WT swim, wild type swim training group; MIF KO sed, MIF KO sedentary group; MIF KO swim, MIF KO swim training group.
Liver steatosis
Histopathological analysis indicated that livers in sedentary mice were characterized by hepatocyte fat infiltration, macro- and micro-vesicular steatosis that was indicators of NAFLD. In addition, WT-Sed mice showed more steatosis than MIF KO-Sed mice (Figure 1D). HFD-swim group had a reduction in the steatosis in comparison to HFD-sed group. No significant difference in the steatosis was found between WT-swim and MIF KO-swim mice (Figure 1E).
Swim training reduce MIF levels in the liver of HFD-induced NAFLD
As shown in Figure 2A and 2B, WT group showed higher MIF expression than MIF KO group both in mRNA and protein levels. Swim training reduced MIF levels in the liver of HFD-induced NAFLD. We then assessed the levels of miR-451 and MIF in the serum of patients with NAFLD. Figure 2C and 2D demonstrated the downregulation of miR-451and upregulation of MIF in patients with NAFLD compared to healthy subjects. In line with this finding, it was shown that swim training increased miR-451 expression in both NFD and HFD mice, and the level of MIF was decreased in swim group mice (Figure 2E and 2F).
Figure 2.
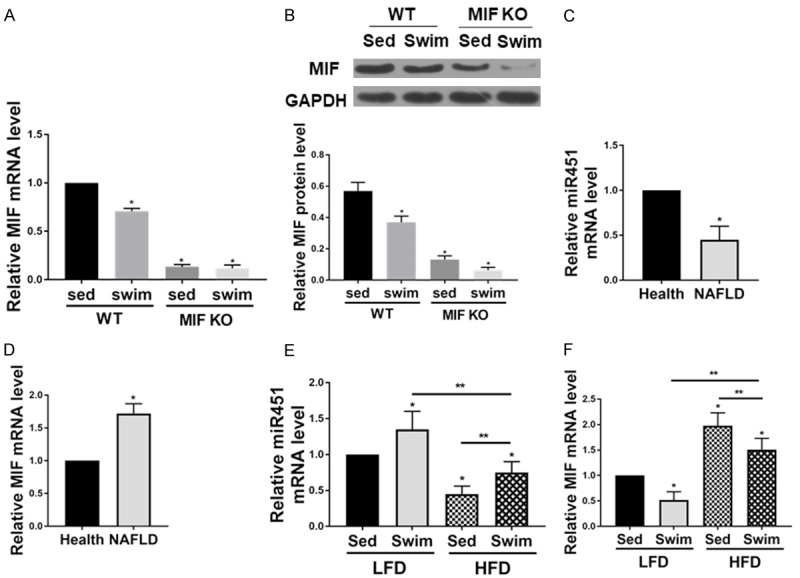
The expression of miR-451 and MIF in NAFLD. Swim training reduce MIF levels in the liver of HFD-induced NAFLD. A. MIF mRNA level in the liver of HFD-induced NAFLD was reduced because of swim training. B. MIF protein level in the liver of HFD-induced NAFLD was lower after swim training (n=6 for each group; *P < 0.05 vs. WT sed group). C and D. The miR-451 and MIF level in the blood between health and NAFLD (n=20 for each group; *P < 0.05 vs. health); E and F. The miR-451 and MIF level in the mice blood (n=8 for each group; *P < 0.05 vs. health). WT sed, Wild type sedentary group; WT swim, wild type swim training group; MIF KO sed, MIF KO sedentary group; MIF KO swim, MIF KO swim training group.
MIF is a target of miR-451
To further elucidate the interaction between MIF and miR-451, TargetScan analysis was performed, which verified the binding between MIF and miR-451 (Figure 3A). Further, we performed dual-luciferase assay in HEK293T cell line, due to the cell line’s superb transfection efficiency. Cells were transfected with renilla reporters that contained either a wild-type (MIF-3’UTR) or mutated 3’UTR of MIF (MIF-3’UTR Mutant, as shown in Figure 3B), along with pre-miR-451 mimics for miR-451 ectopic expression. Analysis of renilla luciferase activity indicated that ectopic expression of miR-451 inhibited the expression of wild-type MIF 3’UTR, but not MIF 3’UTR mutant (Figure 3C). In contrast, ectopic expression of the negative control, pre-miR-NT mimic, had no effect on the expression of the wild-type or mutant MIF-3’UTR constructs (Figure 3C). We then transfected miR-451 mimic or miR-451 inhibitor into the hepatocytes isolated from HFD-WT mice. As shown in Figure 3D, miR-451 mimic reduced MIF level and its inhibitor stimulated MIF expression.
Figure 3.
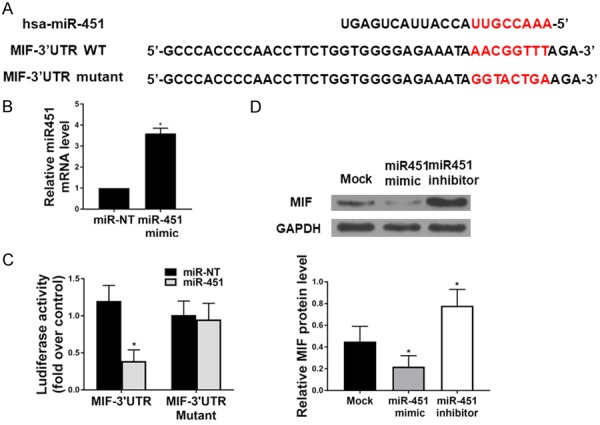
miR-451 targets the 3’UTR of MIF to regulate its expression. A. Sequence alignment of human miR-451 with the 3’UTR of MIF in TargetScan analysis. Seed sequence of miR-451, which corresponds to the binding site within the 3’UTR of MIF is highlighted in red color. B. qRT-PCR was used to verify the transfection efficiency of miR-451 in cells (*P < 0.05). C. MiR-451 over-expression significantly reduced luciferase activity in cells transfected with wild-type 3’UTR MIF construct, but miR-NT had no effect on luciferase activity in cells transfected with either the wild-type or mutant 3’UTR MIF reporter constructs (n=4 for each group). D. The protein level of MIF.
Swim training-induced MIF reduction prevents HFD-induced liver Akt activation
Here we used western blot to analyze the protein expression in liver tissues, and the result showed that Akt activation in liver was significantly higher in WT mice on HFD than that in MIF KO mice on HFD (Figure 4A). Moreover, Akt activation was promoted, as evidenced by the increased phosphorylation of GSK3β, a direct downstream target of Akt. In contrast, MIF KO markedly dampened HFD-induced Akt activation. Swim training inhibited high fat diet-induced Akt pathway activation in liver tissues of both WT and MIF KO mice.
Figure 4.
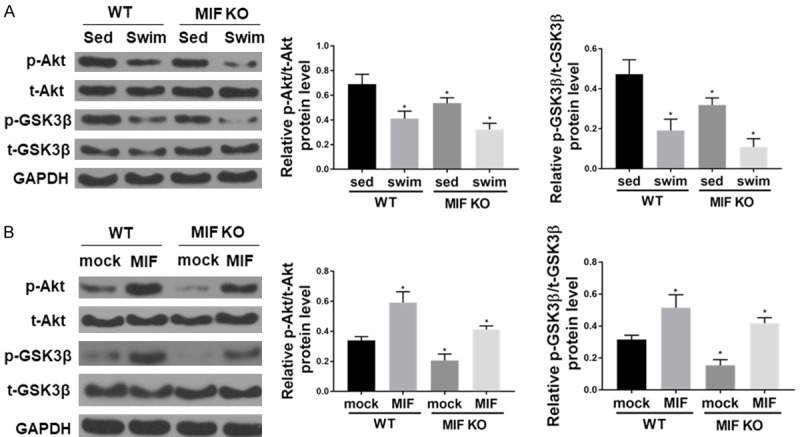
Swim training-induced MIF decreasing prevents Akt activation caused by HFD in liver. A. The protein levels of pAkt, Akt, pGSK-3β, GSK-3β, and GAPDH (as loading control) in liver tissues (n=6 for each group; *P < 0.05 vs. WT sed). B. Western blot shows that recombinant mouse MIF (rmMIF, 50 ng/ml, 6 hr) induces Akt pathway activation in hepatocyte isolated from HFD mice (n=6 for each group; *P < 0.05 vs. WT mock).
To further confirm the role of MIF in regulating Akt signaling, primary hepatocytes isolated from HFD-WT mice were incubated with recombinant mouse MIF (rmMIF) (50 ng/ml) and Akt phosphorylation was evaluated after 6 hours. Our data showed that rmMIF significantly increased Akt phosphorylation levels without affecting total Akt expression (Figure 4B). Furthermore, GSK-3β phosphorylation levels were significantly increased by rmMIF in primary hepatocytes.
Swim-induced MIF reduction alleviates the suppression of hepatocyte autophagy caused by HFD
We proceeded to examine liver autophagy in WT and MIF KO mice on HFD with or without swim training. The protein levels of LC3B II and LC3B I to LC3B II conversion were decreased in liver from WT mice on HFD. On contrary, accumulation of p62, a degradation target for autophagy, was observed in liver from WT mice on HFD. Swim training enhanced autophagy in WT and MIF KO (Figure 5A).
Figure 5.
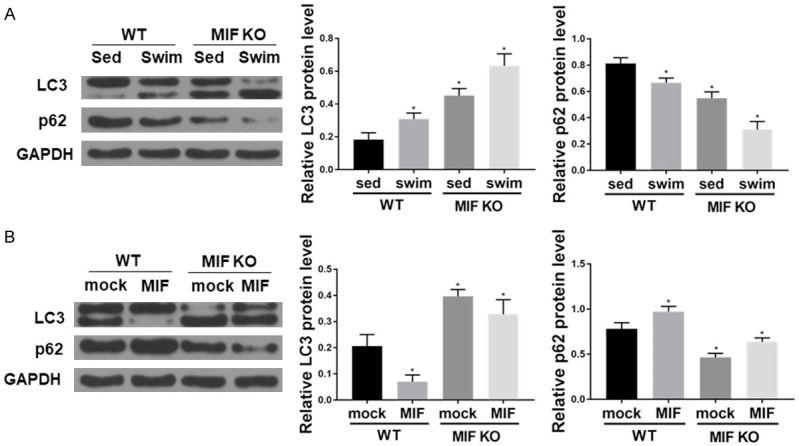
Swim training-induced MIF reduction rescues HFD-induced suppression in hepatocyte autophagy. A. The protein levels of LC3BI/II, p62, and GAPDH (as loading control) in liver tissues (n=6 for each group; *P < 0.05 vs. WT sed). B. Western blot shows that recombinant mouse MIF (rmMIF, 50 ng/ml, 6 hr) inhibits autophagy in hepatocyte isolated from HFD mice (n=6 for each group; *P < 0.05 vs. WT mock).
To further corroborate the finding that MIF KO rescued HFD-induced autophagy suppression, adult hepatocyte from WT and MIF KO mice were treated with rmMIF (50 ng/ml) and we found that MIF also inhibited autophagy (Figure 5B).
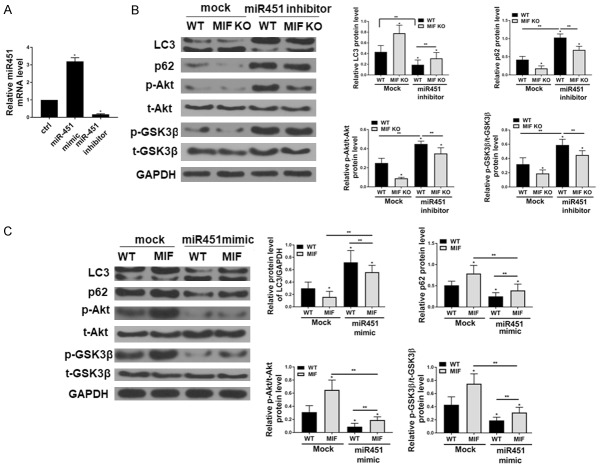
MiR-451 plays an important role in MIF-mediated Akt activation and autophagy in hepatocytes from HFD mice
Primary hepatocytes isolated from HFD-WT and HFD-MIF KO mice were transfected with mock or miR-451 inhibitor, which resulted in upregulation and downregulation of miR-451 levels, respectively (Figure 6A). It was shown that Akt/GSK signaling and autophagy was activated by miR-451 inhibition (Figure 6B). Nonetheless, these effects were abrogated by MIF-KO. Further, as shown in Figure 6C, miR-451 mimic transfection reduced MIF-induced Akt/GSK activation and autophagy activation.
Figure 6.
MiR-451 participates MIF-mediated Akt/GSK and autophagy in hepatocyte from mice on HFD. A. The transfection efficiency of MiR-451 mimic and inhibitor. B. The protein levels of p-Akt, p-GSK3β, LC3BI/II, p62, and GAPDH acts as loading control (n=4 for each group; *P < 0.05 vs. WT sed). C. Western blot shows that recombinant mouse MIF (rmMIF, 50 ng/ml, 6 hr) inhibits autophagy by mediating Akt/GSK in hepatocyte isolated from HFD mice (n=4 for each group; *P < 0.05 vs. WT mock).
Discussions
Obesity has long been recognized as a risk factor of NAFLD [2]. Exercise is considered an efficient way of reducing obesity. In our study, we show that swimming significantly reduced body weight of mice on HFD, which is consistent with previous reports [10,11]. A 20-percent decrease in body mass was also induced by swimming in mice with MIF KO. Concomitantly, TC and TG levels were also lower in MIF KO mice with or without swimming. Our result suggested that MIF KO might be helpful to decrease body mass and TC and TG levels. In the experiment of high fat diet-induced cardiac anomalies, TG level was not changed due to MIF deficiency, which was different from our result [17]. This might be explained that the tissues of MIF deficiency were different. Our subject was liver tissue, while their tissue was myocardium.
Further, to understand the genetic underpinnings of exercise-induced weight loss and its correlation to MIF, we showed that miR-451 was down-regulated in blood samples from mice fed with HFD. TargetScan analysis showed that a binding site exist between miR-451 and MIF [17]. MIF was previously shown to exert antagonizing effects on miR-451 [18,19]. MiR-451 is found as an important diagnostic molecule in several diseases, including osteosarcoma [20], gastric cancer [21], lung cancer [22], cardiac hypertrophy [23], etc. For example, miR-451 was significantly downregulated in NPC cell lines and clinical tissues, and its low expression was associated with poorer overall survival [18]. MIF was verified as a direct target of miR-451, and MIF regulated nasopharyngeal carcinoma cell growth and invasion [24]. In recent studies, regulatory role of miR-451 in autophagy has also been unveiled in the study of cardiac hypertrophy induced by HFD [23,25]. These data provide insight on the mechanism of MIF in regulating obesity and NAFLD and underscore miRNAs as potent mediators in human malignancies.
MIF has been shown to play an important role in the development of obesity and diabetes [12]. However, whether MIF played a role in HFD-induced obesity and was associated with anomalies still remained unknown. One focus of this study was to examine the impact of MIF knockout on HFD-induced obesity, obesity-associated NAFLD, and the underlying mechanisms involved with a focus on Akt signaling and autophagy. Herein, we showed that MIF KO attenuated Akt signaling. Akt has been found that it plays regulatory role in diverse processes, such as cell metabolism, survival and tumorigenesis [26,27]. Activating Akt could inhibit the autophagy-initiating Unc-51-like kinase 1 kinase complex and also directly phosphorylated BECN1, consequently reduced autophagy [33]. MIF over-expression could improve Akt activity in MEF, NIH/3T3, HeLa and several breast cancer cell lines, which led to the inhibition of apoptosis and enhancement of cell survival, namely inhibiting its expression had opposite function [16]. MIF deficiency weakened activation of cardiac autophagy caused by starvation, and repaired autophagic flux in H9C2 cells [34]. In addition, cardiac anomalies induced by high fat die was associated with myocardial Akt activation and autophagy suppression, which were nullified by MIF knockout [28]. Our result was consistent with previous reports.
It was previously shown that Akt-induced autophagy serves as a mediator of MIF in the treatment of Hepatocellular carcinoma. In cardiomyocytes from WT mice, autophagy was shown to be inhibited by exogenous rmMIF through Akt activation. In addition, MIF knockout rescued palmitic acid-induced suppression of cardiomyocyte autophagy, the effect of which could also be nullified by rmMIF. Plenty studies have proved that MIF was negatively regulated by MIR-451, and MIR-451 could suppresses proliferation, migration and promotes apoptosis of various cancer cells by targeting MIF, such as osteosarcoma, colorectal cancer, and gastrointestinal cancer [35]. For example, MIR-451 could inhibit growth of human colorectal carcinoma cells via down-regulating Pi3k/Akt pathway, including the decrease of AKT expression level [36]. Recent article also reported that over-expression of miR-451a could enhance autophagy of breast cancer cells [37]. Moreover, miR-451 was also shown to regulate MIF-mediated Akt/GSK and autophagy [25]. However, the reports about the regulation of MIR-451 on MIF-mediated AKT and autophagy in liver tissue were very few. In addition, the relationship between swimming and MIF expression was unclear. Our experiment found that swimming also could prevent the increase of body weight in mice on high-fat diet and restored hepatocyte autophagy by reducing MIF. Our work not only provided in-depth insight into the function mechanism of MIR-451 and MIF in NAFLD, but also demonstrated swimming could reduce MIF to partly restore autophagic flux.
Furthermore, recent many reports described that autophagy were highly relevant to the pathogenesis of NAFLD, including changing the macroautophagy ability to regulate cellular insulin sensitivity, and metabolism of cellular lipid stores, as well as mediating hepatocyte resistance to injurious stimuli such as oxidants and cytokines and prevent over activation of the innate immune response [11]. These functions of autophagy in pathways were considered important to the development of NAFLD, the current direct evidence of the pathophysiological involvement of hepatic autophagy in NAFLD and its complications, and the possible therapeutic targeting of autophagy in this disease [10].
Conclusions
MIF expression was decreased in the liver of HFD mice during swimming training and directly regulated by miR-451. Swim training suppressed Akt signaling and activated autophagy. The data of the study shed light on the molecular mechanism of NAFLD amelioration induced by swim training.
Disclosure of conflict of interest
None.
References
- 1.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–6. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 2.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 5.Carroll MD, Kit BK, Lacher DA. Total and high-density lipoprotein cholesterol in adults; national health and nutrition examination survey, 2009-2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 6.Bae JC, Suh S, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Kim SW, Hur KY, Kim JH. Regular exercise is associated with a reduction in the risk of NAFLD and decreased liver enzymes in individuals with NAFLD independent of obesity in Korean adults. PLoS One. 2012;7:e46819. doi: 10.1371/journal.pone.0046819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–44. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 8.Hoene M, Lehmann R, Hennige AM, Pohl AK, Häring HU, Schleicher ED, Weigert C. Acute regulation of metabolic genes and insulin receptor substrates in the liver of mice by one single bout of treadmill exercise. J Physiol. 2009;587:241–52. doi: 10.1113/jphysiol.2008.160275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akyildiz M, Gunsar F, Nart D, Sahin O, Yilmaz F, Akay S, Ersoz G, Karasu Z, Ilter T, Batur Y. Macrophage migration inhibitory factor expression and MIF gene- 173 G/C polymorphism in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2010;22:192–8. doi: 10.1097/MEG.0b013e328331a596. [DOI] [PubMed] [Google Scholar]
- 10.Graham A, Falcone T, Nothnick WB. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum Reprod. 2015;30:642–52. doi: 10.1093/humrep/dev005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, Leoni S, Bottazzo GF, Masotti A, Nobili V. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91:283–93. doi: 10.1038/labinvest.2010.166. [DOI] [PubMed] [Google Scholar]
- 12.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q, Zhang HL, Zhou YN. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells. Int J Mol Med. 2014;33:1451–8. doi: 10.3892/ijmm.2014.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29:613–8. doi: 10.3892/ijmm.2012.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the AKT pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–59. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, Ren J. Macrophage migration inhibitory factor (MIF) knockout preserves cardiac homeostasis through alleviating Akt-mediated myocardial autophagy suppression in high-fat diet-induced obesity. Int J Obes (Lond) 2015;39:387–96. doi: 10.1038/ijo.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi Z, Yan F, Shi W, Zhang C, Dong W, Zhao Y, Shen J, Ji X, Liu KJ, Luo Y. AKT-related autophagy contributes to the neuroprotective efficacy of hydroxysafflor yellow A against ischemic stroke in rats. Transl Stroke Res. 2014;5:501–9. doi: 10.1007/s12975-014-0346-x. [DOI] [PubMed] [Google Scholar]
- 19.Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, Sugimoto K, Ohashi K, Teradaira R, Inoue T. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99–103. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Evangelista FS, Muller CR, Stefano JT, Torres MM, Muntanelli BR, Simon D, Alvares-da-Silva MR, Pereira IV, Cogliati B, Carrilho FJ. Physical training improves body weight and energy balance but does not protect against hepatic steatosis in obese mice. Int J Clin Exp Med. 2015;8:10911–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Catta-Preta M, Mendonca LS, Fraulob-Aquino J, Aguila MB, Mandarim-de-Lacerda CA. A critical analysis of three quantitative methods of assessment of hepatic steatosis in liver biopsies. Virchows Arch. 2011;459:477–85. doi: 10.1007/s00428-011-1147-1. [DOI] [PubMed] [Google Scholar]
- 22.Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Böttger T. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13:434–46. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 23.Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12:1153–62. doi: 10.1158/1535-7163.MCT-12-0802. [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Lv GC, Sheng JF, Yang YD. Effect of miRNA-10b in regulating cellular steatosis level by targeting PPAR-alpha expression, a novel mechanism for the pathogenesis of NAFLD. J Gastroenterol Hepatol. 2010;25:156–63. doi: 10.1111/j.1440-1746.2009.05949.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang F, Huang W, Sheng M, Liu T. MiR-451 inhibits cell growth and invasion by targeting CXCL16 and is associated with prognosis of osteosarcoma patients. Tumour Biol. 2015;36:2041–8. doi: 10.1007/s13277-014-2811-2. [DOI] [PubMed] [Google Scholar]
- 26.Su Z, Zhao J, Rong Z, Geng W, Wang Z. MiR-451, a potential prognostic biomarker and tumor suppressor for gastric cancer. Int J Clin Exp Pathol. 2015;8:9154–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Yin P, Peng R, Peng H, Yao L, Sun Y, Wen L, Wu T, Zhou J, Zhang Z. MiR-451 suppresses cell proliferation and metastasis in A549 lung cancer cells. Mol Biotechnol. 2015;57:1–11. doi: 10.1007/s12033-014-9796-3. [DOI] [PubMed] [Google Scholar]
- 28.Kuwabara Y, Horie T, Baba O, Watanabe S, Nishiga M, Usami S, Izuhara M, Nakao T, Nishino T, Otsu K. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res. 2015;116:279–88. doi: 10.1161/CIRCRESAHA.116.304707. [DOI] [PubMed] [Google Scholar]
- 29.Liu N, Jiang N, Guo R, Jiang W, He QM, Xu YF, Li YQ, Tang LL, Mao YP, Sun Y, Ma J. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol Cancer. 2013;12:123. doi: 10.1186/1476-4598-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song L, Su M, Wang S, Zou Y, Wang X, Wang Y, Cui H, Zhao P, Hui R, Wang J. MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC 1. J Cell Mol Med. 2014;18:2266–74. doi: 10.1111/jcmm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, Ruegg C, Hemmings BA. Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2012;2:248–59. doi: 10.1158/2159-8290.CD-11-0270. [DOI] [PubMed] [Google Scholar]
- 32.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Dong X, Zhao R, Zhang R, Xu C, Wang X, Liu C, Hu X, Huang S, Chen L. Cadmium results in accumulation of autophagosomes-dependent apoptosis through activating Akt-impaired autophagic flux in neuronal cells. Cell Signal. 2019;55:26–39. doi: 10.1016/j.cellsig.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Pacheco BD, Leng L, Bucala R, Ren J. Macrophage migration inhibitory factor plays a permissive role in the maintenance of cardiac contractile function under starvation through regulation of autophagy. Cardiovasc Res. 2013;99:412–21. doi: 10.1093/cvr/cvt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamoori A, Wahab R, Vider J, Gopalan V, Lam AK. The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene. 2019;697:165–174. doi: 10.1016/j.gene.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 36.Li HY, Zhang Y, Cai JH, Bian HL. MicroRNA-451 inhibits growth of human colorectal carcinoma cells via downregulation of Pi3k/Akt pathway. Asian Pac J Cancer Prev. 2013;14:3631–4. doi: 10.7314/apjcp.2013.14.6.3631. [DOI] [PubMed] [Google Scholar]
- 37.Liu ZR, Song Y, Wan LH, Zhang YY, Zhou LM. Over-expression of miR-451a can enhance the sensitivity of breast cancer cells to tamoxifen by regulating 14-3-3ζ, estrogen receptor α, and autophagy. Life Sci. 2016;149:104–113. doi: 10.1016/j.lfs.2016.02.059. [DOI] [PubMed] [Google Scholar]