Abstract
Gastric cancer remains the second leading cause of cancer-related deaths worldwide. Adjuvant therapy has been shown to improve survival and is delivered either postoperatively (chemoradiotherapy) or perioperatively (chemotherapy) in Western countries. Debate continues regarding which of these approaches is an optimal strategy. Radioresistance in gastric cancer cells remains a serious concern. B7 homologue 3 (B7-H3, CD276), a newly found member of B7 immunoregulatory family, was found to be expressed in aberrant gastric cancer cells, and played a direct role in gastric cancer progression systems in a previous study. With upregulation or downregulation of B7-H3, it was observed that B7-H3 could increase radiotherapy resistance of gastric cancer cells by modulating apoptosis, cell cycle progression, and DNA double-strand breaks. Furthermore, it was found that B7-H3 could regulate baseline levels of cell autophagy. B7-H3 expression was negatively correlated with LC3-B expression in gastric cancer tissues. It was found that increasing baseline levels of cell autophagy with rapamycin in B7-H3-overexpressing cells could improve their sensitivity to radiation. This protein also exerted its function by modulating apoptosis and DNA double-strand breaks. Overall, it is demonstrated that B7-H3 increases the radiotherapy resistance of gastric cancer cells through regulating baseline levels of cell autophagy.
Keywords: Gastric cancer, B7-H3, radioresistance, autophagy
Introduction
Gastric cancer is the fourth common cancer in the world, despite significant improvements, it remains the second leading cause of cancer-related deaths in the world [1]. Accumulating evidences have demonstrated that chemoradiotherapy was effective for gastric cancer patients, because chemoradiotherapy could promote survival and local control. Radiotherapy has been recognized to play a significant role in treatments of gastric cancer [2,3]. Radioresistance has limited usage of radiotherapy in clinical practices, and radioresistance in gastric cancer cells remains a serious concern [4]. Therefore, novel radiotherapeutic biomarkers are necessary to improve the efficacy of radiotherapy for treating gastric cancer.
B7 homologue 3 (B7-H3, CD276), a novel member of the B7 family of costimulatory proteins, consists of two isoforms: murine 2Ig B7-H3 and human 4Ig B7-H3 [5-7]. The functional role of this protein in the adaptive immune response is controversial [8]. The lack of identification of B7-H3 receptors has hindered further immunological studies [9]. However, the non-immunological role of B7-H3 in cancer progression has received increasing attention. Increasing evidence suggests that B7-H3 promotes the development of many cancer types [10-13]. In a previous report, it was found that B7-H3 is expressed aberrantly in cancer cells and could promote gastric cancer cell migration and invasion [14]. Recent studies have also found that B7-H3 combats apoptosis induced by chemotherapy by delivering signals to pancreatic cancer cells [15]. Until now, no study has assessed the relationship between B7-H3 and radiation sensitivity. It was postulated that B7-H3 was involved in this process. A considerable number of researchers believe that autophagy should be inhibited to improve the efficacy of radiation therapy [16]. Nonetheless, others suggest that autophagy might be a therapeutically relevant objective in patients with cancer who are receiving treatment [17]. Thus far, the role of B7-H3 in radiation and its interaction with autophagy has not been evaluated.
Here, it was shown that B7-H3 can increase the radioresistance of gastric cancer cells by modulating apoptosis, cell cycle progression and DNA double-strand breaks. It was found that B7-H3 overexpression downregulated autophagy in gastric cancer tissue and cells. Finally, it was confirmed that B7-H3 increased the radioresistance of gastric cancer cells through regulating baseline levels of cell autophagy.
Materials and methods
Reagents
The following primary antibodies (anti-human) were used: mTOR, phospho-mTOR, Beclin-1, ATG5, LC3 (Cell Signaling Technology, MA, USA), γ-H2AX (Abcam, UK), GAPDH (Multisciences, China), and CD276 (R&D Systems, MN, USA). The following secondary antibodies were used: horseradish peroxidase-conjugated rabbit anti-goat, goat anti-mouse, and anti-rabbit antibodies (Multisciences, China). Proteins were visualized with an ECL detection kit (Bio-Rad, CA, USA). The following fluorescent secondary antibody was used: Cy3-conjugated anti-mouse IgG (Invitrogen, CA, USA).
Cell culture, lentiviral vectors and irradiation
The human gastric cancer lines, 7901 and MGC-803, were purchased from ATCC. Both cell line models were originally authenticated by the ATCC upon purchase (6-12 months prior to experimental research). ATCC uses morphology, karyotyping, and PCRcased approaches to profile the cytochrome C oxidase I gene (COI analysis) and short tandem repeat (STR) to confirm the identity of human cell lines and to rule out both intra- and interspecies contamination. 7901 and MGC-803 cultured in RPMI 1640 medium (Hyclone, USA) containing 10% heat-inactivated fetal bovine serum (FBS) (Biological industries, Israel) and 1% penicillin/streptomycin (Biological industries, Israel). Cells were incubated at 37°C in a humidified chamber with 5% CO2.
Lentiviral vectors carrying the full-length B7-H3 coding sequence (B7-H3, human, NM_001024736, GenBank) and shRNA targeting B7-H3 or NCshRNA were obtained from Shanghai GeneChem Co. (Shanghai, China).
Cells were exposed to different dosages (2, 4, 6 or 8 Gy) of ionizing radiation using an X-ray linear accelerator (RadSource, Suwanee, GA) at a fixed dose rate of 1.15 Gy/min.
Western blotting
Cells were washed twice with PBS, incubated with protein lysis buffer containing protease inhibitors for 30 min on ice, and then centrifuged for 30 min at 12,000 rpm at 4°C. Equal amounts (10-30 μg) of total protein extracts were subjected to SDS-PAGE and transferred onto PVDF membranes. The membranes were blocked with 5% BSA for 1 h and incubated overnight with primary antibodies at 4°C. Then, they were incubated with the appropriate HRP-conjugated secondary antibody for 2 h at 20°C. The blots were visualized with an ECL detection kit (BIO-RAD, CA, USA) and ChemiScope (Model No. 6300). GAPDH was used as a loading control.
Real-time PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was obtained using a reverse transcription reagent kit (Takara, Otsu, Shiga, Japan). Real-time PCR was performed using Power SYBR Green PCR Master Mix (Takara, Otsu, Shiga, Japan), and the products were detected using a gel documentation system (Bio-Rad, CA, USA). The real-time PCR primers used for mRNA quantification were as follows: GAPDH: 5’-AGAAGGTGGGGCTCATTTG-3’ and 5’-AGGGGCCATCCACAGTCTTC-3’; B7-H3: 5’-TGTCTCATTGCACTGCTGGT-3’ and 5’-CCTCAGCTCCTGCATTCTCC-3’. Relative genomic expression was calculated via the 2-ΔΔCt method.
Clonogenic assay
Gastric cancer cells were re-suspended, seeded into six-well plates at 200-6,000 cells/well for 24 h and then exposed to 0, 2, 4, 6 or 8 Gy X-ray irradiation by linear accelerators at a dose rate of 1.15 Gy/min. After the irradiation, the cells were grown for 7-10 days to allow for colony formation. Then, the cells were fixed and stained using a crystal violet solution. Colonies consisting of 50 or more cells were counted as a clone. The radiation sensitivity enhancement ratio (SER) was measured according to the multi-target single hit model.
Cell apoptosis assay
Cells were pre-infected with adenovirus 24 h before receiving irradiation. Apoptosis was measured using a 7-AAD/Annexin-V double staining apoptosis kit (BD Biosciences, Franklin Lakes, NJ) and flow cytometry (BD Biosciences, Franklin Lakes, NJ).
Cell cycle progression analysis
Cells were harvested by trypsin digestion, pelleted by centrifugation, washed with ice-cold PBS, and then fixed with 70% cold ethanol overnight. A staining solution containing propidium iodide (50 mg/ml) (Sigma-Aldrich, St. Louis, MO) and DNAse-free RNAse (20 mg/ml) was added 30 min before detection. The fraction of the population in each phase of the cell cycle was determined as a function of the DNA content using flow cytometry (BD Biosciences, Franklin Lakes, NJ).
Immunofluorescence
After irradiation, the cells were fixed in 4% paraformaldehyde for 5 min and washed again with PBS three times. The cells were treated with 0.25% Triton X-100 with 0.2% BSA for 5 min and then washed three times with PBS. Next, the cells were blocked with 1% normal horse serum and incubated with specific primary antibodies for 5 h at 4°C. After three PBS washes, the cells were incubated with Cy3-conjugated anti-mouse IgG (1:400; Jackson ImmunoResearch, USA) for 1 h at 37°C and then rinsed three times with PBS for 5 min/wash. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St. Louis, MO). Finally, the cells were observed under a laser confocal microscope IX71 (Olympus, Japan) with a digital camera (Olympus, Japan), and γ-H2AX foci were counted in each cell. At least 100 cells were counted from 10 randomly chosen fields of view.
Transmission electron microscope (TEM) assay
Fix cell suspension or free cells at 4°C by mixing with equal volume of Fixing solution for 2-4 h. Transfer cells to centrifuge tube and spin to get cell pellet. Embed the cell pellet with 1% agarose. Wash in 0.1 M PBS for three times, 15 min each. Post-fix with 1% OsO4 in 0.1 M PBS (pH 7.4) for 2 h at room temperature. Remove OsO4, rinse in 0.1 M PBS (pH 7.4) for three times, 15 min each. Dehydrate as followed: 50% ethanol for 15 min; 70% ethanol for 15 min; 80% ethanol for 15 min; 90% ethanol for 15 min; 95% ethanol for 15 min; Two change of 100% ethanol for 15 min; Finally two changes of acetone for 15 min. Infiltrate. 1:1 acetone: EMBed 812 for 2-4 h; 2:1 acetone: EMBed 812 overnight in dessicator with top off; pure EMBed 812 for 5-8 h; keep in 37°C oven overnight. Embed by baking in 60°C oven for 48 h. Cut ultrathin sections (60-80 nm) with ultramicrotome. Stain sections with uranyl acetate in pure ethanol for 15 min, rinse with distilled water. Then stain with leas citrate for 15 min, rinse with distilled water. Allow sections air-dry overnight. Observe with TEM.
Xenograft studies of nude mice
Five-week-old BALB/c nude mice (specific pathogen-free grade) were divided randomly into 4 groups and injected subcutaneously with 7901-LV-NC and 7901-LV-B7-H3 cells (1×106) with or without irradiation into their left posterior flank region. Two-dimensional measurements were taken with an electronic caliper, and the tumor volumes in mm3 were calculated using the following formula: volume a*b2/2, where a is the longest diameter, and b is the shortest diameter. Tumor volumes (mean ± s.e.m.; mm3) were assessed every 2 days after day 7. Two of the groups were irradiated with a single dose of 10 X-ray irradiation by linear accelerators at a dose rate of 1.15 Gy/min on day 7. After three weeks, all mice were euthanized. Tissues were removed and fixed in 10% formalin for hematoxylin and eosin (H&E) staining. All procedures were approved by the Animal Care and Use Committee of Soochow University.
Patients and clinical specimens
Tissue samples from 150 gastric cancer patients were analyzed immunohistochemically. Patients were selected randomly from all those undergoing radical gastric cancer resection between January 2014 and June 2016 at the Department of Pathology of the Second Affiliated Hospital of Soochow University. None had received chemotherapy or radiation therapy before surgery. Gastric carcinoma diagnoses were confirmed via H&E staining after surgical resection. This study was approved by the Ethics Committee of our hospital, and all patients provided written informed consent prior to enrolment.
Immunohistochemical staining
Immunohistochemical staining was performed as reported previously [14]. In brief, IHC was performed on selected slides using the ChemMateTM Envision/HRP technique. The sections were deparaffinized and dehydrated, and endogenous peroxidase activity was blocked using H2O2. The sections were incubated with B7-H3 and LC3 primary antibodies, followed by the appropriate secondary antibody, and visualized with diaminobenzidine (DAB). Finally, the slides were counterstained with hematoxylin.
Results
Lentivirus-mediated B7-H3 silencing/overexpression in gastric cancer cells
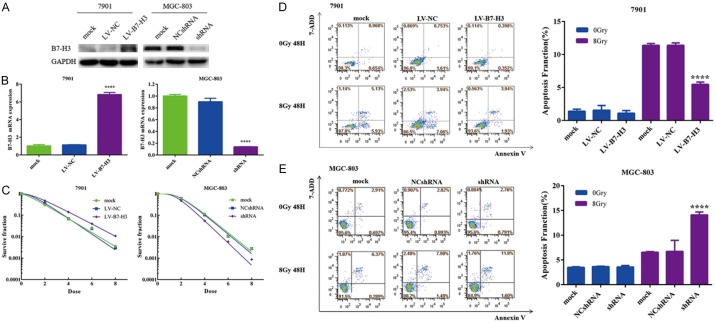
SGC-7901 cells were selected to construct stably transduced cell lines overexpressing B7-H3 by lentivirus. MGC-803 cells were selected to construct stably transduced cell lines with silenced B7-H3 expression. Western blotting and real-time PCR were used to analyze B7-H3 expression in mock-, LV-NC- and LV-B7-H3-infected 7901 cells and mock-, NCshRNA- and shRNA-infected MGC-803 cells. 7901 LV-B7-H3 cells exhibited higher B7-H3 plasma protein levels than 7901 mock and 7901 LV-NC cells. MGC-803 shRNA cells exhibited lower B7-H3 plasma protein levels than MGC-803 mock and MGC-803 NCshRNA cells (Figure 1A). mRNA levels exhibited a similar pattern (Figure 1B). The results showed the high efficiency of the lentiviral infection.
Figure 1.
Lentivirus-mediated B7-H3 silencing/overexpression can be induced in gastric cancer cells, and B7-H3 increases the radioresistance of gastric cancer cells. Protein (A) and mRNA (B) levels of B7-H3: SGC-7901 cells were selected to construct stably transduced cell lines overexpressing B7-H3 via lentivirus. MGC-803 cells were selected to construct stably transduced cell lines with B7-H3 silencing (****P<0.0001). B7-H3 can increase the radioresistance of gastric cancer cells (C). Apoptosis was significantly greater in the 7901-LV-B7-H3 cell population after radiation than in the 7901 mock and 7901-LV-NC cell populations (****P<0.0001) (D). Compared with the MGC-803 mock and MGC-803 NCshRNA cells after radiation, the MGC-803 shRNA cells after radiation had a clear increase in the apoptosis percentage (****P<0.0001) (E).
B7-H3 increases the radioresistance of gastric cancer cells
A clonogenic survival assay was performed to investigate the impact of B7-H3 on radiosensitivity in gastric cancer cells. The radioresistance of 7901 cells was rescued by B7-H3 silencing, and the radiosensitization of MGC-803 cells was reversed by B7-H3 overexpression (Figure 1C). Thus, the results showed that B7-H3 can increase the radioresistance of gastric cancer cells.
B7-H3 inhibits radiation-induced apoptosis in gastric cancer cells
Apoptosis was then assessed in gastric cancer cells after the knockdown/overexpression of B7-H3 and/or radiation. The proportion of apoptotic cells was determined by flow cytometry after irradiation. As shown in Figure 1D, apoptosis was significantly greater in the 7901-LV-B7-H3 cell population after radiation than in the 7901 mock and 7901-LV-NC cell populations (5.47 ± 0.21% in 7901-LV-B7-H3 cells after radiation versus 11.40 ± 0.20 in 7901-LV-NC cells after radiation, P<0.0001). A similar phenomenon was found in MGC-803 cells. Compared with the MGC-803 mock and MGC-803 NCshRNA cells after radiation, the MGC-803 shRNA cells after radiation had a clearly increased apoptosis percentage (14.09 ± 0.35% in MGC-803 shRNA cells after radiation versus 6.53 ± 0.09% in MGC-803 NCshRNA cells after radiation, P<0.0001) (Figure 1E). These data indicate that B7-H3 can inhibit radiation-induced apoptosis in gastric cancer cells.
B7-H3 increases the radioresistance of gastric cancer cells via DNA double-strand breaks and cell cycle progression
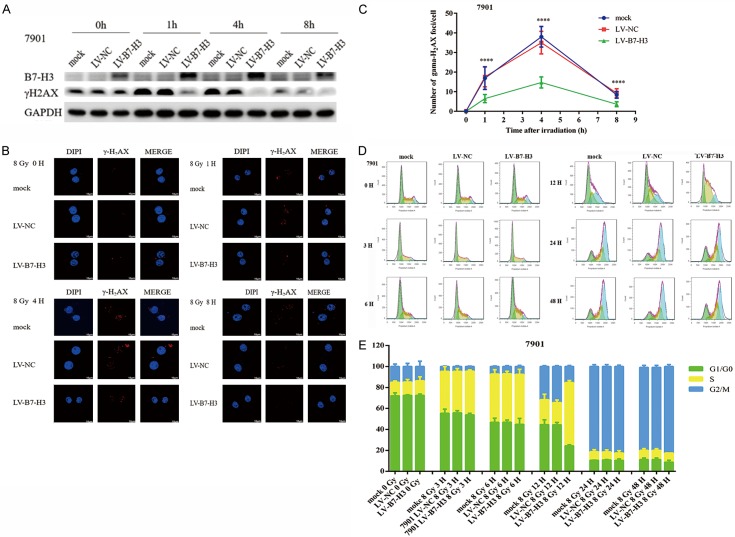
Western blot analysis was subsequently used to examine whether the overexpression of B7-H3 inhibited the formation of nuclear γ-H2AX foci induced by irradiation at 0, 2, 4, 6, and 8 h (Figure 2A). The results revealed that the formation of γ-H2AX foci after 8 Gy X-ray irradiation was significantly inhibited by the overexpression of B7-H3, especially at 4 h. In addition, the formation of γ-H2AX foci after 8 Gy X-ray irradiation was inhibited significantly more by the overexpression of B7-H3 (Figure 2B and 2C), which is consistent with the results from the western blot analysis. Moreover, radiation plus B7-H3 overexpression can cause cells to enter S-phase arrest at 12 h (Figure 2D and 2E). Taken together, these results revealed that B7-H3 can increase the radioresistance of gastric cancer cells by DNA double-strand breaks and cell cycle progression.
Figure 2.
B7-H3 increases the radioresistance of gastric cancer cells via DNA double-strand breaks and cell cycle progression. Western blot analysis was used to examine whether the overexpression of B7-H3 inhibited the formation of nuclear γ-H2AX foci induced by irradiation at 0, 2, 4, 6 and 8 h (A). γ-H2AX foci formation after 8 Gy X-ray irradiation was significantly inhibited by the overexpression of B7-H3, especially at 4 h. (B & C). Radiation plus B7-H3 overexpression can cause cells to enter S-phase arrest at 12 h (D & E).
B7-H3 increases the radioresistance of gastric cancer cells in vivo
Xenografts were established in nude mice to examine whether B7-H3 can increase the radioresistance of gastric cancer in vivo. As shown in Figure 3A and 3B, there was no difference between the 7901 LV-NC group and the 7901 LV-B7-H3 group. However, gastric cancer cell growth was effectively suppressed in the LV-B7-H3 group upon treatment with 10 Gy X-ray irradiation. Mouse tissues were analyzed via H&E staining for further assessment. No major differences in tissue morphology were observed (Figure S1A). These results indicate that B7-H3 can increase the radioresistance of gastric cancer cells in vivo, which is consistent with the in vitro data described above.
Figure 3.
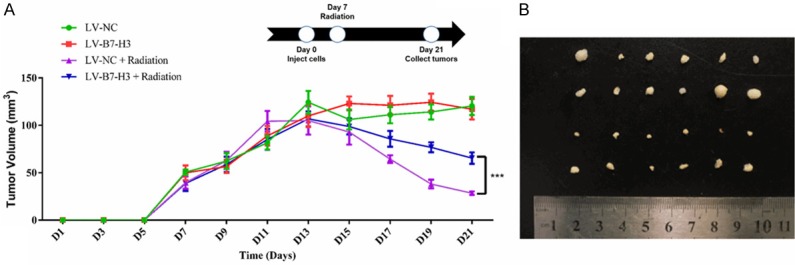
B7-H3 increases the radioresistance of gastric cancer cells in vivo. Xenografts were established in nude mice to examine whether B7-H3 can increase the radioresistance of gastric cancer in vivo. Each group of mice was composed of six male nude mice. Tumor sizes were measured at 2-day intervals. A. There was no difference between the 7901 LV-NC group and the 7901 LV-B7-H3 group. However, gastric cancer cell growth was effectively suppressed in 7901 LV-B7-H3 cells exposed to 10 Gy X-ray irradiation (***P<0.001). B. Tumor xenografts from each group.
B7-H3 and LC3 expression in gastric cancer cells and tissue samples
Western blotting was used to analyze B7-H3 and LC3 expression in mock-, LV-NC- and LV-B7-H3-infected 7901 cells. LV-B7-H3-infected cells exhibited higher B7-H3 plasma protein levels than mock- and LV-NC-infected cells. B7-H3 overexpression downregulated the expression of the autophagy proteins LC3, Atg5 and Beclin-1 (Figure 4A). After 8 Gy X-ray irradiation, protein expression levels were not significantly changed. These results indicate that B7-H3 could regulate baseline levels of cell autophagy.
Figure 4.
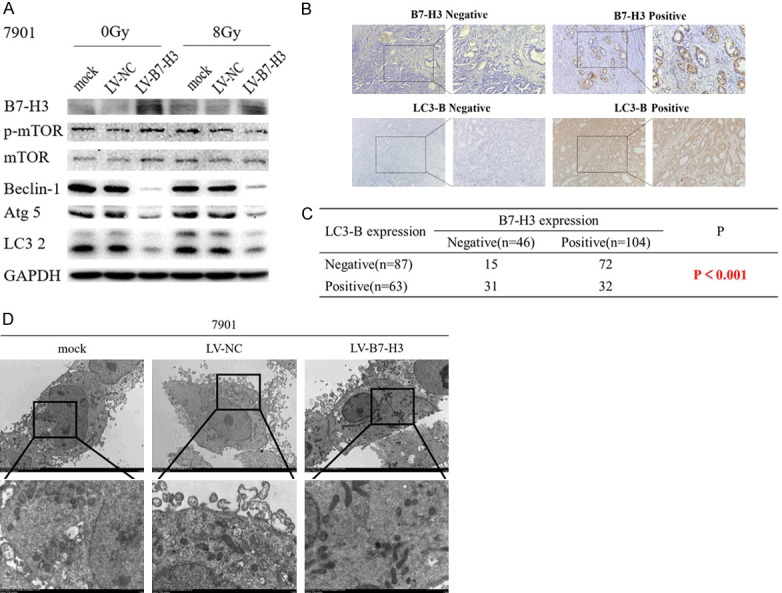
B7-H3 and LC3 expression in gastric cancer cells and tissue samples. A. B7-H3 and autophagy protein expression in mock-, LV-NC- and LV-B7-H3-infected 7901 cells. B7-H3 overexpression downregulated the autophagy proteins LC3, Atg5 and Beclin-1 expression. After 8 Gy X-ray irradiation, protein expression levels were not significantly changed. B. B7-H3 negative and positive and LC3-B negative and positive expression (×200 magnification) in gastric cancer. C. B7-H3 expression is negatively correlated with LC3-B expression in gastric cancer tissue samples (P<0.001). D. Autophagosomes detected by transmission electron microscopy. Mock-, LV-NC- and LV-B7-H3-infected 7901 cells were processed and observed under a transmission electron microscope (2500× & 8000×)
Tissue samples from 150 gastric cancer patients were obtained and analyzed by immunohistochemistry (IHC). IHC staining showed that B7-H3 was expressed in gastric carcinoma cell membranes and cytoplasm. B7-H3 expression was negatively correlated with LC3 expression in gastric cancer tissue samples (P<0.001; Figure 4B and 4C).
Autophagosomes detected by TEM. Mock-, LV-NC- and LV-B7-H3-infected 7901 cells were processed and observed under a transmission electron microscope (2500× & 8000×) (Figure 4D). These results also showed that B7-H3 could regulate baseline levels of cell autophagy.
Rapamycin sensitizes gastric cancer cells to ionizing radiation
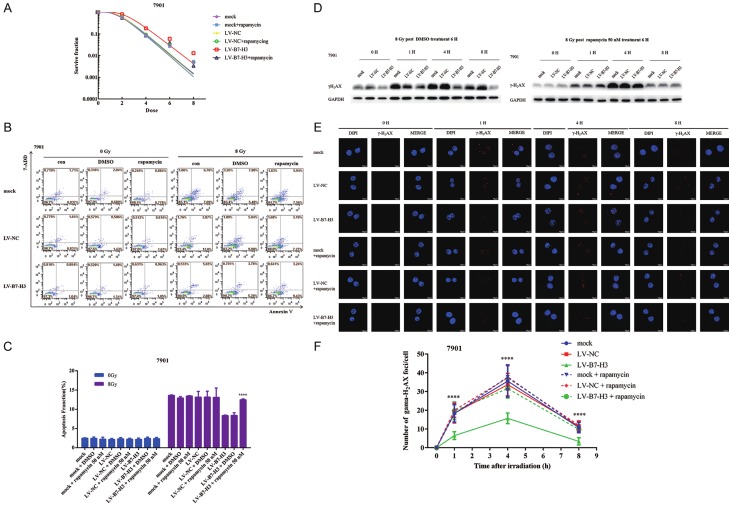
The phosphatidylinositol 3-phosphate kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling pathway is involved in the regulation of autophagy and apoptosis in mammalian cells [18]. First, the effect of rapamycin on the viability of gastric cancer cells was measured with CCK-8 assays. Mock-, LV-NC- and LV-B7-H3-infected 7901 cells were cultured for an additional 24-48 h after rapamycin treatment for 6 h (Figure S1B). Compared with dimethyl sulfoxide (DMSO), 50 nM rapamycin resulted in non-significant decreases in cell viability. Therefore, 50 nM rapamycin was chosen, which did not obviously inhibit cell proliferation. Western blot analysis further confirmed that baseline levels of autophagy were increased, but there were no effects on B7-H3 expression after 50 nM rapamycin treatment for 6 h in gastric cancer cells (Figure S1C).
Next, clonogenic survival assays were performed to investigate the impact of baseline levels of autophagy on radiosensitivity in B7-H3-overexpressing gastric cancer cells (Figure 5A). The results showed that the upregulation of baseline levels of autophagy in B7-H3-overexpressing cells induced by rapamycin can make them sensitive to radiation.
Figure 5.
B7-H3 increases the radiotherapy resistance of gastric cancer cells through regulating baseline levels of cell autophagy. A. The upregulation of baseline autophagy levels in B7-H3 cells by rapamycin can make them sensitive to radiation. B, C. Apoptosis was significantly lower in 7901-LV-B7-H3 + rapamycin cells after radiation than in 7901 LV-B7-H3 and 7901-LV-B7-H3 + DMSO cells after radiation (****P<0.0001). D. The upregulation of baseline autophagy levels in cells overexpressing B7-H3 promoted the formation of nuclear γ-H2AX foci induced by irradiation. E, F. Immunofluorescence was used to confirm the formation of γ-H2AX foci in the nuclei.
B7-H3 increases the radiotherapy resistance of gastric cancer cells through regulating baseline levels of cell autophagy
Apoptosis of gastric cancer cells was assessed after the upregulation of baseline cell autophagy levels in mock-, LV-NC- and LV-B7-H3-infected 7901 cells and/or radiation. The proportion of apoptotic cells was also determined by flow cytometry after irradiation. As shown in Figure 5B, apoptosis was significantly lower in the 7901-LV-B7-H3 + rapamycin cell population after radiation than in the 7901 LV-B7-H3 and 7901-LV-B7-H3 + DMSO cell populations after radiation (14.57 ± 0.1192% in 7901-LV-B7-H3 + rapamycin cells after radiation versus 8.078 ± 0.2106 in 7901-LV-B7-H3 + DMSO cells after radiation, P<0.0001, Figure 5C). These data demonstrated that the upregulation of baseline cell autophagy levels in B7-H3-overexpressing cells by rapamycin can make them sensitive to radiation.
Western blot analysis was used to examine whether the upregulation of baseline autophagy levels in cells overexpressing B7-H3 promoted the formation of nuclear γ-H2AX foci induced by irradiation (Figure 5D). Immunofluorescence was also used to confirm the formation of γ-H2AX foci in the nuclei (Figure 5E and 5F). The results revealed that the upregulation of baseline autophagy levels in cells overexpressing B7-H3 by rapamycin could make them sensitive to radiation via DNA double-strand breaks.
Discussion
Gastric cancer is a highly common malignancy [19]. The prognosis of patients with locally advanced gastric cancer remains poor despite radical surgical resection. Moreover, most newly diagnosed patients are at an advanced stage, approximately 50% of whom have already lost their chance for surgery [20]. Therefore, comprehensive treatments, especially radiotherapy, have recently received much attention, and novel radiotherapeutic biomarkers are necessary to improve efficacy of radiotherapy for gastric cancer [4].
B7-H3, an important costimulatory molecule, is a type I transmembrane protein that shares 20%~27% amino acid identity with other B7 family members [21]. As an immune-related molecule, the non-immunological role of B7-H3 in cancer progression has received increasing attention. A number of studies have shown that B7-H3 promotes the development of many cancer types, such as lung adenocarcinoma [22], brain glioma [11], breast cancer [23] and gastric cancer [14]. Recent studies have found that B7-H3 can reverse chemotherapy resistance in human cancer [15,24]. Our study focused on the relationship between B7-H3 and radiation sensitivity, which has not been previously reported. First, B7-H3 was overexpressed in 7901 cells and silenced in MGC-803 cells via lentiviral vector infection. It was observed that B7-H3 could increase the radiotherapy resistance of gastric cancer cells by modulating apoptosis, cell cycle progression and DNA double-strand breaks.
Autophagy is responsible for intracellular degradation and protein recycling, which play a significant role in tumor suppression and anticancer therapy [25]. The role of baseline levels of cell autophagy in radiotherapy is unclear. Here, it was found that B7-H3 could regulate baseline levels of cell autophagy and that B7-H3 expression was negatively correlated with LC3 expression in gastric cancer tissue samples. Autophagy modulation is a double-edged sword in cancer development [26].
Despite concerted efforts over the past few decades, the exact role of autophagy in the cellular radiation response remains controversial [27-30]. Most of the studies have reported that elevated levels of autophagy were associated with the radioresistance of various types of cancer [31-34]. An increasing number of tumor suppressors have been shown to induce high levels of autophagy [35]. In contrast, there is evidence suggesting that autophagy can promote cell death [36,37]. Combined treatment with Akt inhibitors and radiation has been shown to induce autophagy in cancers, thus enhancing the radiosensitization of cancer cells [37]. Y Kuwahara, et al. reported that the enhancement of autophagy is a potential modality for tumors refractory to radiotherapy [38]. In this study, it was demonstrated that B7-H3 increased the radiotherapy resistance of gastric cancer cells through the regulation of baseline levels of cell autophagy. Our study suggested that the activation of autophagy may overcome tumor radioresistance, which was consistent with previous reports [39-41]. However, the molecular mechanisms through which autophagy promotes tumor suppression are poorly understood. This molecular mechanism still needs more in-depth research.
Conclusions
In summary, it is found that B7-H3 can increase the radioresistance of gastric cancer cells by modulating apoptosis, cell cycle progression and DNA double-strand break repair. Furthermore, it is found that B7-H3 can regulate baseline levels of cell autophagy and that its expression is negatively correlated with LC3-B expression in gastric cancer tissue samples. It is found that the upregulation of baseline autophagy levels in B7-H3 cells by rapamycin can make them sensitive to radiation. B7-H3 also exerts its function by modulating apoptosis and DNA double-strand breaks. Overall, it is demonstrate that B7-H3 increases the radiotherapy resistance of gastric cancer cells through regulating baseline levels of cell autophagy.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (Nos. 81672970, 81673100 and 81872552), The Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group Project Funding, the Project of Suzhou Science and Technology Development Plan (Nos. SZS201618 and SS201753), the Health and Family Planning Commission Fund Project of Jiangsu Province (No. CXTDA2017016 and QNRC2016873), Suzhou Key Medical Center (No. LCZX201505), the Second Affiliated Hospital of Soochow University Preponderant Clinic Discipline Group Project Fund, the Graduate Student Scientific Research Innovation Projects of Jiangsu Province (No. KYCX18_2534), the Key Scientific Development Program of China (No. 2016YFC0904702), the Natural Science Foundation of Jiangsu Province (NO. BK20160338), and the projects of Suzhou Technology Bureau (Nos. SYS201552 and SYS2018054).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330–1344. doi: 10.1016/j.ejca.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS. Clinical overview: adjuvant therapy of gastrointestinal cancer. Cancer Chemother Pharmacol. 2004;54(Suppl 1):S4–11. doi: 10.1007/s00280-004-0880-4. [DOI] [PubMed] [Google Scholar]
- 3.Coburn NG, Govindarajan A, Law CH, Guller U, Kiss A, Ringash J, Swallow CJ, Baxter NN. Stage-specific effect of adjuvant therapy following gastric cancer resection: a population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500–507. doi: 10.1245/s10434-007-9640-0. [DOI] [PubMed] [Google Scholar]
- 4.Liu JS, Che XM, Chang S, Qiu GL, He SC, Fan L, Zhao W, Zhang ZL, Wang SF. beta-elemene enhances the radiosensitivity of gastric cancer cells by inhibiting Pak1 activation. World J Gastroenterol. 2015;21:9945–9956. doi: 10.3748/wjg.v21.i34.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF, Stockl J, Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol. 2004;172:2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 6.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol. 2002;168:6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 7.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, Moretta A, Bottino C. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos M, Hedderich DM, Friess H, Kleeff J. B7-h3 and its role in antitumor immunity. Clin Dev Immunol. 2010;2010:683875. doi: 10.1155/2010/683875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Guo G, Song J, Cai Z, Yang J, Chen Z, Wang Y, Huang Y, Gao Q. B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer. 2017;8:816–824. doi: 10.7150/jca.17759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han S, Shi X, Liu L, Zong L, Zhang J, Chen Q, Qian Q, Chen L, Wang Y, Jin J, Ma Y, Cui B, Yang X, Zhang Y. Roles of B7-H3 in cervical cancer and its prognostic value. J Cancer. 2018;9:2612–2624. doi: 10.7150/jca.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Wang Z, Zhang C, Liu X, Li G, Liu S, Sun L, Liang J, Hu H, Liu Y, Zhang W, Jiang T. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109:2697–2705. doi: 10.1111/cas.13744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inamura K, Takazawa Y, Inoue Y, Yokouchi Y, Kobayashi M, Saiura A, Shibutani T, Ishikawa Y. Tumor B7-H3 (CD276) expression and survival in pancreatic cancer. J Clin Med. 2018;7 doi: 10.3390/jcm7070172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang T, Wang F, Wu JY, Qiu ZC, Wang Y, Liu F, Ge XS, Qi XW, Mao Y, Hua D. Clinical correlation of B7-H3 and B3GALT4 with the prognosis of colorectal cancer. World J Gastroenterol. 2018;24:3538–3546. doi: 10.3748/wjg.v24.i31.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Yang X, Wu Y, Zhao K, Ye Z, Zhu J, Xu X, Zhao X, Xing C. B7-H3 promotes gastric cancer cell migration and invasion. Oncotarget. 2017;8:71725–71735. doi: 10.18632/oncotarget.17847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Wang J, Zhou J, Zhan S, Huang Y, Wang F, Zhang Z, Zhu D, Zhao H, Li D, Chen G, Zhu X, Zhao X. B7-H3 combats apoptosis induced by chemotherapy by delivering signals to pancreatic cancer cells. Oncotarget. 2017;8:74856–74868. doi: 10.18632/oncotarget.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo JY, Xia B, White E. Autophagy-mediated tumor promotion. Cell. 2013;155:1216–1219. doi: 10.1016/j.cell.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Bravo-San Pedro JM, Demaria S, Formenti SC, Kroemer G. Activating autophagy to potentiate immunogenic chemotherapy and radiation therapy. Nat Rev Clin Oncol. 2017;14:247–258. doi: 10.1038/nrclinonc.2016.183. [DOI] [PubMed] [Google Scholar]
- 18.Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol. 2014;13:78. doi: 10.1186/1475-2840-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Fei Q, Gu J, Yin L, He X. Progress of preoperative and postoperative radiotherapy in gastric cancer. World J Surg Oncol. 2018;16:187. doi: 10.1186/s12957-018-1490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 22.Yu TT, Zhang T, Lu X, Wang RZ. B7-H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther. 2018;11:4693–4700. doi: 10.2147/OTT.S169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cong F, Yu H, Gao X. Expression of CD24 and B7-H3 in breast cancer and the clinical significance. Oncol Lett. 2017;14:7185–7190. doi: 10.3892/ol.2017.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Chen Z, Ning K, Jin J, Han X. Inhibition of B7-H3 reverses oxaliplatin resistance in human colorectal cancer cells. Biochem Biophys Res Commun. 2017;490:1132–1138. doi: 10.1016/j.bbrc.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Li W, Wang C, Leng X, Lian S, Feng J, Li J, Wang H. Inhibition of autophagy enhances apoptosis induced by proteasome inhibitor bortezomib in human glioblastoma U87 and U251 cells. Mol Cell Biochem. 2014;385:265–275. doi: 10.1007/s11010-013-1835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pang XL, He G, Liu YB, Wang Y, Zhang B. Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation. World J Gastroenterol. 2013;19:1736–1748. doi: 10.3748/wjg.v19.i11.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaurasia M, Bhatt AN, Das A, Dwarakanath BS, Sharma K. Radiation-induced autophagy: mechanisms and consequences. Free Radic Res. 2016;50:273–290. doi: 10.3109/10715762.2015.1129534. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Qin Z, Liang Z. The prosurvival role of autophagy in Resveratrol-induced cytotoxicity in human U251 glioma cells. BMC Cancer. 2009;9:215. doi: 10.1186/1471-2407-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med. 2012;53:260–270. doi: 10.1016/j.freeradbiomed.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 34.Bartoletti-Stella A, Mariani E, Kurelac I, Maresca A, Caratozzolo MF, Iommarini L, Carelli V, Eusebi LH, Guido A, Cenacchi G, Fuccio L, Rugolo M, Tullo A, Porcelli AM, Gasparre G. Gamma rays induce a p53-independent mitochondrial biogenesis that is counter-regulated by HIF1alpha. Cell Death Dis. 2013;4:e663. doi: 10.1038/cddis.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu LJ, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z, Hei TK. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proc Natl Acad Sci U S A. 1999;96:4959–4964. doi: 10.1073/pnas.96.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Jiang X, Liu D, Na Y, Gao GF, Xi Z. Autophagy protects LNCaP cells under androgen deprivation conditions. Autophagy. 2008;4:54–60. doi: 10.4161/auto.5209. [DOI] [PubMed] [Google Scholar]
- 38.Kuwahara Y, Oikawa T, Ochiai Y, Roudkenar MH, Fukumoto M, Shimura T, Ohtake Y, Ohkubo Y, Mori S, Uchiyama Y, Fukumoto M. Enhancement of autophagy is a potential modality for tumors refractory to radiotherapy. Cell Death Dis. 2011;2:e177. doi: 10.1038/cddis.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist Updat. 2007;10:135–143. doi: 10.1016/j.drup.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Turcotte S, Giaccia AJ. Targeting cancer cells through autophagy for anticancer therapy. Curr Opin Cell Biol. 2010;22:246–251. doi: 10.1016/j.ceb.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalby KN, Tekedereli I, Lopez-Berestein G, Ozpolat B. Targeting the prodeath and prosurvival functions of autophagy as novel therapeutic strategies in cancer. Autophagy. 2010;6:322–329. doi: 10.4161/auto.6.3.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.