Abstract
Glioblastoma (GBM) is one of most malignancy tumors worldwide. Temozolomide (TMZ) is an important chemotherapy drug in GBM therapy. However, acquired TMZ-resistance frequently happens in GBM therapy and leads to high percentage of GBM recurrence. In our study, we demonstrated that Snail is upregulated in recurrent GBM tumors, and promotes the GBM cells resistant to TMZ induced apoptosis. Enhanced expression of Snail compromises the apoptosis induced by TMZ, and increases the cell migration and invasion. Reversely, depletion of Snail by siRNA has the opposite effects. In addition, we confirmed that the expression of Snail is modulated by STAT3 activation, since phospho-STAT3 level is relatively higher in recurrent GBM tumors and TMZ resistant cells. Knockdown of STAT3 turns down the expression of Snail in protein and mRNA level, and thereby sensitized the resistant GBM cells to TMZ treatment. Interestingly, the activation of STAT3 in GBM resistant cells is modulated by IL-6 secretion. Suppression of IL-6 abandons the STAT3 activation, and reduces its binding with Snail promoter. Inhibition of IL-6 by its antibody enhanced the killing effects of TMZ both in vivo and in vitro. Overall, our results provided a rational to overcome the TMZ resistant in GBM treatment by targeting IL-6-STAT3-Snail pathway.
Keywords: Snail, STAT3, IL-6, temozolomide, glioblastoma
Introduction
Glioblastoma multiforme (GBM), as one of most leading causes of human malignancies, is the most common and most aggressive primary brain tumor in humans [1]. The current median survival of GBM is only approximately 14 months [2]. Up to date, temozolomide (TMZ) is the mainly used oral alkylating agent to treat GBM and astrocytomas [3,4]. However, most GBM tumors (up to 50%) are refractory to TMZ therapies, which lead to poor survival of GBM patients [2]. The adaptive TMZ resistant GBM cells differ from their parent cells at the molecular level. Therefore, better understanding of biological and molecular mechanisms of TMZ resistance is desperately needed.
Epithelial to mesenchymal transition (EMT) has been suggested to play vital role in the development of drug resistance in multiple cancers from increasing evidences, including routine chemotherapy and targeted therapy [5,6]. Malignant cancer cells often acquire high migratory mobility and invasive ability during process of EMT, specifically disseminating from the primary tumor location and invasion into other organs [7]. Several related mechanisms regarding evoking of EMT have been explored before, including a loss of intercellular cohesion, up-regulation of extracellular matrix components, increased resistance to apoptosis, as well as increased rate of cellular migration and invasion [8,9]. The same cellular remodeling and signaling networks may also contribute to the development of drug resistance in tumor cells [10]. EMT related regulators have been found to correlate with drug resistance in models of drug resistant breast and ovarian cancers [11,12], contribute to chemotherapeutic drugs resistance in lung and bladder cancers [13,14]. Recently, it was also reported that mesenchymal differentiation or transformation promotes radiation resistance or anti-VEGF resistance in glioblastoma [15]. As the key modulator of EMT, SNAI1 (Snail) was well documented in regulation of tumor progression including GBM [16]. However, the function of Snail mediated EMT in GBM chemotherapy is still unknown.
We hypothesized that Snail might have a role in TMZ resistance in GBM. Using the TMZ-resistant GBM cell line model, we demonstrate increased migration and invasion in resistant compared to sensitive cells, that are reverted following knockdown of Snail. We also demonstrated that Snail is upregulated in TMZ resistant tumors, suggesting that these genes may act as biomarkers of chemotherapy resistance. Mechanically, we found that the extra secretion of IL-6 in resistant GBM promotes the transcription of Snail by targeting STAT3. Inhibition of IL-6 by its antibody can overcome the TMZ resistant in GBM, which provided a novel therapy strategy in GBM treatment.
Materials and methods
GBM patients and primary cell culture
Studies involving patient samples were approved by the Jilin University review board. 16 paired (primary and recurrent) GBM tissue samples collected from consented GBM patients, who received standard TMZ-based treatment. Most of the tumor specimens were obtained directly from the surgery. A piece of each was stored at -80°C for later RNA extraction and another piece fixed with formalin and embedded in paraffin for IHC staining. The primary cells derived from primary and recurrent GBM tumors were cultured as previous described [17].
Cell culture and reagents
The GBM cell lines, U-87, A172 were purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). The cell culture condition is at 37°C with 5% carbon dioxide. Cell culture medium is mixed of Dulbecco’s modified Eagle medium (DMEM) consisting of Ham’s F12 medium (1:1) (Invitrogen) with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA). To generate the TMZ resistant cell lines, A172 and U87 were treated with TMZ concentration gradient progressive methods. Briefly, the logarithmic phase A172 or U87 cells were treated with low concentration of TMZ (5 µM) for 48 h. Then the upper cell suspension containing drugs and dead cells was discarded. Cells were collected and re-cultured in TMZ-free culture medium to get recovered before next higher concentration of TMZ treatment, until cells developed resistance to 10 µM TMZ treatment. TMZ was purchased from Sigma-Aldrich.
Transfection of siRNA and plasmid
Lipofectamine® 2000 (Invitrogen) was used for transfection of small interfering RNA (siRNA) and related plasmids [18]. Plasmid expressing the gene Snail was purchased from Addgene (#16218, Cambridge, MA, USA). siRNAs against Snail and STAT3 were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
Real time PCR
RNeasy kit (Qiagen) was used for preparing the total RNA, and iScript cDNA synthesis kit (BioRad) was used for reverse transcribed into cDNA from mRNA. The targeted mRNA expression levels were measured with SYBR Green and a ABI-7900 system (Applied Biosystems) with Snail primers: Forward: 5’-TCA GAA TTC ATG CCG CGC TCT TTC CTC GTC AGG AAG CC-3’, Reverse: 5’-ACT GGA TCC TCA GCG GGG ACA TCC TGA GCA GCC GGAC-3’.
Cell viability assay and apoptosis analysis
1000 cells per well GBM cells were plated in 96-well plates and treated with different dosages of TMZ for 24 h. After drug treatment, plates were examined by the cell viability assay kit (Promega) at the indicated times. Results are reported from at least triplicate samples as the mean ± standard deviation.
Hoechst 33258 staining (3.7% formaldehyde, 0.5% Nonidet P-40, and 10 μg/ml Hoechst 33258 (Invitrogen)) following with microscopic visualization of condensed chromatin and micronucleation was used to analyze GBM cells undergoing apoptosis as described [19].
For analysis of IL-6 level from tumor cells in vivo, 1 × 106 Hey-A8 and 1 × 106 SKOV3.ip1 cells were injected into the peritoneal cavity of female nude mice. The tumors were harvested after 28 days, and the and then evaluated using immunohistochemical, ELISA.
Chromatin Immunoprecipitation (CHIP) assay
Chromatin Immunoprecipitation was performed by CHIP assay kit (Sigm-Aldrich) as described by manufacturer. In brief, cells were crosslinked with 1% formaldehyde for 10 minutes at 37°C, quenched with glycine and PBS, and then sonicated to generate 300-600 bp DNA fragments. Immunoprecipitation was performed with the antibodies indicated, and IgG was used as a control. The PCR primers (5’-TTT CCC TCG TCA ATG CCA CGC-3’ and 5’-TTT GTC ACC TCC GCG CCA-3’), which spans the -484 to -82 region of Snail promoter, included the putative STAT3 and p65 binding site.
Invasion assay and wound healing assay
The invasion of tumor cells was analyzed with cell Invasion Assay Kit (8 μm pore size, Chemicon, MA, USA) as described in the manufacture’s protocol. After 24 h, cotton swab was used to remove the non-invading cells. Then we take photographs of the invaded cells on the lower side of the upper chamber were stained with crystal violet. Final invasion results were calculated by counting the cell numbers under the light microscope for three times.
CytoSelectTM 24-well cell invasion assay kit (Cell Biolabs, San Diego, CA) was used to carry on the wound healing assay according to the manufacturer’s protocol. GBM cells were cultured at the top of the insert. After cells formed a monolayer, the insert was removed to generate wound gap in the middle. To analyze of migration distance, the wound gap was observed at 24 h.
Western blotting and ELISA
RIPA buffer (Thermo Scientific) was used for total protein extraction and BCA Assay (Thermo Scientific) was used for quantification. Equal amounts of the proteins (depending on experiment) were loaded on NuPage 4-12% gradient polyacrylamide gels (Invitrogen) and i-Blot system (Invitrogen) was used for transferring via polyvinylidine fluoride (PVDF) membranes. The blots were then incubated in the primary antibodies; Snail, Vimentin, Caspase-3, STAT3, phospho-STAT3 (Cell Signaling), phospho-p65, p65 (Santa Cruz) and β-actin (Millipore) were used according to the manufacturer’s instructions.
To evaluate the secretion of IL-6 in GBM cells or tumor, 2 × 105 cells or 10 mg tumor samples were homogenized and tested for the presence of human IL-6 by ELISA (R&D Systems).
Xenograft model
The animal studies were approved by the Institutional Animal Ethics Committee (IEC) of Jilin University and experiments were performed in accordance with the Animal Ethics guidelines of Jilin University. Female NOD-SCID mice were used for in vivo tumorigenicity assays. Briefly, 1 × 106 of parental or TMZ-resistant A172 cells were injected subcutaneously in NOD-SCID mice (n = 5) and mice were periodically observed for tumor development. After 7 days, the mice were treated with different reagents via intraperitoneal injection, including TMZ (25 mg/kg), Anti-IL6 (100 mg/kg), TMZ+Anti-IL6 (25 mg/kg and 100 mg/kg respectively), or PBS as a negative control (Control). The drug injection was administrated 3 times a week for total two weeks. The tumor volume was measured each other day, and determined using the formula: 4/3π (√major axis/2 × minor axis/2). After sacrifice, tumors were dissected and followed with western blot or TUNEL staining by using fixed in 10% formalin and embedded in paraffin.
Statistical analysis
Results are reported as the mean ± standard deviation. Significance was tested by T-Test or ANOVA with post-hoc tests using GraphPad 3.0 software (San Diego, CA).
Results
TMZ resistant primary GBM cells showed high expression of Snail
To gain the function of Snail in TMZ resistant GBM, we analyzed the expression of Snail in tumor samples from 16 pair of primary and recurrent GBM samples from patients received TMZ therapy. Our results showed that the expression of Snail was significantly higher in the recurrent GBM tumor samples (Figure 1A), suggesting high expression of Snail might contributes to TMZ resistance. To gain more function of Snail, we generated two GBM primary cells from primary GBM tumors (GBM1) and recurrent tumors (GBM2). As predicted, we found that recurrent GBM cells (GBM2) is more resistant to TMZ induced cell death (Figure 1B). Since apoptosis is the major cell death caused by TMZ, we analyzed the apoptosis in TZM treated GBM1 and GBM2 by Hoechst 333255 staining and western blot of cleavage caspase-3. TMZ treatment induced less apoptosis and caspase-3 cleavage in GBM2 cells than that in GBM1 cells (Figure 1C, 1D). Consistently, the expression level of Snail in GBM1 is lower than that in GBM2 cells (Figure 1D). Since Snail is an important regulator of EMT [20], we also analyzed the cell migration and cell invasion by wound-healing assay and transwell assay. Our data showed that GBM2 cells have higher cell migration and invasion (Figure 1E, 1F). Consistently, the expression of vimentin, an EMT marker, was suppressed in GBM1 cells in response to TMZ treatment, but increased and stabilized in GBM2 cells (Figure 1D). Collectively, our data indicated that the expression of Snail promotes GBM recurrent after TMZ treatment, and contributes to TMZ resistance.
Figure 1.
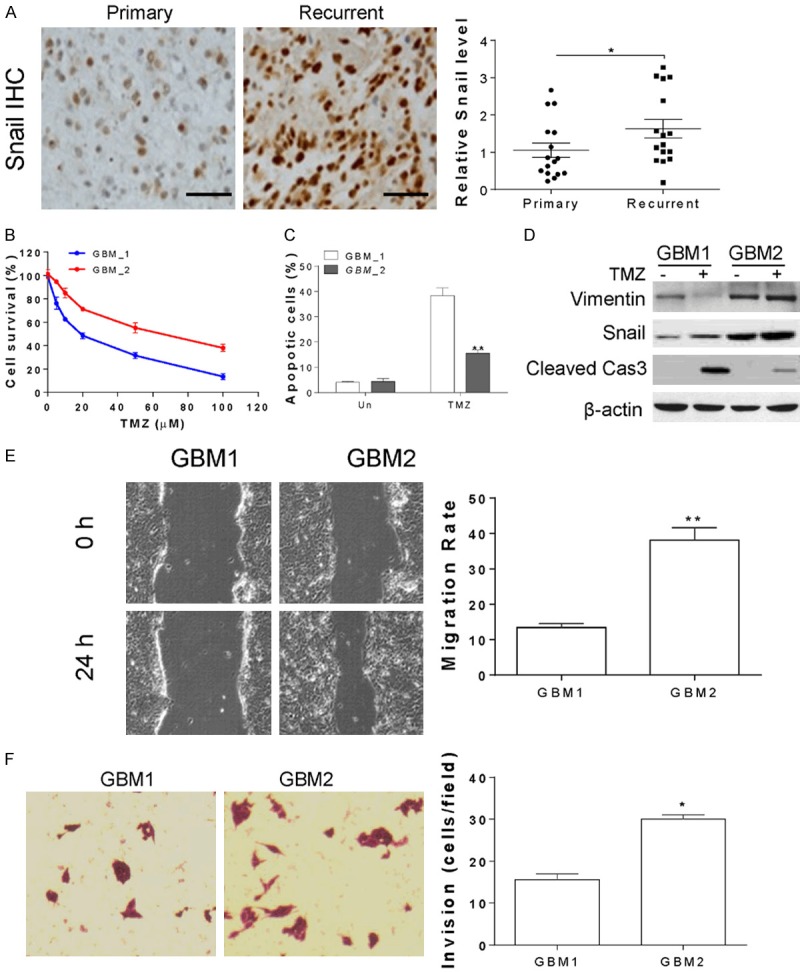
The expression of Snail is higher in TMZ resistant GBM primary cells. A. The relative Snail expression level in 16 pairs of primary and recurrent GBM tumors. The representative pictures of Snail immunochemistry staining were shown. B. The cell viability of primary cells derived from primary tumors (GBM1) and recurrent tumors (GBM2) treated with different doses of TMZ for 24 h. C. The apoptosis of GBM1 and GBM2 treated with 10 µM TMZ for 24 h. D. The expression of indicated proteins in GBM1 and GBM2 treated with 10 µM TMZ for 24 h. E. The cell migration of GBM1 and GBM2. F. The cell invasion of GBM1 and GBM2. *, P<0.05.
TMZ resistant GBM cells line has higher snail expression
To further confirm the expression of Snail in TMZ resistant GBM, we established two different TMZ resistant GBM cell lines by using multiple cycle of TMZ treatment, including A172 and U87 cells. As expected, the TMZ resistant A172 (A172R) and U87 (U87R) showed less apoptosis (Figure 2A). The TMZ resistant A172 and U87 cells also showed higher expression of Snail than the A172 and U87 parental cells (Figure 2B). We also investigated the migration and invasion of A172 and U87 resistant cells, and found that the resistant cell showed higher migration and invasion rate than the parental cells (Figure 2C, 2D). Furthermore, the expression of vimentin is also higher in the A172 and U87 resistant cells, when comparted with their parental cells (Figure 2B). Therefore, our results further confirmed that Snail is upregulated in TMZ resistant GBM cells.
Figure 2.

TMZ resistant GBM cell lines have higher Snail expression. A. The apoptosis of A172 parental (P) or resistant (R) cells and U87 parental (P) or resistant (R) cells treated with 10 µM TMZ for 24 h. B. The expression of Snail and vimentin in A172P, A172R, U87P, U87R cells. C. The cell migration of A172P, A172R, U87P, U87R cells. D. The cell invasion of A172P, A172R, U87P, U87R cells. **, P<0.01.
Expression of Snail contributes to TMZ resistance in GBM
In the next step, we investigated whether the expression of Snail is necessary for TMZ resistance in GBM. Enhanced expression of Snail by transfection of pCMV-Flag-Snail in A172 parental cells suppressed the cleavage of caspase-3, and enhanced the expression of Vimentin (Figure 3A). Consistently, overexpression of Snail compromised the apoptosis in A172 parental cells treated with TMZ (Figure 3B). The wound-healing assay and transwell assay also suggested that Snail overexpression promotes the migration and invasion of A172 cells (Figure 3C, 3D). Reversely, knockdown of Snail by its siRNA sensitized the A172 TMZ resistant cells to TMZ induced apoptosis, as indicated by cleavage of caspase-3 (Figure 3E), and Hoechst 333255 staining (Figure 3F). Abrogation of Snail expression also reduced the expression of vimentin (Figure 3E), and suppressed the A172 resistant cells migration and invasion (Figure 3G, 3H). Therefore, it can be concluded that Snail mediated EMT promotes the TMZ resistance in GBM cells.
Figure 3.
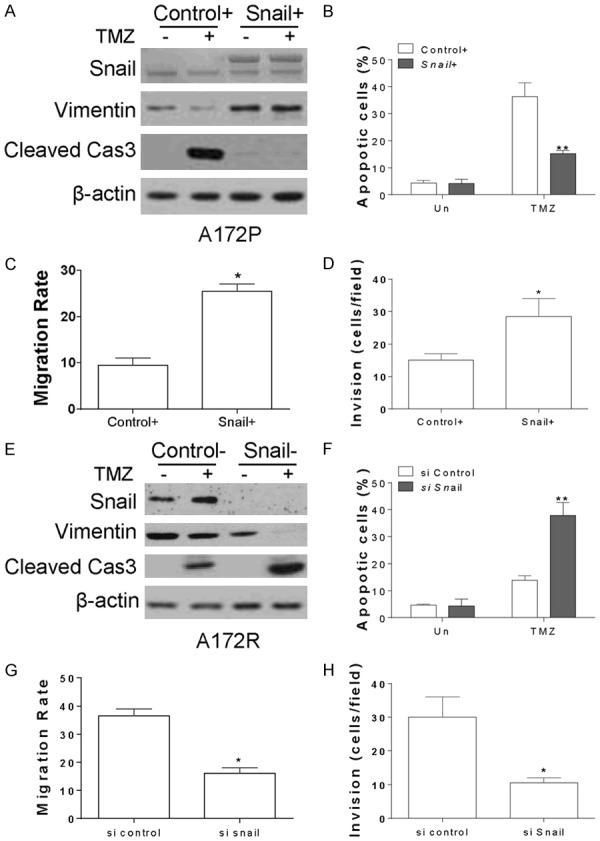
Expression of Snail promotes the TMZ resistance in GBM. (A) A172P cells were transfected with control or pCMV-Flag-Snail, and treated with 10 µM TMZ for 24 h. The expression of indicated proteins was detected by western blot. (B) The apoptosis of A172P cells treated as in (A). (C) The cell migration of A172P cells transfected with control or pCMV-Flag-Snail plasmids. (D) The cell invasion of A172P cells transfected with control or pCMV-Flag-Snail plasmids. (E) A172R cells were transfected with control or Snail siRNA, and treated with 10 µM TMZ for 24 h. The expression of indicated proteins was detected by western blot. (F) The apoptosis of A172R cells treated as in (E). (G) The cell migration of A172R cells transfected with control or Snail siRNA. (H) The cell invasion of A172R cells transfected with control or Snail siRNA. **, P<0.01.
The expression of Snail is modulated by STAT3 in GBM
Since the abnormal expression of Snail contributes to TMZ resistance in GBM, it would be interesting to study the mechanism for its upregulation in GBM resistant cells or tumor. As the Snail expression is usually modulated in mRNA level, we firstly investigated the mRNA level of Snail in the primary or recurrent GBM tumors, and found that the mRNA expression of Snail is significantly higher in recurrent tumors (Figure 4A). Consistently, the mRNA level in TMZ resistant cells is also much higher than the parental cells (Figure 4B), further confirmed that the expression of Snail in TMZ resistant GBM is modulated in its mRNA level. It was reported that the mRNA expression of Snail can be modulated by p65 or STAT3 [15,21]. In our study, we found that the only the phosphorylation of STAT3 (p-STAT3) is higher in TMZ resistant GBM cells (Figure 4C). Furthermore, the binding of STAT3 with Snail promoter is also higher in TMZ resistant A172 cells than the A172 parental cells (Figure 4D), suggesting STAT3 might be the upstream transcription factor of Snail in TMZ resistant GBM. In contrast, no difference of p65 binding with Snail promoter in A172 resistant and parental cells was found (Figure 4D), ruling out the function of p65 in modulating the Snail mRNA in TMZ resistant GBM cells. To confirm the role of STAT3 in modulating the expression of Snail, the siRNA for STAT3 was transfected into A172 resistant cells. Silence of STAT3 abolished the expression of Snail in protein and mRNA level (Figure 4E, 4F). Depletion of STAT3 also re-sensitized the A172 resistant cells to TMZ induced apoptosis (Figure 4E, 4G), and suppressed the migration and invasion of resistant cells (Figure 4E, 4H). Therefore, our results indicated that STAT3 is the upstream transcription factor for Snail expression in TMZ resistant GBM.
Figure 4.
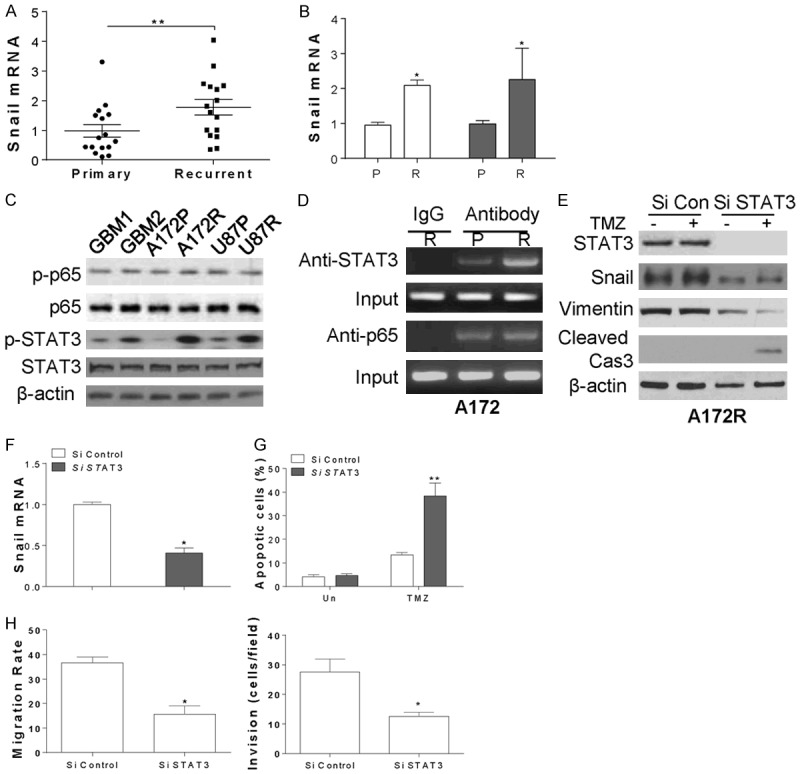
STAT3 mediates the Snail expression in TMZ resistant GBM cells. (A) The relative Snail mRNA expression level in 16 pairs of primary and recurrent GBM tumors. (B) The relative Snail mRNA expression level in A172P, A172R, U87P and U87R cells. (C) The expression of indicated proteins in GBM1, GBM2, A172P, A172R, U87P and U87R cells. (D) The binding of STAT3 or p65 with Snail promoter was detected by CHIP assay. (E) A172R cells were transfected with control or STAT3 siRNA, and treated with 10 µM TMZ for 24 h. The expression of indicated proteins was detected by western blot. (F) The mRNA level of Snail in A172R cells transfected with control or STAT3 siRNA. (G) The apoptosis of A172R cells treated as in (E). (H) The cell migration and invasion of A172R cells transfected with control or Snail siRNA. **, P<0.01.
Secretion of IL-6 promotes the expression of Snail in TMZ resistant GBM
Since secretion of IL-6 promotes Snail related changes in tumor cells [22], we further investigated whether secretion of IL-6 contribute to TMZ resistant in GBM. We found that the IL-6 secretion in recurrent GBM tumors is much higher than that in primary tumors (Figure 5A). Similarly, the IL-6 level in TMZ resistant GBM cells is also higher than that in parental cells (Figure 5B). Treating the A172 or U87 resistant cell with IL-6 antibody (anti-IL6) suppressed the phosphorylation of STAT3, as well as the expression the Snail and vimentin (Figure 5C). Inhibition of IL-6 by anti-IL6 also suppressed the mRNA level of Snail (Figure 5D), as well as the binding of STAT3 with Snail promoter (Figure 5E), indicating that IL-6 stimulated the transcription activity of STAT3 in Snail promoter. Furthermore, pre-treatment of anti-IL6 re-sensitized the A172 resistant cell to TMZ induced apoptosis (Figure 5F), and suppressed the A172 resistant cells migration and invasion (Figure 5G). Collectively, our results suggested that inhibition of IL-6 might be a strategy to overcome the TMZ resistance in GBM.
Figure 5.
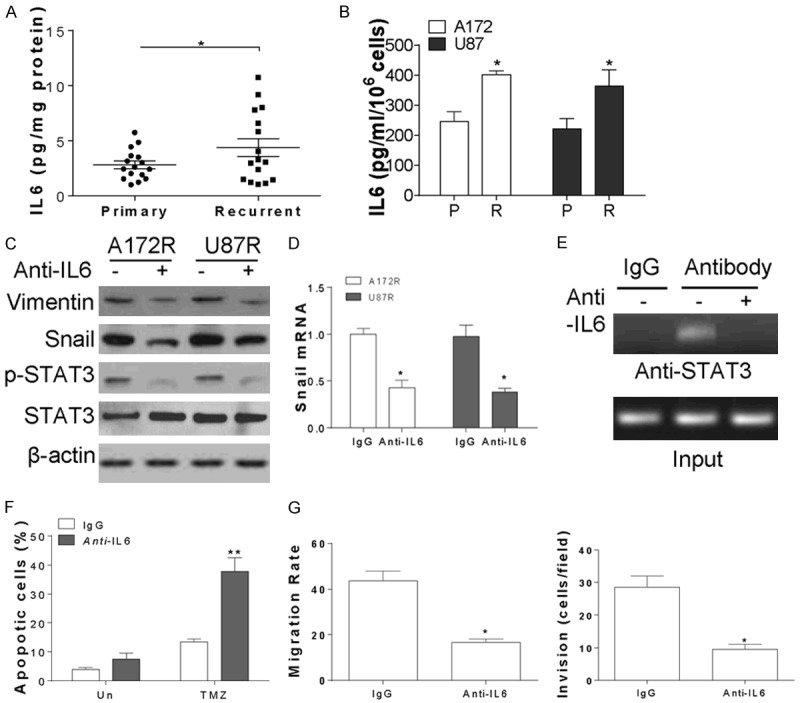
IL-6 secretion in TMZ resistant GBM cells activated the transcription of Snail by STAT3. A. The secretion of IL-6 in 16 pairs of primary and recurrent GBM tumors. B. The secretion of IL-6 in A172P, A172R, U87P and U87R cells. C. The expression of indicated proteins in A172R and U87R cells treated with 10 ng/ml IL-6 antibody. D. The mRNA level of Snail in A172R and U87R cells treated with 10 ng/ml IL-6 antibody. E. The binding of STAT3 with Snail promoter in A172R cells treated IL-6 antidody was detected by CHIP assay. F. The apoptosis in A172R cells treated with 10 µM TMZ with or without 10 ng/ml IL-6 antibody combination. G. The cell migration and invasion of A172R cells treated with 10 ng/ml IL-6 antibody. *, P<0.05; **, P<0.01.
Anti-IL6 overcome TMZ resistance in GBM in vivo
To explore if TMZ resistance in GBM benefit from the combination treatment of TMZ and IL-6 inhibition in vivo. We implanted A172 parental and resistant cells subcutaneously into the flanks of the BALB/c nude mice. After 7 days, the mice were randomly divided into 6 groups and administered an intraperitoneal injection of TMZ, anti-IL6, TMZ+anti-IL6, or PBS as a negative control (Control). 17 days later, the tumor from the A172 resistant cells has less response and lager tumor size than the tumors from A172 parental cells (Figure 6A, 6B). The tumor growth was greatly suppressed by combined treatment of TMZ with anti-IL6, compared with the TMZ or anti-IL6 single treatment in resistant tumors (Figure 6A, 6B). The caspase 3 activation in different tumors after treatment was also analyzed. The data showed that TMZ treatment actually induced cleavage of caspase-3 in A172 parental tumors, which was abrogated in A172 resistant tumors (Figure 6C). The combination of TMZ with FLA rescued the caspase-3 activation in A172 resistant tumor (Figure 6C). Consistently, the expression of Vimentin and Snail was higher in A172 resistant tumors, which was reduced by anti-IL6 treatment (Figure 6C). TUNEL staining results indicated that the combination treatment can re-activate the apoptosis in A172 resistant tumors (Figure 6D). Therefore, inhibition of IL-6 could re-sensitize the GBM resistant cells to TMZ treatment in vivo.
Figure 6.
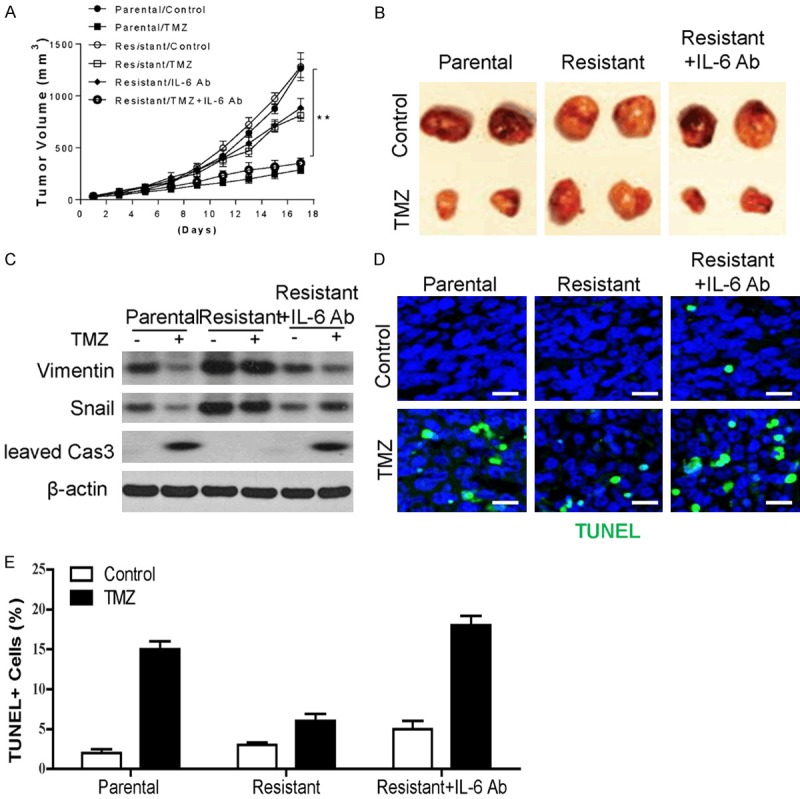
Targeting IL-6 re-sensitized the TMZ resistant GBM to TMZ treatment in vivo. (A) Tumor growth of A172 parental or TMZ resistant cells xenografted NOD-SCID mice (n = 5) received intraperitoneal injection of TMZ (25 mg/kg), anti-IL6 (100 mg/kg), TMZ+anti-IL6 (25 mg/kg and 100 mg/kg respectively), or PBS as a negative control (Control). (B) The representative tumor in each group. (C) The expression of indicated proteins in each group of tumor. (D) The TUNEL staining of tissue sections from different groups of tumors. (E) Quantification of staining in (D). *, P<0.05; **, P<0.01.
Discussion
Improved understanding of signaling networks that regulate chemoresistance in GBM has encouraged the adaptation of targeted molecular therapies to treat GBM [23]. It was well-established that Snail play an important role in EMT during both embryogenesis and tumor metastasis [24]. More and more studies revealed that Snail mediated EMT also take effects in chemoresistance in various cancers, including lung cancer, ovarian cancer, breast cancer, et al [5,6,9]. In this study, we showed a novel role of Snail in regulation of chemoresistance in GBM. We found that the expression of Snail correlated to the TMZ resistance in GBM tumors and cell lines, suggested that the expression of Snail can be a biomarker for the chemoresistance in GBM. The expression of Snail in GBM resistant cells is modulated by STAT3, which is stimulated by the over secretion of IL-6. Inhibition of IL-6 overcomes the TMZ resistance, and re-sensitizes the GBM to TMZ induced apoptosis in vitro and in vivo. Generally, our data presented here provided a new mechanism for TMZ resistance in GBM, and indicated that suppression of IL-6 might be an alternative method to overcome the TMZ resistance in GBM.
Although previous studies pointed the important role of EMT in tumor development and metastasis, it may also contribute to a cellular ability to evade the effects of chemotherapies [5]. In cancer progression, EMT has been suggested to regulate the invasion of tumor cells and facilitates metastasis by converting a non-motile cancerous epithelial cell into a motile mesenchymal cell, which disseminate from the tumor mass and enter the circulatory or lymphatic system [25]. As to the drug resistance, EMT may rely on many of the same transcription factors that function in embryogenesis and metastasis. However, the details of their regulation are largely unknown. In our study, we found that Snail mediated EMT is involved in TMZ resistance in GBM. The expression of Snail is relative higher in TMZ resistant GBM, and the TMZ resistant GBM cells showed higher percentage of migration and invasion. Depletion of Snail or inhibition its upstream effector, IL-6/STAT3, re-sensitized the GBM resistant cells to TMZ treatment. Consistently, upregulation of Snail has been correlated with resistance to radiation and paclitaxel in ovarian cancer [6]. It was also reported that elevated Snail expression promotes glial-mesenchymal transition after irradiation in malignant glioma, and increases the recurrence of GBM [20]. Therefore the idea that the EMT gene, Snail, play a significant role in TMZ resistance in GBM is supported by previous evidence.
As to the expression of Snail, STAT3 activation was found to be necessary for elevated levels of Snail in TMZ resistant GBM. It was previously reported that constitutive active STAT3 frequently expressed in high-grade gliomas [26]. Increased expression of STAT3 is also found in adaptive TMZ resistant U87 cells [21]. Several anti-STAT3 agents have been discovered and evaluated in cultured cancer cells and in xenografts due to the potential anticancer target therapy of STAT3, such as the antitumor activity of anti-STAT3 platinum compounds, CPA1 and CPA7 in colon tumors [27], and AG490 in GBM cells [28]. One recent study also found that WP1066 presents better anti-proliferative efficacy on U87MG malignant gliomas than AG490 [29]. The EMT transcription factors Snail was also regulated by inhibition of STAT3 in murine Tu-2449 glioma cells [30]. In human GBM cells, the similar regulation pattern contributed to TMZ resistance, decreased expression of the Snail can be achieved with knockdown of STAT3. Concomitantly, STAT3 silencing decreased the migratory and invasive behavior of the tumor cells, and therefore abrogated the TMZ resistance. Therefore, STAT3 inhibition might yield additional benefit beyond the arrest of diffuse infiltration in GBM therapy.
IL-6 is a cytokine playing an important role in the inflammation, and exerts crucial effects on the growth of some types of cells [31]. However, is not solely synthesized and released by inflammatory cells. IL-6 derived from tumor cells is also able to regulate some pathological processes related to the growth, invasion and apoptosis of cancer cells [32,33]. In this study, we found that IL-6 secretion was at a high level in TMZ resistant GBM, which was consistent with previous findings [34]. Furthermore, our results also revealed IL-6 secretion promotes the activation of STAT3 and enhanced the expression of Snail, which contributes to the TMZ resistance in GBM. Other than promoting the infiltration of immune cells into the tumor [35], IL-6 was also reported to enhance STAT3 phosphorylation, which elevates anti-apoptosis of tumor cells. It was recently reported that humanized anti-IL6 monoclonal antibodies have been evaluated in clinical trials [36], and demonstrated anti-tumor effect in GBM [34]. These data in combination with our results of TMZ combined with IL-6 antibody treatments in GBM xenografts. The antitumor effect of TMZ is schedule-dependent with multiple administrations being more effective than a single treatment. Our results suggest that IL-6 antibody may be useful combination choice for TMZ treatment against GBM.
Taken together, our data revealed a novel function of Snail in TMZ resistance in GBM. The modulation of Snail in TMZ resistant GBM is tightly controlled by IL-6/STAT3 pathway. Moreover the expression of Snail, IL-6 might be a useful marker for TMZ resistance in GBM. Future efforts should be taken on targeting the IL-6/STAT3/Snail pathway, which might benefit the GBM chemotherapy.
Acknowledgements
Natural Science Foundation of Jilin Provincial Science and Technology Department (20190201030JC).
Disclosure of conflict of interest
None.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Huse JT, Holland EC. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat Rev Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 3.Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal brain tumor group. J. Clin. Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 4.Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013:CD007415. doi: 10.1002/14651858.CD007415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 7.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 8.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Iseri OD, Kars MD, Arpaci F, Atalay C, Pak I, Gunduz U. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed Pharmacother. 2011;65:40–45. doi: 10.1016/j.biopha.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Haslehurst AM, Koti M, Dharsee M, Nuin P, Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, Davey S, Squire J, Park PC, Feilotter H. EMT transcription factors snail and slug directly contribute to cisplatin resistance in ovarian cancer. BMC Cancer. 2012;12:91. doi: 10.1186/1471-2407-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang TH, Tsai MF, Su KY, Wu SG, Huang CP, Yu SL, Yu YL, Lan CC, Yang CH, Lin SB, Wu CP, Shih JY, Yang PC. Slug confers resistance to the epidermal growth factor receptor tyrosine kinase inhibitor. Am J Respir Crit Care Med. 2011;183:1071–1079. doi: 10.1164/rccm.201009-1440OC. [DOI] [PubMed] [Google Scholar]
- 14.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, Siefker-Radtke A, Dinney C. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–344. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat KPL, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WFA, Boddeke HWGM, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caja L, Tzavlaki K, Dadras MS, Tan EJ, Hatem G, Maturi NP, Moren A, Wik L, Watanabe Y, Savary K, Kamali-Moghaddan M, Uhrbom L, Heldin CH, Moustakas A. Snail regulates BMP and TGFbeta pathways to control the differentiation status of glioma-initiating cells. Oncogene. 2018;37:2515–2531. doi: 10.1038/s41388-018-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seidel S, Garvalov BK, Acker T. Isolation and culture of primary glioblastoma cells from human tumor specimens. Methods Mol Biol. 2015;1235:263–275. doi: 10.1007/978-1-4939-1785-3_19. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Song X, Yue W, Chen D, Yu J, Yao Z, Zhang L. Fibulin-5 inhibits wnt/beta-catenin signaling in lung cancer. Oncotarget. 2015;6:15022–15034. doi: 10.18632/oncotarget.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D, Ming L, Zou F, Peng Y, Van Houten B, Yu J, Zhang L. TAp73 promotes cell survival upon genotoxic stress by inhibiting p53 activity. Oncotarget. 2014;5:8107–8122. doi: 10.18632/oncotarget.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahabir R, Tanino M, Elmansuri A, Wang L, Kimura T, Itoh T, Ohba Y, Nishihara H, Shirato H, Tsuda M, Tanaka S. Sustained elevation of Snail promotes glial-mesenchymal transition after irradiation in malignant glioma. Neuro Oncol. 2014;16:671–685. doi: 10.1093/neuonc/not239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohsaka S, Wang L, Yachi K, Mahabir R, Narita T, Itoh T, Tanino M, Kimura T, Nishihara H, Tanaka S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol Cancer Ther. 2012;11:1289–1299. doi: 10.1158/1535-7163.MCT-11-0801. [DOI] [PubMed] [Google Scholar]
- 22.Chen W, Gao Q, Han S, Pan F, Fan W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-Twist signaling. Tumour Biol. 2015;36:973–981. doi: 10.1007/s13277-014-2717-z. [DOI] [PubMed] [Google Scholar]
- 23.Ohka F, Natsume A, Wakabayashi T. Current trends in targeted therapies for glioblastoma multiforme. Neurol Res Int. 2012;2012:878425. doi: 10.1155/2012/878425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to iressa and alkylators. Clin Cancer Res. 2008;14:6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turkson J, Zhang S, Palmer J, Kay H, Stanko J, Mora LB, Sebti S, Yu H, Jove R. Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent antitumor activity. Mol Cancer Ther. 2004;3:1533–1542. [PubMed] [Google Scholar]
- 28.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 29.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, Kondo S, Priebe W, Kondo Y. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 30.Priester M, Copanaki E, Vafaizadeh V, Hensel S, Bernreuther C, Glatzel M, Seifert V, Groner B, Kogel D, Weissenberger J. STAT3 silencing inhibits glioma single cell infiltration and tumor growth. Neuro Oncol. 2013;15:840–852. doi: 10.1093/neuonc/not025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Mao Y, Wang J, Zu L, Hao M, Cheng G, Qu Q, Cui D, Keller ET, Chen X, Shen K, Wang J. IL-6 secreted by cancer-associated fibroblasts induces tamoxifen resistance in luminal breast cancer. Oncogene. 2014;33:4450. doi: 10.1038/onc.2014.158. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Ye Y, Zhang X, Song J. Bradykinin stimulates IL-6 production and cell invasion in colorectal cancer cells. Oncol Rep. 2014;32:1709–1714. doi: 10.3892/or.2014.3366. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB, Rich JN. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Lai W, Zhang Y, Liu L, Luo X, Zeng Y, Wu H, Lan Q, Chu Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer. 2014;14:330. doi: 10.1186/1471-2407-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]


