Abstract
Background: Vascular smooth muscle cells (VSMCs) play an important role in foam cell formation, a hallmark of atherosclerosis obliterans (ASO). We recently demonstrated that miR-223 is significantly upregulated both in atherosclerotic arteries and in the serum sample of ASO patients. However, it is still unknown if miR-223 is implicated in the foam cell formation of VSMCs. The current study aimed to investigate the role of miR-223 in the foam cell formation of VSMCs. Methods: Artery and serum samples were collected from ASO patients. Human VSMCs were isolated from the normal arteries of healthy donors. For miR-223 overexpression, miR-223 mimic was transfected into VSMCs using Lipofectamine 2000. Foam cell formation was evaluated by lipid accumulation using Oil Red O staining. Luciferase assay was adopted to confirm the target gene of miRNA. Results: miR-223 was significantly upregulated in both the arteries and serum samples from ASO patients. miR-223 overexpression significantly inhibited the foam cell formation and decreased total intracellular cholesterol levels in VSMCs. miR-223 overexpression induced autophagy of VSMCs. Blocking autophagy by 3-methyladenine or autophagy-related 7 (Atg7) siRNAs attenuated the inhibitory effect of miR-223 overexpression on foam cell formation. Luciferase assay showed that IGF-1R is a direct target of miR-223. miR-223 overexpression reduced protein levels of IGF-1R expression and the phosphorylated form of PI3K and Akt proteins. Conclusions: miR-223 overexpression inhibited foam cell formation in VSMCs, at least partially, via inducing autophagy. The IGF-1R/PI3K/Akt signaling pathway may be also involved in this mechanism.
Keywords: Arteriosclerosis, vascular smooth muscle cells, miR-223, autophagy, foam cell formation
Introduction
Atherosclerosis obliterans (ASO) in the lower extremities is the most common peripheral arterial disease and is the leading cause of leg amputation [1]. During pathogenesis of atherosclerosis, one key step is foam cell formation characterized by intracellular accumulation of cholesterol esters and triglycerides [2,3]. The Foam cells could be derived from macrophages or vascular smooth muscle cells (VSMCs) [2]. Some studies have demonstrated that VSMCs are the major cells to form the foam cells in atherosclerosis lesions [4,5]. Compared with macrophage-derived foam cells, the molecular mechanism underlying VSMCs-derived foam cells in atherosclerosis remains relatively unclear. Elucidating this mechanism may be helpful for developing the strategy of the prevention and treatment of ASO.
Autophagy is an evolutionarily conserved process involved in the degradation of dysfunctional and damaged organelles or proteins in the cytosol, which plays an important role in maintaining cytoplasmic homeostasis [6,7], and glucose, protein and lipid metabolism [8,9]. Autophagy has also been shown to be involved in foam cell formation, in which autophagy induction reduced the foam cell formation in rat VSMCs, but autophagy inhibition increased foam cell formation [10]. These results suggest that autophagy may play an important role in foam cell formation in human VSMCs.
MicroRNAs (miRNAs) are small non-coding RNAs with 18-24 nucleotides in length, and are able to regulate gene expression at the post-translational level [11]. Accumulating evidence has suggested that miRNAs play an important role in the pathogenesis of cardiovascular diseases [12-15]. We have previously demonstrated that the blood cell-secreted miR-223 can enter VSMCs and play a crucial role in VSMC function and atherogenesis [16]. In addition, miR-223 knockdown deteriorates the atherosclerotic lesions in apolipoprotein-E knockout mice [16]. However, it is still unknown if miR-223 is implicated in the foam cell formation of VSMCs. Therefore, the purpose of this study was to investigate the potential role of miR-223 in foam cell formation in human VSMCs.
Materials and methods
Sample acquisition
ASO artery samples were collected from patients with ASO who received lower limb amputation (n = 5, 3 males, 2 females, mean age = 62 ± 5.3 years), and normal artery samples were obtained from healthy organ donors without ASO (n = 5, 3 males, 2 females, mean age = 57 ± 5.6 years). Serum samples were collected from ASO patients (n = 5) and healthy control donors (n = 5). All samples were obtained from three vascular surgeons blinded to the experimental design. This study was performed in compliance with the Declaration of Helsinki and approved by the Research Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University with the informed consent of all subjects.
Cell culture
Human VSMCs were isolated from the normal arteries of healthy donors using the standard method as described in our previous study [17]. VSMCs were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Karlsruhe, Germany) with 10% fetal bovine serum (FBS; Gibco, Karlsruhe, Germany) and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified incubator with an atmosphere containing 5% CO2. VSMCs were verified by staining with an anti-smooth muscle α-actin antibody (Abcam, Burlingame, CA, USA). Cells at passage 3-8 were used for all experiments.
Transfection of miR-223 mimic, small interfering RNA (siRNA) or GFP-LC3
VSMCs were cultured on coverslips in 24-well plates overnight and transfected with GFP-LC3 expression plasmid (1 ug/ml, pEGFP-LC3, Cell Biolabs, San Diego, CA, USA), miR-223 mimic (50 nM; sense: 5-UGUCAGUUUGUCAAAUACCCCA-3; anti-sense: 5-UGGGGUAUUUGACAAACUGACA-3), or negative control oligo (50 nM) using RNAiMAX and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in Opti-MEM medium according to the manufacturer’s instructions. For knockdown of autophagy-related 7 (Atg7), Atg7 siRNA (50 nM) (Applied Biosystems, ThermoFisher Scientific, Waltham, MA Foster City, CA, USA; Cat#4392420, siRNA1 ID: s20650; siRNA2 ID: s20651; siRNA2 ID: s20652) was applied. Transfection medium was replaced by complete medium after 8 hours of transfection. After transfection, the cells were viewed by fluorescence microscopy and LC3 puncta and GFP-LC3 puncta (> 1 μm) was calculated using Image-Pro Plus 6.0 software.
Immunofluorescence of LC3 in VSMCs
VSMCs were seeded on coverslips in 24-well plates overnight, followed by different reagent treatments. Forty-eight hours after treatments, VSMCs were fixed with ice-cold paraformaldehyde for 15 min, followed by permeabilizing with 0.1% Triton X-100 in PBS for 5 min and blocking with 1% BSA for 1 h. Subsequently, cells were incubated with rabbit anti-LC3 antibody (Cell Signaling Technology, Boston, MA, USA) at 4°C overnight. After washing three times with PBS, cells were incubated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA) for 1 h in dark at room temperature. Finally, cells were observed under a fluorescence microscopy and analyzed by Image-Pro Plus 6.0 software [18].
Western blot analysis
Proteins were extracted from VSMCs using RIPA lysis buffer (Cell Signaling Technology, Boston, MA, USA) supplemented with protease inhibitor cocktail (Roche, Basel, Switzerland). Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking, membranes were incubated overnight at 4°C with appropriate primary antibodies. Membranes were washed and incubated with the horseradish peroxidase (HRP)-conjugated secondary antibodies. The protein was detected by ECL and imaged using a GE ImageQuant Las 4000 mini (GE, Fairfield, CT, USA). Western blot quantification was performed by densitometry and normalized to GAPDH. Antibodies against IGF-1R (1:1000), p-AKT/t-AKT (1:2000), p-PI3K/t-PI3K (1:2000), LC3 (1:500), Beclin-1 (1:2000), Atg7 (1:1000), GAPDH (1:2000) and p62 (1:2000) were purchased from Cell Signaling Technology (Boston, MA, USA).
Oil Red O staining and intracellular total cholesterol content measurement
Lipid accumulation was quantified by Oil Red O staining [19]. Cultured VSMCs with different treatments were plated on 6-well plates. To induce foam cell formation, VSMCs were incubated with 80 μg/ml oxidized low-density lipoprotein (ox-LDL) for 24 h. After that, the cells were washed with PBS, fixed by 4% paraformaldehyde and stained with Oil Red O (Sigma-Aldrich Co., USA). Stained cells were photographed under a microscope at 200× magnification. Intracellular total cholesterol content was detected by Cholesterol Quantitation Kit (Sigma-Aldrich Co., USA) according to manufacturer’s instructions. Briefly, intracellular cholesterol was extracted with isopropyl alcohol from VSMCs treated with ox-LDL. After centrifugation at 1,500× g for 10 min, the supernatant was applied to detect total cholesterol by enzymatic assay. Sediment was lysed by lysis buffer on ice, followed by centrifugation. The supernatants then were used to determine total cellular protein levels by Bradford method. The intracellular total cholesterol content was presented as mg of cholesterol per mg of cellular protein. The results were normalized to the control.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s protocol. For miRNA, total RNA was subjected to reverse transcription with miRNA RT reagent kit (Takara, Dalian, China). Real-time PCR was performed to detect the expression level of mir-223 using SYBR PrimeScript miRNA Real-Time PCR Kit (Takara, Dalian, China) according to the manufacturer’s instruction. For mRNA, first-strand cDNA synthesis was performed using the PrimeScript RT reagent kit (Takara, Dalian, China). Real-time PCR was performed using the PrimeScript Real Time Master Mix (Takara, Dalian, China) according to the manufacturer’s recommendations. The primer sequences used for PCR were:
IGF-1R, 5’-ACCTCATTGGCCATGGAAACA-3’ (forward) and 5’-GACAATGACGGCAGCCAGAC-3 (reverse); GAPDH, 5’-GCACCGTCAAGGCTGAGAAC-3’ (forward) and 5’-TGGTGAAGACGCCAGTGGA-3’ (reverse); mir-223, 5’-CTGGTCCTAGTCAAATACCCCA-3’.
3’UTR luciferase assay
PsiCHECKTM-2 luciferase reporter plasmids (Promega, Madison, WI, USA) containing putative or mutated IGF-1R 3’UTR was co-transfected with negative oligo control (50 nM), miR-223 mimic (50 nM) or miR-223 inhibitor (50 nM; 5-UGGGGUAUUUGACAAACUGACA-3; an antisense sequence of miR-223) into HEK 293 cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Cells were harvested 48 h after transfection and luciferase activity was measured by using the Dual-Luciferase Reporter Assay System Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
Statistical analysis
All data was presented as the mean ± SD. Student’s t-test or one-way analysis of variance was conducted for the statistical analysis to identify significant differences. The difference was considered statistically significant at P < 0.05. GraphPad Prism version 5.0 (GraphPad, San Diego, CA, USA) and SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) were applied for our statistical analyses.
Results
miR-223 expression was significantly upregulated in arteries and serum from patients with ASO
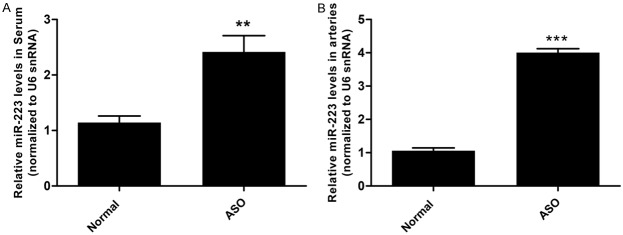
To assess the miR-223 expression in arteries and serum, arteries and serum samples were collected from 5 ASO patients and 5 normal controls. The levels of miR-223 were determined using qRT-PCR. The results showed that the level of miR-223 was significantly upregulated in both arteries and serum of ASO patients as compared with the normal control samples (Figure 1) miR-223 was upregulated by 3.77-fold in ASO arteries and 2.10-fold in ASO serum, respectively.
Figure 1.
miR-223 was upregulated in arteries and serum of ASO patients. A. The levels of miR-223 were higher in ASO arteries as compared with normal arteries determined by qRT-PCR (n = 5). B. The levels of miR-223 in serum were higher in ASO patients as compared with normal controls determined by qRT-PCR (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
miR-223 overexpression inhibited the foam cell formation in ox-LDL-treated human VSMCs
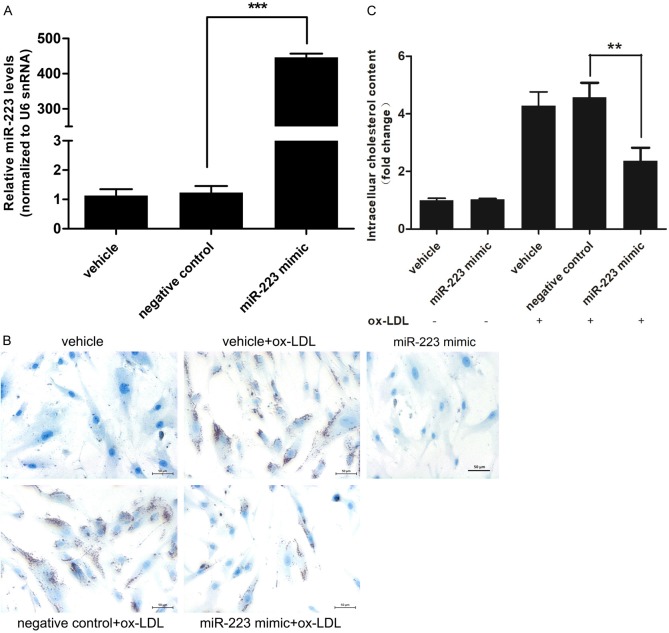
To investigate the role of miR-223 in foam cell formation, we overexpressed miR-223 by transfecting miR-223 mimic into VSMCs (Figure 2A) and observed the effect on VSMCs loaded with ox-LDL (80 μg/ml). Oil Red O staining showed that miR-223 overexpression significantly inhibited the foam cell formation of VSMCs (Figure 2B). The total intracellular cholesterol contents were decreased by 1.93 folds (Figure 2C) as compared with the control group.
Figure 2.
miR-223 mimic inhibited the foam cell formation in ox-LDL-treated VSMCs. A. The level of miR-223 in VSMCs was markedly increased after transfection of miR-223 mimic (50 nmol/L) (n = 6). B. Lipid droplet accumulation (red color by Oil Red O staining) in ox-LDL (80 μg/ml)-treated-VSMCs after transfection with negative control oligo (50 nmol/L) or miR-223 mimic (50 nmol/L) (n = 4). C. Fold change of intracellular cholesterol contents of ox-LDL (80 μg/ml)-treated-VSMCs after transfection with negative control oligo (50 nmol/L) or miR-223 mimic (50 nmol/L) (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
miR-223 overexpression induced autophagy in VSMCs
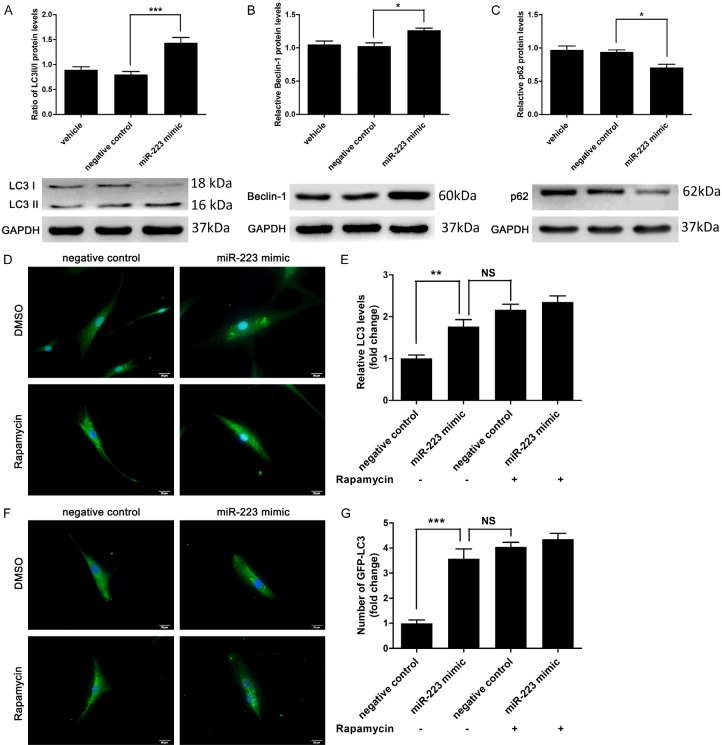
It has been reported that activation of cellular autophagy could decrease the levels of total cholesterol and cholesterol esters in both macrophages and VSMCs treated with ox-LDL, as well as inhibit the foam cell formation [10,20]. To address if autophagy participates in the effect of miR-223, the levels of autophagy-related proteins was assessed by Western blot. miR-223 overexpression remarkably increased LC3II/LC3I ratio and beclin-1 expression but decreased p62 in VSMCs (Figure 3A-C). The immunofluorescence staining for endogenous LC3 showed that miR-223 overexpression significantly enhanced the autophagy formation as compared with the negative control group (Figure 3D and 3E). miR-223 overexpression significantly increased the number of GFP-LC3 puncta in VSMCs (Figure 3F and 3G).
Figure 3.
miR-223 induced autophagy in VSMCs. A-C. miR-223 increased the expression of the ratio of LC3II/LC3I and beclin-1 expression whereas the expression of p62 was decreased, as determined by western blot analysis (n = 6). D and E. Immunostaining and fluorescence microscopy analysis of LC3 (green) and nuclear (blue) showed that miR-223 mimic (50 nmol/L) markedly induced autophagy formation in VSMCs (scale bar: 20 μm; n = 5). Rapamycin (10 nmol/L, an inducer of autophagy) was used as a positive control for autophagy of VSMCs. F and G. Fold change of GFP-LC3 puncta in VSMCs co-transfected with GFP-LC3 (1 ug/ml) and miR-223 mimic (50 nmol/L), as determined by fluorescence microscopy analysis and quantification (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
Inhibitory effect of miR-223 overexpression on foam cell formation in VSMCs was related to its effect on autophagy
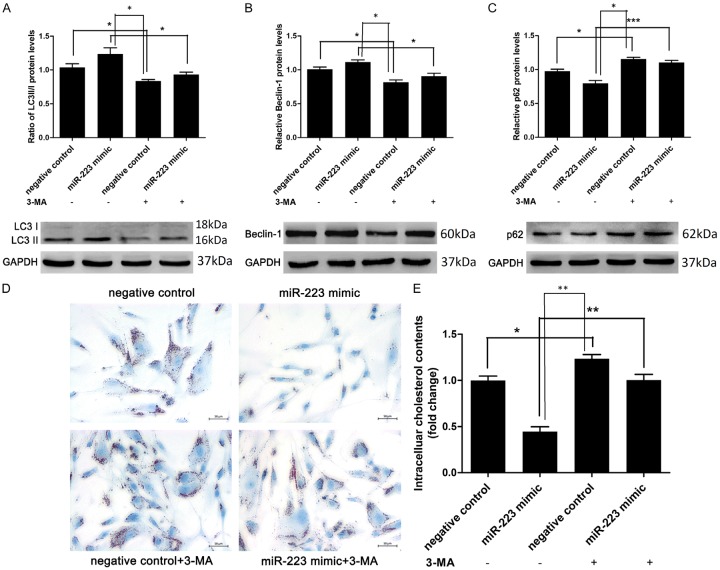
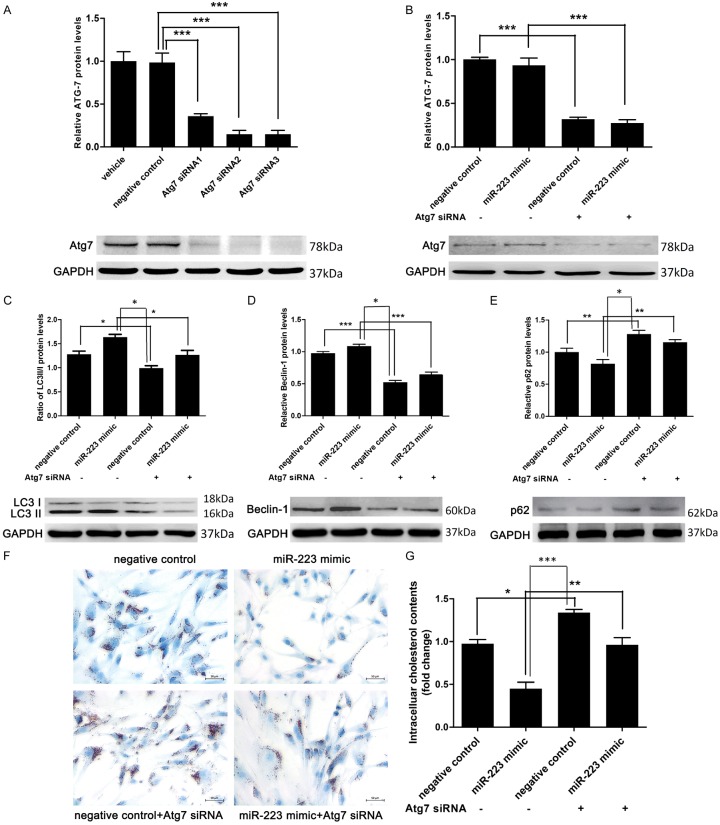
To investigate the role of autophagy in the inhibitory effect of miR-223 on foam cell formation in VSMCs, we blocked the autophagy in VSMCs by autophagy inhibitor 3-methyladenine (3-MA, 5 mmol/L, an autophagy inhibitor) (Figure 4A-C). Oil Red O staining and intracellular cholesterol measurement revealed that the inhibitory effect of miR-223 overexpression could be partially blocked by autophagy inhibition (Figure 4D and 4E). In another set of experiment, VSMCs were co-transfected with Atg7 siRNA to block the autophagy (Figure 5A). Transfection with Atg7 siRNA in VSMCs remarkably decreased protein expression of Atg7, LC3-II, and beclin-1, but increased the p62 level. These results indicated that Atg7 knockdown inhibited autophagy (Figure 5B-E). Moreover, blocking autophagy via Atg7 knockdown in VSMCs attenuated the inhibitory effect of miR-223 on foam cell formation (Figure 5F and 5G), indicating the miR-223 inhibited the foam cell formation via repressing autophagy.
Figure 4.
3-MA enhanced VSMCs foam cell formation by inhibition of miR-223-induced autophagy. A-C. LC3II/LC3I ratio and beclin-1 level were decreased whereas the level of p62 was increased by 3-MA, as determined by western blot analysis (n = 5). D and E. Lipid droplet accumulation and intracellular cholesterol contents measurement (n = 4 for both) in ox-LDL-treated VSMCs treated with negative control (50 nmol/L), miR-223 mimic (50 nmol/L) or 3-MA (5 mmol/L). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
Atg7 siRNA increased VSMCs foam cell formation by inhibition of miR-223-induced autophagy. A and B. The protein expression level of Atg7 was significantly decreased by Atg7 siRNA, as detected by western blot analysis (n = 5). C-E. LC3II/LC3I ratio and beclin-1 level were decreased whereas the expression of p62 was minimally increased by Atg7 siRNA, as determined by western blot analysis (n = 5). F and G. Lipid droplet accumulation and intracellular cholesterol contents measurement (n = 4 for both) in ox-LDL-treated VSMCs transfected with negative control (50 nmol/L), miR-223 mimic (50 nmol/L) or Atg7 siRNA (50 nmol/L). *P < 0.05, **P < 0.01, ***P < 0.001.
IGF-1R is a direct target of miR-223 in VSMCs
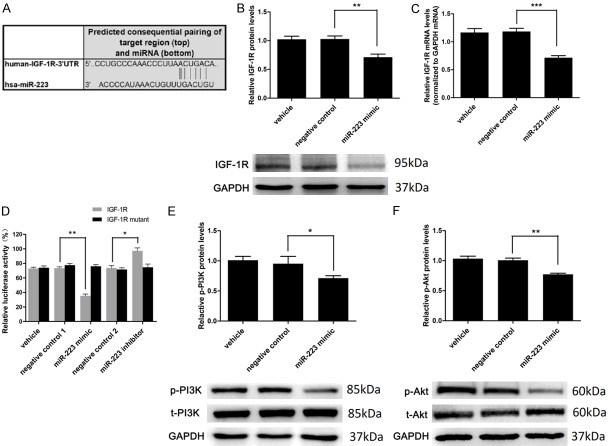
To investigate the molecular mechanism for miR-223-induced activation of autophagy, we attempted to identify potential target genes of miR-223. Four miRNA target prediction software tools (TARGETSCAN, miRBD, TargetMiner and microrna.org) predicted that IGF-1R is a potential target of miR-223 as its mRNA 3’-UTR sequences complementary to miR-223 (Figure 6A). Hence, we address if miR-223 affects the expression of IGF-1R. Overexpression of miR-223 significantly decreased the protein (Figure 6B) and mRNA (Figure 6C) levels of IGF-1R in VSMCs. To investigate whether IGF-1R is the direct target of miR-223, luciferase assay containing the 3’UTR of IGF-1R with the putative miR-223 binding site was performed. The results showed that the luciferase activity was significantly decreased by miR-223 overexpression (Figure 6D). When the IGF-1R 3’UTR was mutated, the inhibitory effect of miR-223 on luciferase activity was remarkably abrogated. By contrast, transfection of miR-223 inhibitor significantly enhanced the luciferase activity (Figure 6D). Taken together, these results indicated that IGF-1R is a direct target of miR-223.
Figure 6.
IGF-1R is a direct target of miR-223. A. IGF-1R with a binding site of miR-223 in 3’UTR predicted by TARGETSCAN. B. IGF-1R protein expression was significantly downregulated by the miR-223 mimic (50 nmol/L), as determined by western blot analysis (n = 5). C. The miR-223 mimic (50 nmol/L) downregulated the expression of IGF-1R mRNA, as determined by qRT-PCR (n = 8). D. Relative luciferase activity of the wild-type and mutant-type IGF-1R 3’UTR in HEK 293 cells transfected with miR-223 (50 nmol/L) or negative control (50 nmol/L) or miR-223 inhibitor (50 nmol/L) (n = 3). E and F. miR-223 mimic downregulates the expression of p-PI3K and p-AKT normalized to t-PI3K and t-AKT, as determined by western blot analysis (n = 5). *P < 0.05, **P < 0.01, ***P < 0.001.
The effect of mir-223 expression on PI3K-Akt pathway
PI3K-Akt is a downstream signaling pathway of IGF-1R which is related to cellular autophagic activity [21]. Therefore, we investigate if mir-223 has an effect on PI3K-Akt pathway. We found that miR-223 overexpression reduced protein levels of IGF-1R expression and the phosphorylated form of PI3K and Akt proteins (Figure 6E and 6F), suggesting an inhibition on the PI3K-Akt pathway.
Discussion
miR-223 is a hematopoietic lineage cell-specific miRNA [22] and its expression was thought to be negligible in VSMCs. However, our recent study has demonstrated that blood cell-secreted miR-223 in serum can enter VSMCs and regulate proliferation and apoptosis of VSMCs [16]. However, it remains to be determined if miR-223 participates in the foam cell formation of VSMCs. In this study, we investigated the role of miR-223 in the foam cell formation of VSMCs. The results showed that miR-223 was significantly upregulated in both the arteries and serum samples from ASO patients. miR-223 overexpression significantly inhibited the foam cell formation and decreased total intracellular cholesterol levels in VSMCs. miR-223 overexpression induced autophagy of VSMCs. Blocking autophagy by 3-methyladenine or Atg7siRNAs attenuated the inhibitory effect of miR-223 overexpression on foam cell formation. Luciferase assay showed that IGF-1R is a direct target of miR-223. miR-223 overexpression reduced protein levels of IGF-1R expression and the phosphorylated form of PI3K and Akt proteins. Taken together, these findings suggested that miR-223 overexpression inhibited foam cell formation in VSMCs through inducing autophagy. The IGF-1R/PI3K/Akt signaling pathway may be also involved. To our best knowledge, this is the first study reporting the role of miR-223 in foam cell formation of VSMCs and the underlying molecular mechanism.
Autophagy has been shown to play a major role in several important biological processes, such as embryogenesis, development, aging, and cell death [23]. Previous studies have revealed that activation of cellular autophagy could reduce the levels of total cholesterol and cholesterol esters, thus inhibition of the foam cell formation in both macrophages and VSMCs [10,20,24]. Based on these observations, we proposed that autophagy may be involved in the inhibitory effect of miR-223 overexpression on foam cell formation in VSMCs. Our results showed that miR-223 overexpression decreased the foam cell formation and enhanced autophagy in VSMCs. In addition, blocking autophagy attenuated the inhibitory effect of miR-223 overexpression on foam cell formation, strongly suggesting that autophagy directly accounted for the inhibitory effect of miR-223 overexpression on foam cell formation in VSMCs. miR-223-induced autophagy is a protective mechanism from the foam cell formation in VSMCs. Furthermore, our finding could provide an additional molecular mechanism for our previous observation that the atherosclerotic lesions in apoE knockout mice on a Western diet are significantly exacerbated by miR-223 knockdown [16].
Our miRNA target prediction analysis showed that IGF-1R is a potential target of miR-223. IGF-1/PI3K/Akt signaling pathway has been shown to involve in the foam cell formation in macrophage [25]. In addition, previous studies have demonstrated that IGF-1R/PI3K/Akt is a critical signal pathway of autophagy in VSMCs [21,24]. Therefore, we hypothesized that IGF-1/PI3K/Akt signaling may be also implicated in the effect of miR-223 on foam cell formation and autophagy in VSMCs. Our results revealed that miR-223 overexpression significantly downregulated IGF-1R in both protein and mRNA levels, and luciferase assay confirmed that IGF-1R was identified as a target gene of miR-223. Furthermore, its downstream signal pathway PI3K/Akt was also suppressed by miR-223 overexpression in VSMCs. These results suggested that IGF-1/PI3K/Akt signaling may be involved in the effect of miR-223 in VSMCs. However, it remains to be investigated if IGF-1/PI3K/Akt signaling directly accounts for miR-223 overexpression-induced inhibition on foam cell formation and activation of autophagy in VSMCs. In the future study, IGF-1R knockdown should be applied to examine whether the miR-223-induced autophagy activation and foam cell formation are blocked. On the other hand, miRNAs typically have more than one target gene, thereby there may be still some unknown target genes involving in the inhibitory effect of miR-223 on foam cell formation in VSMCs. Moreover, the in vitro findings of the current study remain to be validated in an in vitro animal model. All these limitations should be addressed in the future study.
In summary, our findings suggested that miR-223 overexpression inhibited foam cell formation in VSMCs, at least partially, via inducing autophagy. The IGF-1R/PI3K/Akt signaling pathway may be involved in the underlying mechanism.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (81270378; 81070258; 81370368; 81600336) and the Pearl River S&T Nova Program of Guangzhou (201806010006), and was partially supported by Natural Science Foundation of Guangdong Province (2015A030310477; 2016A030310133), “Three and three” Project of the First Affiliated Hospital of Sun Yat-Sen University, Young teacher training program of Sun Yat-sen University (17ykpy26), the Guangzhou Science and Technology Plan Projects (2013B021800261; 201508020116), Guangdong Department of Finance grant (2014SC104) and Guangdong Engineering Laboratory for Diagnosis and Treatment of Vascular Disease grant (2015B090903064).
Disclosure of conflict of interest
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics - 2014 update: a report from the American heart association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Mechanisms of foam cell formation in atherosclerosis. J Mol Med. 2017;95:1153–1165. doi: 10.1007/s00109-017-1575-8. [DOI] [PubMed] [Google Scholar]
- 3.Siegel-Axel D, Daub K, Seizer P, Lindemann S, Gawaz M. Platelet lipoprotein interplay: trigger of foam cell formation and driver of atherosclerosis. Cardiovasc Res. 2008;78:8–17. doi: 10.1093/cvr/cvn015. [DOI] [PubMed] [Google Scholar]
- 4.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan P, Xia C, Duan C, Li S, Mei Z. Biological characteristics of foam cell formation in smooth muscle cells derived from bone marrow stem cells. Int J Biol Sci. 2011;7:937–946. doi: 10.7150/ijbs.7.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovsan J, Bashan N, Greenberg AS, Rudich A. Potential role of autophagy in modulation of lipid metabolism. AJP Endocrinol Metab. 2010;298:E1–E7. doi: 10.1152/ajpendo.00562.2009. [DOI] [PubMed] [Google Scholar]
- 10.Li BH, Yin YW, Liu Y, Pi Y, Guo L, Cao XJ, Gao CY, Zhang LL, Li JC. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death Dis. 2014;5:e1182. doi: 10.1038/cddis.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue M, Zhuo Y, Shan B. MicroRNAs, long noncoding RNAs, and their functions in human disease. Methods Mol Biol. 2017;1617:1–25. doi: 10.1007/978-1-4939-7046-9_1. [DOI] [PubMed] [Google Scholar]
- 12.Bernardo BC, Gao XM, Winbanks CE, Boey EJH, Tham YK, Kiriazis H, Gregorevic P, Obad S, Kauppinen S, Du XJ, Lin RC, McMullen JR. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci U S A. 2012;109:17615–17620. doi: 10.1073/pnas.1206432109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, Hare JM, Olson EN, van Rooij E. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Zhao H, Tan Z, Zhang C, Fu X. Bottleneck limitations for microRNA-based therapeutics from bench to the bedside. Pharmazie. 2015;70:147–154. [PubMed] [Google Scholar]
- 15.Zhou S, Jin J, Wang J, Zhang Z, Freedman JH, Zheng Y, Cai L. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018;39:1073–1084. doi: 10.1038/aps.2018.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Z, Qin S, Li W, Wu W, Yang J, Chu M, Li X, Huo Y, Schaer GL, Wang S, Zhang C. An endocrine genetic signal between blood cells and vascular smooth muscle cells: role of microRNA-223 in smooth muscle function and atherogenesis. J Am Coll Cardiol. 2015;65:2526–2537. doi: 10.1016/j.jacc.2015.03.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, Liu Y, Cheang TY, Huang XL, Wang SM. MicroRNA-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arterioscler Thromb Vasc Biol. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Yang K, Zhou L, Wu M, Wu Y, Zhu M, Lai XM, Chen T, Feng L, Li M, Huang C, Zhong Q, Huang X. MicroRNA-155 promotes autophagy to eliminate intracellular mycobacteria by targeting Rheb. PLoS Pathog. 2013;9:e1003697. doi: 10.1371/journal.ppat.1003697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li BH, Liao SQ, Yin YW, Long CY, Guo L, Cao XJ, Liu Y, Zhou Y, Gao CY, Zhang LL, Li JC. Telmisartan-induced PPARγ activity attenuates lipid accumulation in VSMCs via induction of autophagy. Mol Biol Rep. 2015;42:179–186. doi: 10.1007/s11033-014-3757-6. [DOI] [PubMed] [Google Scholar]
- 20.Mei S, Gu H, Ward A, Yang X, Guo H, He K, Liu Z, Cao W. p38 mitogen-activated protein kinase (MAPK) promotes cholesterol ester accumulation in macrophages through inhibition of macroautophagy. J Biol Chem. 2012;287:11761–11768. doi: 10.1074/jbc.M111.333575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Gong Y, Wang Z, Jiang L, Chen R, Fan X, Zhu H, Han L, Li X, Xiao J, Kong X. Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med. 2014;18:542–553. doi: 10.1111/jcmm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vian L, Di Carlo M, Pelosi E, Fazi F, Santoro S, Cerio AM, Boe A, Rotilio V, Billi M, Racanicchi S, Testa U, Grignani F, Nervi C. Transcriptional fine-tuning of microRNA-223 levels directs lineage choice of human hematopoietic progenitors. Cell Death Differ. 2014;21:290–301. doi: 10.1038/cdd.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayat MA. Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging, volume 1. Elsevier Science. 2014 [Google Scholar]
- 24.Jia G, Cheng G, Gangahar DM, Agrawal DK. Insulin-like growth factor-1 and TNF-α regulate autophagy through c-jun N-terminal kinase and Akt pathways in human atherosclerotic vascular smooth cells. Immunol Cell Biol. 2006;84:448–454. doi: 10.1111/j.1440-1711.2006.01454.x. [DOI] [PubMed] [Google Scholar]
- 25.Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC, Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, Fu YC, Tang CK. APP-A negatively regulates ABCA1, ABCG1 and SR-B1 expression by inhibiting LXRα through the IGF-I-mediated signaling pathway. Atherosclerosis. 2012;222:344–354. doi: 10.1016/j.atherosclerosis.2012.03.005. [DOI] [PubMed] [Google Scholar]