Abstract
Inhibition of the sodium glucose co-transporter 2 (SGLT2) reduces cardiovascular morbidity, and mortality in patients with type 2 diabetes mellitus (T2DM) with atherosclerotic, cardiovascular disease. So far, a link between common genetic variations of the SGLT2 encoding gene SLC5A2 and glucose homeostasis as well as cardiovascular disease has not been established. The present study, therefore, aimed to investigate SLC5A2 single nucleotide polymorphisms (SNPs) in relation to type 2 diabetes and coronary artery disease (CAD) and prospectively the incidence of cardiovascular events. We genotyped the SLC5A2 tagging SNPs rs9934336, rs3813008, and rs3116150 in a total of 1684 high risk cardiovascular patients undergoing coronary angiography, including 400 patients with T2DM. Additionally, we performed a meta-analysis combining results from the present study and the literature. Variant rs9934336 was significantly associated with decreased HbA1c (P = 0.023). Further, rs9934336 was significantly inversely associated with the presence of T2DM in univariate (OR = 0.82 [0.68–0.99]; P = 0.037) as well as in multivariate analysis (OR = 0.79 [0.65–0.97]; P = 0.023). The association between rs9934336 and T2DM was confirmed in a meta-analysis including results from two previous observations which by themselves had failed to show a significant association of the polymorphism with T2DM (OR = 0.86 [0.78–0.95]; P = 0.004). Polymorphisms rs3813008 and rs3116150 were associated neither with glycemic parameters nor with T2DM. None of the SNPs tested was significantly associated with the baseline presence of CAD or the incidence of cardiovascular events. We conclude that genetic variation within the SLC5A2 gene locus is significantly related to the manifestation of T2DM.
Keywords: cardiovascular disease, SGLT2, single nucleotide polymorphisms, type 2 diabetes
Introduction
To preserve energy, glomerular filtered glucose is physiologically reabsorbed in the proximal tubule by the high-capacity sodium glucose co-transporter 2 (SGLT2) and the high-affinity SGLT1 transporter. This reabsorption frees the filtrate almost totally from glucose, cotransports sodium and is increased with a higher filtered glucose load like in hyperglycemia and therefore in diabetes [1].
The SGLT2 molecules – formerly mainly of nephrological interest [2] – gained the attention of diabetologists when specific inhibitors became available [3]. Interest also was shared by cardiologists when the results of the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA-REG-OUTCOME) trial revealed that the SGLT2 inhibitor empagliflozin, compared with standard antidiabetic treatment, reduced cardiovascular mortality and morbidity in type 2 diabetic patients with established cardiovascular disease [4,5]. The Canagliflozin Cardiovascular Assessment Study essentially confirmed these findings with the SGLT2 inhibitor canagliflozin [6]. So far, the underlying mechanisms behind the cardiovascular protection observed in clinical trials of SGLT2 inhibitors remain incompletely understood and there is a great scientific interest in potential pathways linking SGLT2 to diabetes and cardiovascular disease. Genetic analyses in many other fields have substantially helped to understand mechanisms and to reveal causal relationships [7–9].
SGLT2 is encoded by the SLC5A2 gene on chromosome 16 and is expressed almost exclusively in the proximal renal tubule. It has been hypothesized – but never proven – that a loss of function mutation in SLC5A2 could protect from T2DM by elimination of glucose in an insulin-independent mode via glucosuria. Testing common variants in the SLC5A2 gene in large patient cohorts could shed some new light on the association between SLC5A2 and the pathogenesis of T2DM. However, until now, the association between common genetic variations in SLC5A2 and glucose homeostasis or T2DM has hardly been investigated and the few published reports provided inconclusive results [10,11]. Moreover, the association between SLC5A2 variants and coronary atherosclerosis as well as with the risk of future cardiovascular events is unclear.
We therefore analyzed three tagging SNPs of the SLC5A2 gene in a large cohort of patients characterized by coronary angiography, including a high proportion of T2DM. We explored whether SLC5A2 SNPs were associated with (i) fasting glucose, the post-challenge glucose increase in oral glucose tolerance tests and HbA1c; (ii) the prevalence of T2DM, and (iii) baseline coronary artery disease (CAD) as well as incidence of cardiovascular events during follow-up. In addition, we performed a meta-analysis including data from earlier studies to further elucidate the impact of genetic variants of the SLC5A2 gene on the presence of diabetes.
Materials and methods
Study subjects
We enrolled a total of 1684 consecutive Caucasian patients, who were referred to elective coronary angiography for the evaluation of established or suspected stable CAD at the academic teaching hospital Feldkirch, a tertiary care center in Western Austria, as described previously [12,13].
Hypertension was defined according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [14], and T2DM was diagnosed according to World Health Organization criteria [15]. Height and weight were recorded, and body mass index (BMI) was calculated as body weight (kg)/height (m2). The metabolic syndrome was diagnosed according to National Cholesterol Education Program ATP-III criteria [16]. Coronary angiography was performed with the Judkins technique [17]. Stenosis of the coronary arteries was considered significant when lumen narrowing was ≥50% [18]. The extent of coronary atherosclerosis was defined as the number of significant coronary stenoses in a given patient, as described previously [19,20]. During a mean (±SD) follow-up period of 7.6 ± 2.7 years, fatal as well as non-fatal cardiovascular events were recorded. Cardiovascular events were defined as a composite of coronary death (fatal myocardial infarction, sudden cardiac death, mortality from congestive heart failure due to CAD), fatal ischemic stroke, non-fatal myocardial infarction, non-fatal ischemic stroke and need for aorto-coronary bypass surgery, percutaneous transluminal coronary angioplasty, or revascularization in the carotid or peripheral arterial beds. This primary study endpoint has been a priori chosen in analogy to a large cardiovascular outcome trial, the Heart Protection Study [21].
The Ethics Committee of the Medical University of Innsbruck approved the present study; all participants gave written informed consent and all clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki.
Laboratory analyses
Venous blood samples were collected after an overnight fast of 12 h before angiography was performed. Serum triglycerides and total cholesterol were determined on a Cobas 6000 or 8000 (Roche, Basel, Switzerland).
Levels of fasting plasma glucose were measured enzymatically from venous fluoride plasma samples with the hexokinase method (Roche Basel, Switzerland) on a Hitachi 717 or 911 (Mountain View, CA, U.S.A.). Glycosylated hemoglobin was determined as HbA1c by high-performance liquid chromatography on a Menarini-Arkray KDK HA 8140 (Kyoto, Japan).
Oral glucose tolerance tests were performed after an oral 75g glucose challenge. Serum insulin was measured by an enzyme immunoassay on an AIA 1200 (Tosoh, Foster City, CA, U.S.A.). Further, beta-cell function and insulin resistance were estimated from fasting plasma glucose and serum insulin using homeostasis model assessment [22].
Genotyping
Three SLC5A2 SNPs were selected due to their given roles as tagging SNPs. According to data of the 1000 Genomes Project, pilot 1 [23], or HapMap SNP database, release 27 [24], these SNPs cover all variants with a minor allele frequency ≥ 0.05 and pairwise r2 ≥ 0.8 within the SLC5A2 gene including 2 kb of the 5′ flanking region and 1 kb of the 3′ flanking region. Genotyping of these variants was carried out by the 5′ nuclease assay using TaqMan® MGB probes on a LightCycler® 480 Real-Time PCR System (F. Hoffmann-La Roche Ltd, Basel, Switzerland). TaqMan® MGB probes together with corresponding PCR primers were provided by the Assay-on-demand™ service (Thermo Fisher Scientific, Waltham, MA, U.S.A.). Genotypes were automatically determined by LightCycler® software 1.5 followed by a visual control of accurate genotype classification.
Statistics
Differences in categorical study variables were tested for statistical significance with the chi-squared test; for continuous variables t-tests and Kruskal–Wallis tests were applied. The Kolmogorov–Smirnov test was used as a test for normality. Non-normally distributed variables were log-transformed prior to analysis. Data are presented as means ± SD of not log-transformed values. Association between analyzed SLC5A2 SNPs with T2DM or CAD was determined by univariable and multivariable logistic regression analysis. Hazard ratios (HR) for the incidence of vascular events were derived from Cox proportional hazards models. Multiple linear regression analysis was used for assessing the association between genetic variants and continuous variables.
P-values < 0.05 were considered as significant. Statistical analyses were performed with the software package IBM SPSS 22 for Windows (IBM Corp., Armonk, NY, U.S.A.). Hardy–Weinberg equilibrium was assessed using chi-squared test with one degree of freedom.
Meta-analysis
We performed a systematic literature search in the PubMed and the Cochrane libraries, including all published literature up to December 4th, 2018. The following search terms were used: Diabetes AND (SLC5A2 OR SGLT2) AND (‘single nucleotide polymorphism’ OR ‘genetic variation’). Records providing genotype frequencies of SLC5A2 SNPs for patients with T2DM and without T2DM were considered. The quality of enrolled studies was independently assessed by two investigators according to the Newcastle-Ottawa Scale (NOS) for quality assessment of case–control studies [25]. The maximum possible score is nine stars and studies with stars equal to or higher than five were considered to be of sufficient quality.
Odds ratios (ORs) together with their corresponding 95% confidence intervals (CIs) were adopted from previous reports or calculated based on reported genotype frequencies by logistic regression analysis for an additive model of inheritance. Meta-analysis was performed with the Comprehensive Meta-Analysis 3.3 statistical software (Biostat, Inc., Englewood, NJ, U.S.A.). Heterogeneity among studies was evaluated with the Cochran Q test and Higgins I2 statistic [26]. Fixed effects models were used in cases of low inter-study heterogeneity (I2 < 50%, P-value > 0.05) to estimate the pooled additive ORs and 95% CIs. Otherwise, random effects models were applied. For publication bias assessing, rank correlation test by Begg and Mazumdar as well as Egger’s regression test were applied.
Results
Patient characteristics
Clinical and biochemical characteristics of the total study population, of patients with and of subjects without T2DM, and of patients with and of subjects without significant CAD are given in Table 1. Overall, the characteristics of our patients were representative for patients undergoing coronary angiography for the evaluation of CAD, with a high proportion of male gender and a high prevalence of a history of smoking, MetS, hypertension, and T2DM. Age, BMI, MetS, any lesions of the coronary arteries, significant coronary artery stenoses, total cholesterol and triglyceride levels as well as use of antihypertensive therapy were significantly associated with the presence of T2DM. Age, male gender, MetS, T2DM, total cholesterol, triglyceride levels as well as use of statins, anticoagulant therapy orantihypertensive therapy were significantly associated with the presence of significant CAD. Genotyping of SLC5A2 SNPs was successful in all patients and all analyzed variants were in Hardy–Weinberg equilibrium. Individual genotypes together with clinical data are given in Supplementary Table S1.
Table 1. Clinical and biochemical characteristics of the total study and of patients with and without type 2 diabetes mellitus.
| All patients (n = 1684) | Non-diabetic patients (n = 1284) | Patients with T2DM (n = 400) | P-value | Patients without significant CAD (n = 710) | Patients with significant CAD (n = 974) | P-value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 63.7 | 63.3 | 65.1 | 0.001 | 62.5 ± 10.6 | 64.6 ± 10.6 | <0.001 |
| Male gender (%) | 66.7 | 66.1 | 68.5 | 0.378 | 52.4 | 77.1 | <0.001 |
| BMI (kg/m2) | 27.6 ± 4.3 | 27.1 ± 4.1 | 29.1 ± 4.8 | <0.001 | 27.9 ± 4.6 | 27.3 ± 4.1 | 0.007 |
| Metabolic syndrome (%) | 44.2 | 36.6 | 68.5 | <0.001 | 40.8 | 46.6 | 0.019 |
| Hypertension (%) | 82.6 | 81.6 | 85.9 | 0.050 | 80.8 | 83.9 | 0.103 |
| Type 2 diabetes (%) | 23.8 | – | – | – | 18.5 | 27.6 | <0.001 |
| History of smoking (%) | 59.2 | 57.5 | 66.0 | 0.002 | 51.3 | 65.5 | <0.001 |
| Any lesion of the coronary arteries (%) | 81.7 | 79.6 | 88.3 | <0.001 | 56.5 | 100.0 | <0.001 |
| Significant stenosis (%) | 57.8 | 54.9 | 67.3 | <0.001 | – | – | – |
| Cholesterol (mg/dl) | 204.0 ± 46.2 | 207.9 ± 45.2 | 191.3 ± 47.4 | <0.001 | 208.0 ± 44.5 | 200.1 ± 47.3 | 0.002 |
| Triglycerides (mg/dl) | 149.0 ± 96.9 | 141.9 ± 89.9 | 171.6 ± 113.7 | <0.001 | 142.6 ± 96.4 | 153.7 ± 97.0 | 0.003 |
| eGFR (ml/min/1.73 m2) | 93.4 ± 19.1 | 93.7 ± 18.8 | 92.2 ± 20.1 | 0.134 | 94.1 ± 18.1 | 92.8 ± 19.8 | 0.107 |
| Statin therapy (%) | 32.5 | 30.5 | 39.9 | 0.034 | 35.1 | 54.2 | <0.001 |
| ASA therapy (%) | 68.5 | 57.5 | 71.9 | 0.104 | 66.1 | 70.3 | 0.074 |
| Anticoagulant therapy (%) | 7.5 | 6.8 | 9.7 | 0.057 | 10.2 | 5.5 | <0.001 |
| Antihypertensive therapy (%) | 72.0 | 69.6 | 79.8 | <0.001 | 66.6 | 76.0 | <0.001 |
Data of patient characteristics are given as means ± standard deviations or percentage as indicated. Coronary angiography was performed with the Judkin’s technique and stenoses narrowing ≥ 50% were defined as significant CAD. Differences in categorical study variables between non-diabetic patients and patients with T2DM were tested for statistical significance with the chi-squared test; for continuous variables, t-tests were applied. Non-normally distributed variables (age, BMI, triglycerides, eGFR) were log-transformed prior to statistical analysis.
Abbreviations: ASA, acetylsalicylic acid; BMI, body mass index; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; T2DM, type 2 diabetes mellitus.
Association of SLC5A2 variants with parameters of glucose homeostasis
The association between SLC5A2 variants and parameters of glucose homeostasis is shown in Table 2. Variant rs9934336 was significantly associated with decreased HbA1c, fasting glucose, and 120 min glucose concentrations during oral glucose tolerance tests. Variant rs3813008 was significantly linked to 120 min glucose during oral glucose tolerance tests. No significant associations between variant rs3116150 and the investigated traits of glucose homeostasis were observed.
Table 2. Association between analyzed SLC5A2 single nucleotide polymorphisms and traits of glucose hemostasis in the total study cohort.
| n | HbA1c (%) | Fasting glucose (mmol/l) | 120 min glucose (mmol/l) | Fasting insulin (µU/ml) | HOMA IR | HOMA BCF | |
|---|---|---|---|---|---|---|---|
| rs9934336 | |||||||
| GG | 939 (55.8%) | 6.13 ± 1.05 | 6.3 ± 2.06 | 8.04 ± 4.37 | 13.14 ± 30.92 | 4.13 ± 14.77 | 104.42 ± 117.4 |
| GA | 638 (37.9%) | 6.06 ± 0.96 | 6.16 ± 1.88 | 7.7 ± 4.11 | 11.47 ± 10.61 | 3.44 ± 4.91 | 98.98 ± 71.26 |
| AA | 107 (6.4%) | 5.94 ± 0.78 | 5.93 ± 1.29 | 7.11 ± 3.19 | 10.98 ± 8.13 | 3.12 ± 3.11 | 96.49 ± 52.93 |
| P-value | 0.023 | 0.044 | 0.049 | 0.235 | 0.075 | 0.584 | |
| rs3813008 | |||||||
| GG | 1267 (75.2%) | 6.09 ± 1.01 | 6.2 ± 1.9 | 7.66 ± 4.03 | 12.56 ± 26.91 | 3.92 ± 12.94 | 102.73 ± 103.75 |
| GA | 395 (23.5%) | 6.09 ± 0.95 | 6.28 ± 2.07 | 8.35 ± 4.62 | 11.64 ± 10.03 | 3.36 ± 3.18 | 99.42 ± 81.47 |
| AA | 22 (1.3%) | 6.36 ± 1.46 | 6.71 ± 2.91 | 9.47 ± 5.89 | 12.59 ± 9.76 | 4.62 ± 6.28 | 90.42 ± 54.84 |
| P-value | 0.355 | 0.221 | 0.019 | 0.626 | 0.880 | 0.329 | |
| rs3116150 | |||||||
| GG | 940 (55.8%) | 6.06 ± 0.99 | 6.18 ± 1.96 | 7.92 ± 4.23 | 11.51 ± 9.68 | 3.41 ± 4.15 | 99.81 ± 80.95 |
| GA | 634 (37.6%) | 6.12 ± 1.01 | 6.27 ± 1.96 | 7.69 ± 4.17 | 13.76 ± 37.3 | 4.44 ± 17.94 | 105.56 ± 124.23 |
| AA | 110 (6.5%) | 6.18 ± 1.05 | 6.28 ± 1.84 | 8.05 ± 4.25 | 11.66 ± 8.12 | 3.51 ± 3.42 | 97.82 ± 68.3 |
| P-value | 0.147 | 0.239 | 0.513 | 0.377 | 0.255 | 0.898 | |
Analyses were performed by multiple linear regression analysis using sex, age, and body mass index as covariates under an additive model of inheritance. All variables were log-transformed prior to statistical analysis. Data are presented as mean ± SD of non-log-transformed values.
Abbreviations: HbA1c, hemoglobin A1c; HOMA BCF, homeostasis model of assessment beta-cell function; HOMA IR, homeostasis model assessment of insulin resistance; SD, standard deviation.
Association of SLC5A2 variants with type 2 diabetes
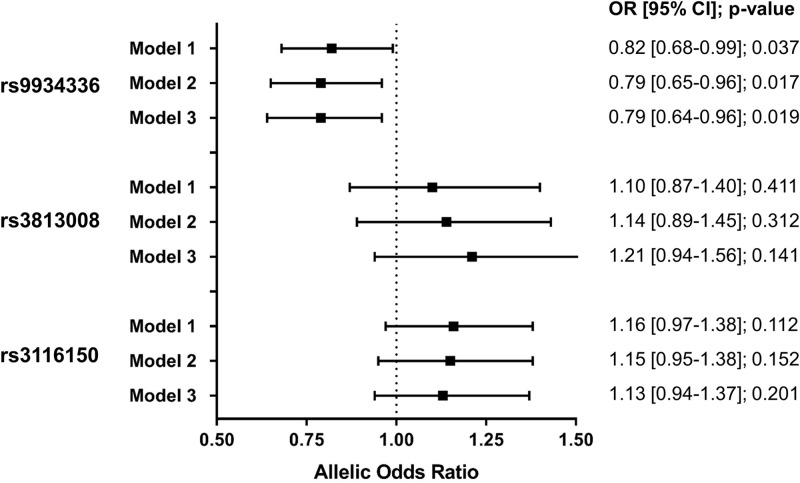
The association between the analyzed SLC5A2 variants and T2DM is displayed in Figure 1. Variant rs9934336 was significantly associated with T2DM univariately as well as in multivariable logistic regression analyses adjusting for sex, age, BMI, the metabolic syndrome, and hypertension. Variants rs3813008 and rs3116150 did not show any significant correlation with T2DM.
Figure 1. Association between analyzed SLC5A2 variants and type 2 diabetes mellitus determined by logistic regression analysis.
Model 1, unadjusted; model 2, adjusted for sex, age, and body mass index; model 3, additionally adjusted for metabolic syndrome and hypertension. CI, confidence interval; OR, odds ratio.
Results from meta-analysis
A systematic review of the literature identified four original articles [10,11,27,28] and one review article [29] for the given search terms. Two original articles [10,11] provided genotype frequencies of SLC5A2 SNPs for patients with and subjects without T2DM and were scheduled for the meta-analysis (Supplementary Figure S1).
The study by Enigk et al. [11] included two study cohorts comprising 786 non-diabetic Sorbs (a self-contained population in Eastern Germany) and 106 Sorbs with T2DM in the first and 1683 non-diabetic subjects and 359 T2DM patients from Berlin in the second cohort.
However, only for the first cohort genotyping data in conjunction with the presence or absence of T2DM were reported. Therefore, the second cohort was not considered in the meta-analysis. The study by Zimdahl et al. [10] included 2229 individuals without diabetes, but at increased risk for type 2 diabetes (defined as a family history of type 2 diabetes, BMI of at least 27 kg/m2, impaired fasting glycemia, and/or previous gestational diabetes) and 979 patients with T2DM included in placebo-controlled phase III clinical trials, which assessed the safety and efficacy of the SGLT2-inhibitor empagliflozin.
According to NOS criteria both studies were of sufficient quality (eight and six stars for the study by Enigk et al. [11] and Zimdahl et al. [10], respectively) and were, therefore, included in our meta-analysis. Consequently, meta-analysis of these two previous studies together with the present study included a total of 5196 non-diabetic subjects and 1738 individuals with T2DM. Genotype distributions among studies included in meta-analysis are given in Supplementary Table S1.
Heterogeneity assessment showed no significant inter-study heterogeneity for variants rs9934336 and rs3813008 (I2 = 0.0%; P = 0.510 and I2 = 0.0%; P = 0.682, respectively); therefore fixed effects models were used to estimate the pooled additive ORs and 95% CIs. A significant heterogeneity between studies was observed for variant rs3116150 (I2 = 91.2%; P ≤ 0.001), so a random effects model was used. Begg and Mazumdar’s rank correlation test as well as Egger’s regression test indicated that there were no publication bias in any performed meta-analysis (all P-values >0.05; Supplementary Table S2).
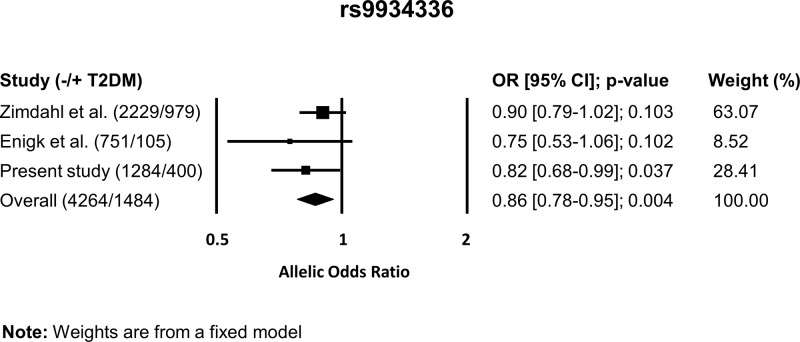
ORs of the individual studies as well as the pooled OR are shown for rs9934336 in Figure 2. The association between rs9934336 and T2DM did not reach statistical significance in the two previously performed studies. However, in the meta-analysis the pooled OR of rs9934336 of the previously published observations together with the data from our study was highly significantly associated with T2DM, reaching an approximately 10-fold increase in the level of statistical significance as compared with the data from our study alone. Notably, the association between rs9934336 and T2DM in the non-definite previously published single observations remained significant after exclusion of the present study from the meta-analysis (OR = 0.88 [0.78–0.99]; P = 0.036. Also after individual removal of the other studies the pooled OR remained significant (OR = 0.80 [0.68–0.95]; P = 0.009 and 0.87 [0.79–0.97]; P = 0.012 by removing the study by Zimdahl et al. and Enigk et al., respectively).
Figure 2. Meta-analysis of the association between rs9934336 and type 2 diabetes mellitus.
Weights are from a fixed model. Abbreviations: CI, confidence interval; OR, odds ratio.
No significant association of variant rs3813008 and rs3116150, respectively, with T2DM was observed in the meta-analysis (OR = 1.01 [0.89–1.14]; P = 0.870 and OR = 0.92 [0.63–1.34]; P = 0.667, respectively; Figures S3 and S4). It should be noted, however, that in the study by Zimdahl et al. the association between rs3116150 and T2DM was significant, but contrary to the (non-significant) effect of the other studies, probably leading to the observed inter-study heterogeneity among studies.
Associations of SLC5A2 variants with coronary atherosclerosis and with the incidence of cardiovascular events
None of the tested SLC5A2 genetic variants was significantly associated with the presence of significant CAD at baseline, either in univariate (Table 3) or in multivariable logistic regression analysis (Supplementary Table S3). To further evaluate their quantitative contribution to angiographically characterized coronary atherosclerosis, we investigated the association of these genetic variants with the extent of coronary atherosclerosis. No significant association of SLC5A2 SNPs with the extent of coronary atherosclerosis were found (Supplementary Table S4).
Table 3. Association of SLC5A2 variants with significant coronary artery disease.
| SNP | Genotype frequencies (controls/cases) | OR [95% CI] | P-value | ||
|---|---|---|---|---|---|
| AA | AB | BB | |||
| rs9934336 | 396/543 | 264/374 | 50/57 | 0.97 [0.83–1.14] | 0.701 |
| rs3813008 | 532/735 | 167/228 | 11/11 | 0.96 [0.78–1.18] | 0.680 |
| rs3116150 | 397/543 | 263/371 | 50/60 | 0.98 [0.84–1.15] | 0.814 |
Association between analyzed SLC5A2 variants and significant coronary stenosis determined by univariable logistic regression analysis under an additive model of inheritance.
Abbreviations: A, major allele, B, minor allele; CI, confidence interval; OR, odds ratio; SNP, single nucleotide polymorphism.
Prospectively, a follow-up rate of 98.6% was achieved. From 1684 patients investigated at baseline, 1661 therefore entered the prospective analyses. Prospectively, cardiovascular events occurred in 30.1% of our patients during the follow-up period of 7.6 ± 2.7 years. None of the tested genetic variants was significantly associated with the incidence of future cardiovascular events, either in univariate (Table 4) or in multivariable Cox regression analysis (Supplementary Table S5).
Table 4. Association of SLC5A2 variants with the incidence of future cardiovascular events.
| SNP | Genotype frequencies (controls/cases) | HR [95% CI] | P-value | ||
|---|---|---|---|---|---|
| AA | AB | BB | |||
| rs9934336 | 624/300 | 442/189 | 73/33 | 0.94 [0.82-1.09] | 0.410 |
| rs3813008 | 855/394 | 269/121 | 15/7 | 0.96 [0.80-1.15] | 0.650 |
| rs3116150 | 637/288 | 432/195 | 70/39 | 1.05 [0.92-1.21] | 0.478 |
Association between analyzed SLC5A2 variants and the incidence of future cardiovascular events was determined by univariable Cox regression analysis under an additive model of inheritance.
Abbreviations: A, major allele, B, minor allele; CI, confidence interval; HR, hazard ratio; SNP, single nucleotide polymorphism.
To investigate the interaction effect of SLC5A2 SNPs and diabetes on the cardiovascular risk we conducted tests for interaction and performed subgroup analyses with respect to the presence or absence of T2DM. Neither in non-diabetic subjects nor in patients with T2DM SLC5A2 SNPs were significantly associated with the prevalence of significant coronary atherosclerosis (Supplementary Table S6) or the incidence of future vascular events (Supplementary Table S7). Thus, no significant interaction terms between SLC5A2 SNPs and diabetes regarding the cardiovascular risk were observed (all Pinteraction values ≥ 0.05).
Discussion
The present work is novel in several aspects. First, we for the first time observed a significant association of SLC5A2 SNPs with T2DM. Second, together with previously published data from the literature, which by themselves did not reveal this association we performed a meta-analysis which confirmed our novel finding. Third, this is the first investigation prospectively addressing the association of SLC5A2 SNPs with cardiovascular events. We found a significant association between the common genetic variant rs9934336 of the SGLT2-encoding gene SLC5A2 and fasting glucose, post-challenge glucose, HbA1c and with the presence of T2DM in angiographied coronary patients. In contrast, no significant association between this SNP and CAD or with the incidence of cardiovascular events could be observed over 7.6 ± 2.7 years of follow-up.
Results from single investigations on SNPs are now rightfully interpreted with caution because they previously often could not be reproduced in other cohorts. In this regard, other single studies failed to detect the association between rs9934336 and T2DM. Several reasons may have contributed to the different strength of association among studies: First, differences in patients’ characteristics (including e.g. age, gender distribution, etc.) between studies may have confounded the association between SLC5A2 SNPs and T2DM. Furthermore, our study population consists of angiographied coronary patients and results obtained from this high coronary risk cohort are not necessarily applicable to other populations. On the other side, the study by Zimdahl et al. [10] included subjects without diabetes, but at increased risk for type 2 diabetes. Therefore, genetic differences between non-diabetic subjects at high diabetic risk and patients with diabetes may be attenuated in the present study. Subjects investigated by Enigk et al. [11] are part of a self-contained population, the Sorbs. Here, it can be speculated that the genetic background of diabetes differs between ethnicities.
However, our meta-analysis including previously published data concerning SLC5A2 variants and T2DM [10,11] supports our findings demonstrating a significant association between rs9934336 and T2DM. Notably, in this meta-analysis rs9934336 was associated with the presence of T2DM both with and without inclusion of our data.
The observations we made can be attributed to a distinct pathophysiological mechanism. Like in familial renal glucosuria, lowered function of SGLT2 leads to glucose spillage over the urine and prevents larger glucose increases, particularly in the postprandial state. In the long-term, this mechanism should be expected to protect from the development of T2DM. This idea is supported by the benign natural history of familial renal glucosuria [2] and of renal glucosuria in pregnancy [30].
The magnitude of glucose excretion is increased up to 74% of the filtered load [1] in diabetic patients administered empagliflozin but has not been investigated for the genetic variant rs9934336. Glucose excretion can be expected to be much less pronounced with the genetic variant than with the pharmacological intervention. This could explain why in contrast with the EMPAREG-OUTCOME trial, where empagliflozin led to a significant reduction in cardiovascular events and total mortality, no significant reduction in cardiovascular events was noted in our investigation, neither in the total patient cohort nor in in subgroup analyses stratified by the diabetic state, although we, similar to EMPAREG-OUTCOME, investigated a high-risk patient population. Here it should be noted, that sample size, particularly in subgroup analysis, appears small and, therefore, from our data we cannot exclude that an association between rs9934336 and cardiovascular events could emerge in a much larger study. Of note, however, no data on the association of the polymorphism with CAD or with cardiovascular events are available from the literature. In face of the results of outcome studies showing cardiovascular risk reductions with SGLT2 inhibitors, it would be of particular interest to further investigate the association between genetic variation at the SLC5A2 gene locus and cardiovascular disease in larger study cohorts in order to definitely clarify this issue.
However, it should be considered that also in trials investigating cardiovascular outcomes with SGLT2 inhibitors [4–6], these medications improved heart failure outcomes and cardiovascular mortality but not typical atherothrombotic endpoints such as myocardial infarctions or strokes. Most likely the beneficial impact of SGLT2 inhibitors on cardiovascular outcomes is not carried by an anti-atherosclerotic effect which is very well in line with the lack of an association between SLC5A2 SNPs and CAD in our study.
We observed a significant association between rs9934336 and T2DM. However, it may be questioned whether the assumed moderate impact of common genetic variants on glucose levels via its effect on SGLT2 function is sufficient to protect from diabetes. In this context it must not be forgotten that a genetic variant exerts its effect from birth on over a lifetime and that even slight effects can have a huge impact on long term prognosis. Here, a comparison with loss-of-function mutations in other metabolic fields appears tempting. Noteworthy, it has been shown that common variants in the genes encoding for Niemann–Pick C1-like 1 and Proprotein Convertase Subtilisin Kexin 9, critical proteins in cholesterol metabolism only slightly decrease LDL cholesterol but have a huge impact on the lifelong incidence of cardiovascular events [31–33]. Similarly, it appears plausible that live-long exposure to slightly reduced plasma glucose concentrations and to a slightly increased loss of calories in the urine caused by genetic variants lowering SGLT2 activity may protect from the development of T2DM.
An in-depth molecular genetic consideration appears necessary. Variant rs9934336 is located in intron 1, 121 base-pairs upstream of the 5′ end of exon 2. In silico splice site analysis using Human Splicing Finder 3.0 tool (www.umd.be/HSF3) [34] showed that the variant creates a new intronic splicing enhancer site, but probably has no impact on splicing due to its deep intronic position. Therefore, a potential impact of rs9934336 on SGLT2 protein structure remains unclear and it appears more likely that a variant highly correlated with it may contribute to putatively decreased SGLT2 activity.
Our study has strengths and limitations. Important strengths of this work are a broad characterization of glucose metabolism including besides fasting glucose HbA1c, in oral glucose tolerance tests, and measures of insulin resistance and beta-cell function. Further, the population of angiographied coronary patients we chose to investigate allowed us to study the association of polymorphisms both with coronary atherosclerosis and with the incidence of cardiovascular events. Further, our high-risk patient population has been of particular interest in randomized clinical trials using SGLT2 inhibitors [4,6]. Furthermore, we have not accounted for multiple testing in our study. Of note, the Bonferroni correction would probably have been too conservative owing to the given close correlation among included SLC5A2 SNPs and investigated phenotypes (T2DM, coronary atherosclerosis, cardiovascular events). Therefore, associations between analyzed SNPs and clinical outcome are given for a nominal significance level. Notably, the observed significant association between variant rs9934336 and T2DM obtained by meta-analysis would have survived Bonferroni correction (Pcorrected = 0.036). As stated above, we cannot exclude that some associations not found to be significant in our study may reach statistical significance with a larger sample size. Clearly, replication of our observations in large independent studies is warranted. It should be further mentioned that the observed associations between determined genotypes and glycemic traits might have been influenced by the prevalence of T2DM or its treatment. Furthermore, like in other studies on common SLC5A2 variants [10,11,28], data on the extent of urinary glucose excretion are not available in our study, which however of course does not diminish the validity of our results. From our data we also cannot exclude that SLC5A2 polymorphisms are associated with microvascular complications. SLC5A2 SNPs were associated with the presence of T2DM in our patients as well as in our meta-analysis. Whether SLC5A2 SNPs in a pure diabetes cohort are associated with microvascular diabetes complications is an important question that should be addressed in large cohorts of patients with diabetes.
Conclusions
In conclusion, thus, we have presented novel data pointing to a significant role of a SLC5A2 genetic variant in glucose homeostasis. The variant rs9934336 is associated with decreased presence of diabetes in cardiac patients. Our finding is supported by a meta-analysis including previously published data. Hitherto neglected, the SGLT2 transport mechanism should gain in-depth research interest with larger studies of genomics, downstream effects and functional consequences of the protective SNP described here. A new field of clinical research for diabetologists, nephrologists, and cardiologists emerges.
Supporting information
Supplementary Figure S1. Flow chart of the literature selection process in the meta-analysis.
Supplementary Table S1. Genotype distributions among studies included in meta-analyses.
Supplementary Table S2. Evaluation of publication bias.
Supplementary Table S3. Association between SLC5A2 SNPs and significant coronary atherosclerosis - results from multivariable logistic regression analyses.
Supplementary Table S4. Associations between SLC5A2 SNPs and the extend of significant coronary lesions.
Supplementary Table S5. Association between SLC5A2 SNPs and significant coronary atherosclerosis - results from multivariable Cox regression analyses.
Supplementary Table S6. Association of SLC5A2 variants with significant coronary artery disease in non-diabetic patients and patients with type 2 diabetes.
Supplementary Table S7. Association of SLC5A2 variants with future cardiovascular events in non-diabetic patients and patients with type 2 diabetes.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- EMPA-REG-OUTCOME
Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- SGLT2
sodium glucose co-transporter 2
- SNP
single nucleotide polymorphism
- T2DM
type 2 diabetes mellitus
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
Author Contribution
H.D. and A.M. designed the study, interpreted the data, and wrote the manuscript. A.M. performed statistical analyses. A.L. and C.H.S. contributed to design and discussion. K.G. performed molecular analyses. C.H.S. and A.V. were responsible for patient recruitment and provided clinical characteristics. A.L., E.M.B., C.H.S., and P.F. reviewed/edited the manuscript.
References
- 1.Al-Jobori H., Daniele G., Cersosimo E., Triplitt C., Mehta R., Norton L.. et al. (2017) Empagliflozin and kinetics of renal glucose transport in healthy individuals and individuals with type 2 diabetes. Diabetes 66, 1999–2006 10.2337/db17-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santer R. and Calado J. (2010) Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin. J. Am. Soc. Nephrol. 5, 133–141 10.2215/CJN.04010609 [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E. and Solini A. (2012) SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat. Rev. Endocrinol. 8, 495–502 10.1038/nrendo.2011.243 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S.. et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 5.Wanner C., Lachin J.M., Inzucchi S.E., Fitchett D., Mattheus M., George J.. et al. (2018) Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137, 119–129 10.1161/CIRCULATIONAHA.117.028268 [DOI] [PubMed] [Google Scholar]
- 6.Neal B., Perkovic V., Mahaffey K.W., de Zeeuw D., Fulcher G., Erondu N.. et al. (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.Paternoster L., Tilling K. and Davey Smith G. (2017) Genetic epidemiology and Mendelian randomization for informing disease therapeutics: conceptual and methodological challenges. PLoS Genet. 13, e1006944. 10.1371/journal.pgen.1006944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tada H., Kawashiri M. and Yamagishi M. (2017) Clinical perspectives of genetic analyses on dyslipidemia and coronary artery disease. J. Atheroscler. Thromb. 24, 452–461 10.5551/jat.RV17002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenson R.S., Brewer H.B., Barter P.J., Björkegren J.L.M., Chapman M.J., Gaudet D.. et al. (2017) HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat. Rev. Cardiol. 15, 9–19 10.1038/nrcardio.2017.115 [DOI] [PubMed] [Google Scholar]
- 10.Zimdahl H., Haupt A., Brendel M., Bour L., Machicao F., Salsali A.. et al. (2017) Influence of common polymorphisms in the SLC5A2 gene on metabolic traits in subjects at increased risk of diabetes and on response to empagliflozin treatment in patients with diabetes. Pharmacogenet. Genomics 27, 135–142 10.1097/FPC.0000000000000268 [DOI] [PubMed] [Google Scholar]
- 11.Enigk U., Breitfeld J., Schleinitz D., Dietrich K., Halbritter J., Fischer-Rosinsky A.. et al. (2011) Role of genetic variation in the human sodium-glucose cotransporter 2 gene (SGLT2) in glucose homeostasis. Pharmacogenomics 12, 1119–1126 10.2217/pgs.11.69 [DOI] [PubMed] [Google Scholar]
- 12.Muendlein A., Saely C.H., Rhomberg S., Sonderegger G., Loacker S., Rein P.. et al. (2009) Evaluation of the association of genetic variants on the chromosomal loci 9p21.3, 6q25.1, and 2q36.3 with angiographically characterized coronary artery disease. Atherosclerosis 205, 174–180 10.1016/j.atherosclerosis.2008.10.035 [DOI] [PubMed] [Google Scholar]
- 13.Vonbank A., Saely C., Rein P., Sturn D. and Drexel H. (2013) Current cholesterol guidelines and clinical reality: a comparison of two cohorts of coronary artery disease patients. Swiss Med. Wkly. 143, w13828. [DOI] [PubMed] [Google Scholar]
- 14.Scanlon P.J., Faxon D.P., Audet A.M., Carabello B., Dehmer G.J., Eagle K.A.. et al. (1999) ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J. Am. Coll. Cardiol. 33, 1756–1824 10.1016/S0735-1097(99)00126-6 [DOI] [PubMed] [Google Scholar]
- 15.Alberti K.G. and Zimmet P.Z. (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15, 539–553 [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 17.Judkins M.P. (1967) Selective coronary arteriography. I. A percutaneous transfemoral technic. Radiology 89, 815–824 10.1148/89.5.815 [DOI] [PubMed] [Google Scholar]
- 18.Drexel H., Aczel S., Marte T., Benzer W., Langer P., Moll W.. et al. (2005) Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care 28, 101–107 10.2337/diacare.28.1.101 [DOI] [PubMed] [Google Scholar]
- 19.Drexel H., Amann F.W., Rentsch K., Neuenschwander C., Luethy A., Khan S.I.. et al. (1992) Relation of the level of high-density lipoprotein subfractions to the presence and extent of coronary artery disease. Am. J. Cardiol. 70, 436–440 10.1016/0002-9149(92)91186-8 [DOI] [PubMed] [Google Scholar]
- 20.Drexel H., Amann F.W., Beran J., Rentsch K., Candinas R., Muntwyler J.. et al. (1994) Plasma triglycerides and three lipoprotein cholesterol fractions are independent predictors of the extent of coronary atherosclerosis. Circulation 90, 2230–2235 10.1161/01.CIR.90.5.2230 [DOI] [PubMed] [Google Scholar]
- 21.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F. and Turner R.C. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 22.Collins R., Armitage J., Parish S., Sleigh P., Peto R., Heart Protection Study Collaborative Group (2003) MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 361, 2005–2016 10.1016/S0140-6736(03)13636-7 [DOI] [PubMed] [Google Scholar]
- 23.Durbin R.M., Altshuler D.L., Durbin R.M., Abecasis G.R., Bentley D.R., Chakravarti A.. et al. (2010) A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 10.1038/nature09534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437, 1299–1320 10.1038/nature04226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 26.Higgins J.P.T. and Thompson S.G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 27.Gong S., Guo J., Han X., Li M., Zhou L., Cai X.. et al. (2017) Clinical and genetic features of patients with type 2 diabetes and renal glycosuria. J. Clin. Endocrinol. Metab. 102, 1548–1556 10.1210/jc.2016-2332 [DOI] [PubMed] [Google Scholar]
- 28.Ordelheide A.-M., Böhm A., Kempe-Teufel D., Wagner R., Machicao F., Heni M.. et al. (2017) Common variation in the sodium/glucose cotransporter 2 gene SLC5A2 does neither affect fasting nor glucose-suppressed plasma glucagon concentrations. PLoS One 12, e0177148. 10.1371/journal.pone.0177148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tönjes A. and Kovacs P. (2013) SGLT2: a potential target for the pharmacogenetics of Type 2 diabetes? Pharmacogenomics 14, 825–833 10.2217/pgs.13.61 [DOI] [PubMed] [Google Scholar]
- 30.Drexel H. and Sailer S. (1980) Kinetics of glucose handling in renal glucosuria during pregnancy. Klin. Wochenschr. 58, 1299–1306 10.1007/BF01478138 [DOI] [PubMed] [Google Scholar]
- 31.Kent S.T., Rosenson R.S., Avery C.L., Chen Y-DI, Correa A., Cummings S.R.. et al. (2017) PCSK9 loss-of-function variants, low-density lipoprotein cholesterol, and risk of coronary heart disease and stroke: data from 9 studies of Blacks and Whites. Circ. Cardiovasc. Genet. 10, e001632. 10.1161/CIRCGENETICS.116.001632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ference B.A., Majeed F., Penumetcha R., Flack J.M. and Brook R.D. (2015) Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both. J. Am. Coll. Cardiol. 65, 1552–1561 10.1016/j.jacc.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muendlein A., Leiherer A., Saely C.H., Rein P., Zanolin D., Kinz E.. et al. (2015) Common single nucleotide polymorphisms at the NPC1L1 gene locus significantly predict cardiovascular risk in coronary patients. Atherosclerosis 242, 340–345 10.1016/j.atherosclerosis.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 34.Desmet F.-O., Hamroun D., Lalande M., Collod-Béroud G., Claustres M. and Béroud C. (2009) Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 37, e67. 10.1093/nar/gkp215 [DOI] [PMC free article] [PubMed] [Google Scholar]