Abstract
Nepeta cataria L. has long been used in folk food and medicine for several functions. Essential oils (EOs) were extracted from Nepeta cataria L. by supercritical fluid extraction. The results of animal experiments showed that EOs from Nepeta cataria L. significantly attenuated acetaminophen-induced liver damage. Further study confirmed that EOs were able to increase mRNA expression of uridine diphosphate glucuronosyltransferases (UGTs) and sulfotransferases (SULTs), as well as inhibit CYP2E1 activities, and thereby suppressed toxic intermediate formation. Nrf-2 activation might be involved in EOs-induced up-regulation of Phase II enzymes. Collectively, our data provide evidence that EOs protect the liver against acetaminophen-induced liver injury mainly by accelerating acetaminophen harmless metabolism, implying that EOs can be considered as a potential natural resource to develop hepatoprotective agent.
Keywords: Essential oil, Hepatoprotective, Nepeta cataria L.
Introduction
Nepeta cataria L. is a fragrant annual herb, widely distributed in Asia, Europe and North America since the Eastern Han Dynasty. It has been traditionally and popularly used as both food and medicine for thousands of years [1]. Since ancient times, the people in China have added the leaf of Nepeta cataria L. to noodles and other traditional Chinese foods for anti-microbial, anti-inflammation, cough-relieving, antioxidant and anti-cancer its abilities [2–5]. As a medicine, some clinical therapies are also reported in the literature. The search for other active compounds has led to the discovery and isolation of many phytochemicals and essential oils (EOs) with interesting anti-bacterial activity.
Drug-induced liver injury has become a major topic in the field of Hepatology and Gastroenterology. APAP overdose is the leading cause of drug-induced acute liver failure. Oxidative stress is considered to be the primary cellular event in acetaminophen-induced liver injury [6,7]. When taken at overdose, most of APAP is metabolized by Phase II conjugating enzymes, mainly sulfotransferase (SULT) and UDP-glucuronosyltransferase (UGT), converting it to nontoxic compounds which are then excreted with the urine. Of the remaining APAP, approximately 5–9% is metabolized by the cytochrome P450 enzymes (CYPs), mainly CYP2E1 into the highly reactive intermediate metabolite N-actyl-p-benzoquinone imine (NAPQI). Usually, NAPQI is rapidly detoxified by conjugating with glutathione (GSH). However, when Phase II metabolizing enzymes are saturated after APAP overdose, excessive NAPQ1 deplete GSH, leading to covalent binding of sulfhydryl groups in cellular proteins, this results liver oxidative stress [8,9].
In the present study, we found that EOs from Nepeta cataria L. have a liver protective ability on acetaminophen-induced liver injury.
Materials and methods
Supercritical fluid extraction of essential oil from Nepeta cataria L.
The EO from leaf of Nepeta cataria L. was extracted by supercritical CO2 extraction system. The settlement of parameters was selected on the basis of, temperature, pressure, static time, which had crucial efficiency on the EO extraction of supercritical fluid extraction (SFE). The primary parameters for EO extraction by SFE were selected according to practical experience. Supercritical CO2 was used as the extraction agent. The extraction procedure was operated under the optimized conditions: the extraction pressure was 35 MPa, extraction temperature was 50°C, flow rate of CO2 was 20 l/h, separation pressure was 6 MPa, separation temperature was 40°C and 1.5 h for the variable levels, respectively. The SFE coupled with the 5000-ml extraction vessel was used for EO from 5.0 kg powder of Nepeta cataria L. under the selected conditions. About 165 g of EO was collected.
Experimental animals
The experimental protocol was reviewed and approved by the Ethics Committee of Fourth Military Medical University for the use of laboratory animals. A total of 50 (half male and half female) inbred Kunming mice (18–20 g) aged 4 weeks was obtained from the animal center of Fourth Military Medical University (Shanxi, China). The animals were kept under controlled conditions at temperature 22 ± 2°C, humidity 70 ± 4% with 12-h light–dark cycling.
Animal treatments
Mice were randomly divided into five groups, ten mice were in each group (n=10, five males and five females). Control group: 1% Tween-80 aqueous solution (100 ml/kg); APAP group: 300 mg/kg; EOs-APAP group: EO was emulsified in a 1% Tween-80 aqueous solution, and then 100 ml/kg 1% Tween-80 were oral administration after intraperitoneal injection APAP (300 mg/kg) treatment 1 h; N-acetylcysteine (NAC)-APAP group: NAC (100 mg/kg) emulsified in a 1% Tween-80 aqueous solution and then it were oral administration after intraperitoneal injection APAP treatment 1 h; EOs group: EO was emulsified in a 95% Tween-80 aqueous solution (100 ml/kg). Mice were treated intragastrically with EOs after acetaminophen for 3 days.
Determination of serum alanine aminotransferase and aspartate aminotransferase levels
Enzymatic activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum were evaluated by spectrophotometer using commercial diagnostic kits (Nanjing Jiancheng Institute of Biotechnology, Nanjing, China).
Determination of hepatic reactive oxygen species, superoxide dismutase, catalase and malondiadehyde levels
Frozen liver tissues were homogenized in ice-cold PBS. The supernatants were collected after the homogenate was centrifuged at 3000 g, 4°C for 10 min. Superoxide dismutase (SOD), catalase (CAT) and malondiadehyde (MDA) levels by spectrophotometer using the commercially available assay kits as each manufacturer instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The reactive oxygen species (ROS) levels were assay by fluorescence detector using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The protein concentration in tissue homogenates were measured by Bradford protein assay using bovine serum albumin as the standard (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Determination of related genes expression
Total mRNA was isolated from frozen liver tissues using a Total RNA kit (Tiangen, Beijing, China). Quantitative real-time PCR (qPCR) was carried out for the amplification of cDNA using 2×SYBR Green I PCR Master Mix (Vazyme, Nanjing, China). The PCR procedure consisted of 95°C for 30 s followed by 35 cycles of 95°C for 15 s, 58°C for 30 s and 72°C for 30 s. The PCR primers were used as shown in Table 1. The melting curve analysis was performed on the PCR products to verify primer specificity and product purity. A dissociation curve was performed for each plate to confirm the production of a single product. The relative abundance of each mRNA was calculated with the formula 2−(ΔΔCt), where ΔΔCt = (CtTarget – CtGAPDH) treatment − (CtTarget − CtGAPDH) control.
Table 1. Primers used for qPCR.
| Target gene | Sense 5′–3′ | Antisense 5′–3′ |
|---|---|---|
| Nrf2 | GCTGATGGAGTACCCTGAGGCTAT | ATGTCCGCAATGGAGGAGAAGTCT |
| HO-1 | TGCCAGTGCCACCAAGTTCAAG | TGTTGAGCAGGAACGCAGTCTTG |
| NQO1 | GGAGACAGCCTCTTACTTGCCAAG | CCAGCCGTCAGCTATTGTGGATAC |
| GCLC | TGAGATTTAAGC CCCCTCCT | TTGGGATCAGTCCAGGAAAC |
| GSTA2 | TCAGTAACCTGCCCACAGTGAAG | GCATGTTCTTGACCTCTATGGCTGG |
| UGT1A1 | CACGCTGGGAGGCTGTTAGT | CACAGTGGGCACAGTCAGGTA |
| UGT1A6 | CACGTGCTACCTAGAGGCACAG | GACCACCAGCAGCTTGTCAC |
| UGT1A9 | GAAGAACATGCATTTTGCTCCT | CTGGGCTAAAGAGGTCTGTCATAGTC |
| SULT1A1 | CCCGTCTATGCCCGGATAC | GGGCTGGTGTCTCTTTCAGAGT |
| SULT2A1 | TAGGGAAAAATTTAGGGCCAGAT | TTGTTTTCTTTCATGGCTTGGA |
| CYP2E1 | CACCGTTGCCTTGCTTGTCTG | CTCATGAGCTCCAGACACTTC |
| GAPDH | ACATGGCCTCCAAGGAGTAAGA | GATCGAGT TGGGGCTGTGACT |
Abbreviations: GCLC, glutamate-cysteine ligase catalytic subunit; GSTA2, glutathione S-transferase A 2; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; NQO1, NAD(P)H: quinone oxidoreductase 1.
Determination of pathological changes of liver
Taken a small piece of liver tissue and fixed it in 10% paraformaldehyde. After washing with distilled water, it is subjected to a series of operations such as dehydration, transparent, paraffin embedding and sectioning. The sections were counterstained with Hematoxylin after diaminobenzidine staining. Photomicrographs were taken with a digital camera.
Determination of related protein expression
For nuclear factor erythroid 2-related factor 2 (Nrf2) expression analysis, the extraction and isolation of cytoplasmic and nuclear protein were performed using a Cytoplasmic and Nuclear Protein Extraction Kit (Beyotime, Nanjing, China) according to the manufacturer’s instructions. For CYP2E1 expression analysis, the extraction and isolation of microsomal protein were carried out as described previously [10]. The concentration of protein was determined by BCA assay kit (Beyotime, Nanjing, China). Equal amounts of protein extracts were subjected to SDS/polyacrylamide gel electrophoresis under reducing conditions on concentrate protein gel 5% (pH = 6.8) and separating protein gel 12% (pH = 8.8). The separated proteins were transferred to PVDF membranes using a tank transfer for 2 h at 200 mA in Tris-glycine buffer with 15% methanol. Membranes were blocked with 5% skimmed milk for 3 h and incubated for 12 h with anti-CYP2E1 (1:1500, BOSTER, Wuhan, China), anti-Nrf-2 (1:500, Bioss, Beijing, China), anti-GAPDH (1:1000, BOSTER, Wuhan, China) and anti-Lamin B (1:500, Bioss, Beijing, China) for 2 h at 37 °C. The secondary antibodies (IgG/HRP) were incubated for 2 h at 37°C. The images of the blots were visualized by ECL (Genshare, Xi’an, China).
Statistical analysis
The results were presented as means of at least five measurements, duplicated for each set, having a coefficient of variation less than 5%. One-way ANOVA followed by Duncan’s multiple range test (P<0.05) with SPSS 20.0 (SPSS, Inc., Chicago, IL, U.S.A.).
Results
Effect of EOs on APAP-induced hepatotoxicity
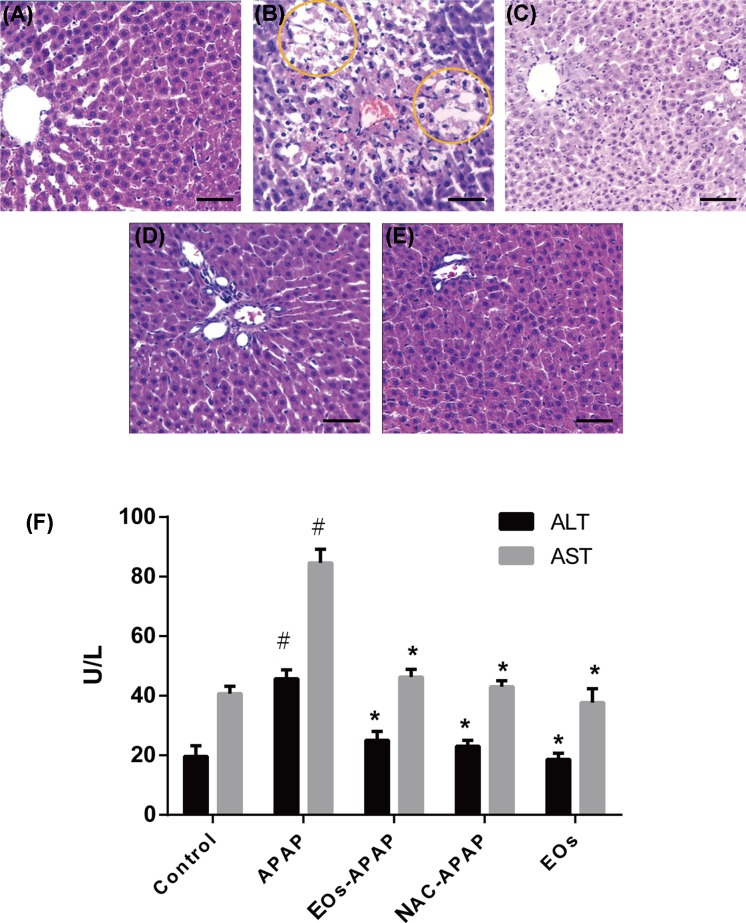
APAP can induce liver injury by oxidative stress and inflammation [11,12]. ALT and AST were important markers of liver injury [13]. ALT and AST levels were significantly increased by 2.67-fold and 2.06-fold in APAP group, respectively, as compared with the control group (Figure 1F), suggestion APAP treatment induced liver injury. The levels of ALT and AST have no significant difference in EOs-APAP and NAC-APAP treatment group, suggestion EOs can prevent APAP-induced liver injury.
Figure 1. Serum markers of liver toxicity and liver changes in mice.
Notes: (A–G) The liver changes in mice. (A) Control group; (B) APAP treatment group; (C) APAP-EOs treatment group; (D) APAP-NAC treatment group; (E) EOs treatment group; bars = 20 μm. (F) The activity of ALT and AST. #Significant compared with control group alone, P<0.05. *Significant compared with model group alone, P<0.05.
Pathological section results shown that control mice had normal hepatic architecture, APAP treatment mice exhibitions hepatocellular injury. More than half of the centrilobular hepatocytes were swollen with marked cytoplasmic vacuolation and condensed nuclei. Treatment with EOs from Nepeta cataria L. markedly attenuated the APAP-induced necrotic lesions (Figure 1A–E). Combine the results of Figure 1, EOs from Nepeta cataria L. have protective ability to prevent APAP-induced liver injury.
Effect of EOs on hepatic antioxidant characters
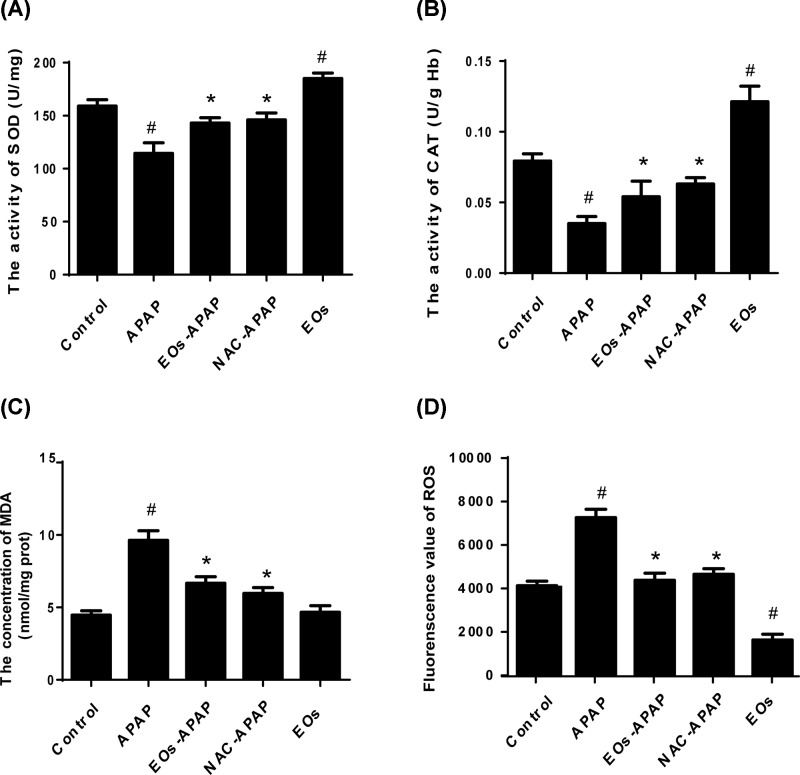
To investigate the effect of EOs on hepatic antioxidant characters, the levels of oxidative stress markers were examined in the model of APAP-induced mice. As shown in Figure 2, APAP treatment significantly increased the content of MDA and ROS by 2.38-fold and 1.92-fold, respectively. And APAP administration resulted in a significantly decreased the activity of SOD and CAT by 0.72-fold and 0.44-fold. Overdose APAP was metabolized by CYP2E1 enzymes into NAPQI, which undergoes chemical and enzymatic conjugation to GSH in APAP toxicity. This could lead to lipid peroxidation, antioxidant enzyme activities could be reduced and the levels of ROS were increased. Here, APAP increased MDA and ROS levels and decreased the activities of SOD and CAT, suggestions APAP-induced hepatic dysfunction is caused by oxidative stress.
Figure 2. Effects of EOs on the oxidative stress markers in liver homogenate.
(A) The activity of SOD. (B) The activity of CAT. (C) The concentration of MDA. (D) Fluorescence value of ROS. #Significant compared with control group alone, P<0.05. *Significant compared with model group alone, P<0.05.
EO-APAP administration prevented the decrease in SOD and CAT by 1.32-fold and 2.2-fold, and reduced the levels of ROS and MDA by 0.53-fold and 0.57-fold, respectively. EOs treatment not significantly increased the activities of SOD and CAT but significantly decreased the levels of ROS compared with the control group (P<0.05), suggestion EOs have the ability of ROS elimination.
Effect of EOs on oxidative stress-related gene expression
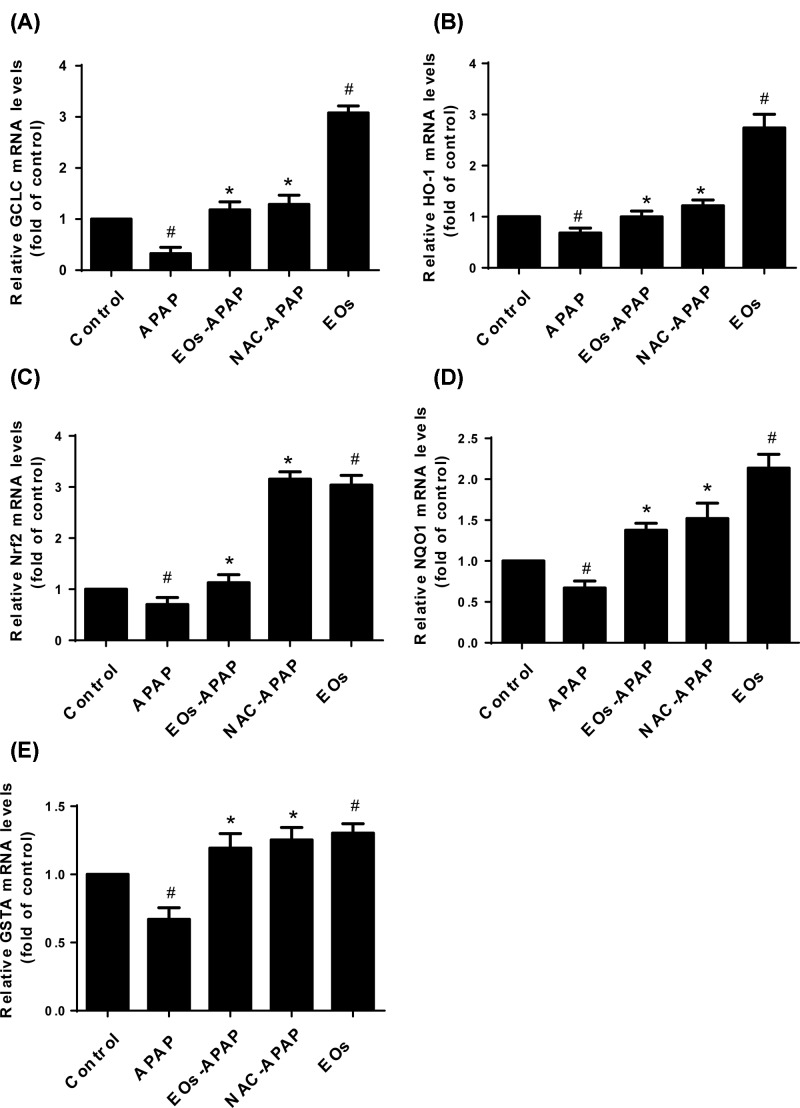
Nrf2 is a kind of transcription factor that modulates endogenous antioxidants and antioxidant enzymes [14–16]. ROS stimulates Nrf2 activation, which binds to the antioxidant response element and further activates transcription of gene encoding for antioxidants and detoxifications like heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and glutathione-synthesizing enzymes, like glutamate-cysteine ligase catalytic subunit (GCLC) [17]. To investigate the effect of EOs on Nrf2, HO-1, NQO1, GCLC and glutathione S-transferase A 2 (GSTA2) gene expression, the mRNA of them was assayed by qPCR. As shown in Figure 3, APAP treatments were significantly decreased the levels of GCLC and GSTA, EOs-APAP treatment alleviate APAP induced decreased levels of GCLC and GSTA. EOs significantly increased the levels of Nrf2, HO-1, NQO1, GCLC and GSTA2, suggestion EOs can mediate antioxidant biological activities.
Figure 3. The levels of mRNA expression of oxidative stress-related gene.
#Significant compared with control group alone, P<0.05. *Significant compared with model group alone, P<0.05.
Effect of EOs on APAP metabolism
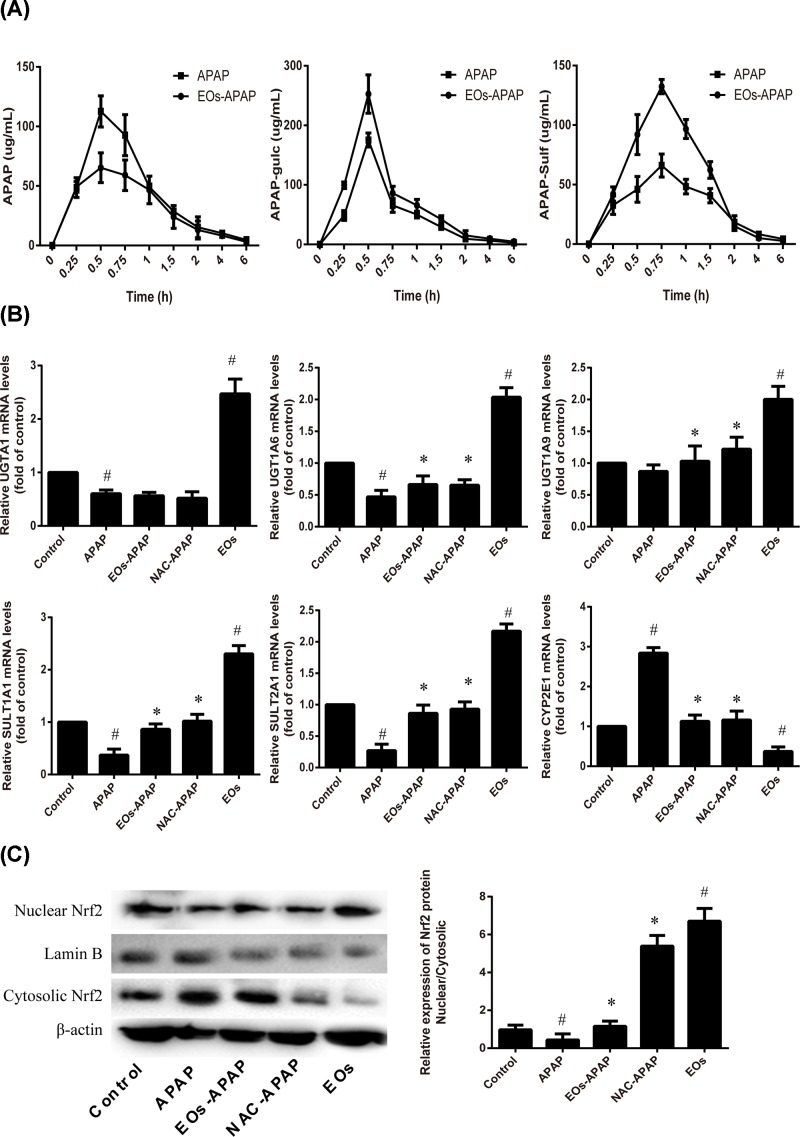
APAP and its major conjugates in plasma were analyzed. As shown in Figure 4A, compared with APAP treatment group, the concentration of APAP was decreased, while APAP-gulc and APAP-sulf were increased in APAP-EOs treatment. The results show that EOs can increase the APAP metabolized into APAP-gluc and APAP-sulf.
Figure 4. Effects of EOs on the APAP metabolic disposition.
(A) EOs can increase the APAP metabolized into APAP-gluc and APAP-sulf. (B) The mRNA expression of major enzymes involved in APAP lucuronidation. (C) The expression of Nuclear Nrf2 and Cyosolic Nrf2 was detected by Western blot. #Significant compared with control group alone, P<0.05. *Significant compared with model group alone, P<0.05.
APAP has two metabolism pathway in liver, one is non-toxicity pathway, APAP was metabolized into APAP-gluc and APAP-sulf by UGT family and SULT family and excreted in the blood and bile [18]. UGT1A6 and UGT1A1 are major enzymes involved in APAP glucuronidation. The increased levels of UGT1A6 and UGT1A1 enhanced the resistance to APAP toxicity [19]. In the present study, the levels of UGT1A1, UGT1A6, UGT1A9, SULT1A1, SULT2A1 were significantly increased after EOs treatments, suggestion EOs can enhance APAP metabolism by non-toxicity pathway. UGT family and SULT family are essential for inducing gene expression by Nrf2 with the consensus of TGAG/CNNNGC (N represents any base) [20]. As the result shows that EOs significantly increased the mRNA expression of Nrf2 (Figure 3C). The activated Nrf2 was transfer from cytoplasm into nuclear, the nuclear/cytosolic relative expression was significantly increased in EOs treatment group, suggestion EOs were induced Nrf2 transfer from cytoplasm to nucleus, then it leads to transcriptional activation of antioxidant enzymes, such as HO-1, SOD, CAT (Figures 2 and 3) and Phase II metabolic enzyme, such as UGT, SULT (Figure 4).
The second metabolism pathway of APAP was toxicity. Overdose APAP was metabolism to NAPQI by CYP2E1. In the present study, the expression of CYP2E1 was significantly increased by APAP treatment and decreased by EOs treatment (Figure 4). CYP2E1 deficient mice were resistant to the liver injury induced APAP, while the transgenic mouse expression human CYP2E1 were susceptible the conversion of APAP to NAPQI. Our results show that EOs inhibit CYP2E1 expression to against the liver injury.
Together, EOs induced the Nrf2 activity, the activated Nrf2 transcriptional activation of UGT and SULT. EOs were acceleration non-toxicity metabolism of APAP into APAP-gluc and APAP-sulf by enhance the levels of UGT and SULT, as well as inhibition of CYP2E1, which decreased the formation of NAPQI.
Discussion
APAP is metabolized by sulfation, glucuronidation and CYP oxidation. The sulfation and glucuronidation pathways are considered detoxification routes, while the CYP oxidation pathway generates NAPQI, a reactive and toxic species. In case of acetaminophen toxicity, the Phase II conjugation enzymes are saturated, and a higher fraction is converted to NAPQI that leads to oxidative stress of liver. ROS is one of major factors in oxidative stress progress. Nrf2, which is likely activated by redox status changes induced by NAPQI. Mechanistically, the activated Nrf2 transfers from cytoplasm to nucleus, then it leads to transcriptional activation of antioxidant enzymes, such as NADPH: quinone oxidoreductase 1 (NQO1), HO-1, glutamate cysteine ligase (GCL) and GSTA, which increased the expression of SOD and CAT [21]. Besides, APAP caused severe liver injury characterized by significantly increased serum AST and ALT levels, ROS and hepatic MDA, as well as liver SOD, CAT depletions.
NAC can be used to detoxify for acetaminophen poisoning. It has the functions of anti-oxidation, scavenging oxygen free radicals, preventing DNA damage, regulating cell metabolic activity, regulating gene expression and signal transduction, and inhibiting the production of inflammatory mediators in the body [22]. NAC has the FDA approval for the treatment of potentially hepatotoxic doses of APAP. Therefore, we used NAC as the positive control drugs.
The first record of Nepeta cataria L. was documented in the ancient Chinese herbal book Ben-Cao-Gang-Mu (Ming Dynasty, by Shizhen Li). The book stated that the fired leaves and tender stems of Nepeta cataria L. could be served as food and tea. EO was extracted by different methods. SFE method was chosen in food industries, and has been extensively used to remove metal ions from various solid and liquid matrices of environmental samples. Different areas culturing Nepeta cataria L. have a variety of different ingredients, and uses for different functions. However, the function of Nepeta cataria L. was unclear.
In our studies, we found that EOs from Nepeta cataria L. up-regulated the expression and activities of Nrf2 and detoxification enzymes including UGTs and SULTs, as well as inhibited the activity of CYP2E1, which decreased plasma concentration of APAP and accelerated APAP harmless metabolism. Our data also showed that the effectiveness of EOs was as good as the NAC.
In summary, EOs from Nepeta cataria L. are effective in APAP-induced liver injury. They can be considered as a potential natural resource to develop hepatoprotective agent and feed additive instead of antibiotics. The results from the present study might supply useful functional food and better the understanding of the antioxidative characteristics of EOs from traditional herbal Nepeta cataria L.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CAT
catalase
- CYP
cytochrome P450 enzyme
- EO
essential oil
- GCLC
glutamate-cysteine ligase catalytic subunit
- GSH
glutathione
- GSTA2
glutathione S-transferase A 2
- HO-1
heme oxygenase-1
- MDA
malondiadehyde
- NAC
N-acetylcysteine
- NAPQI
N-actyl-p-benzoquinone imine
- Nrf2
nuclear factor erythroid 2-related factor 2
- qPCR
quantitative real-time PCR
- ROS
reactive oxygen species
- SFE
supercritical fluid extraction
- SOD
superoxide dismutase
- SULT
sulfotransferase
- UGT
uridine diphosphate glucuronosyltransferase
Funding
This work was supported by the foundation of Natural Science Foundation of Shaanxi Province [grant number SXZRKXJJ-17775]. This work was also supported by grants from the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University [grant number YNQN 2017053]
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
F.Q. provided the idea of this article. J.T. and Prof. F.Q. contributed equally to the data and writing of this article.
References
- 1.Wang M., Cheng K.W., Wu Q. and Simon J.E. (2007) Quantification of nepetalactones in catnip (Nepeta cataria L.) by HPLC coupled with ultraviolet and mass spectrometric detection. Phytochem. Anal. 18, 157–160 10.1002/pca.965 [DOI] [PubMed] [Google Scholar]
- 2.Süntar I., Nabavi S.M., Barreca D., Fischer N. and Efferth T. (2018) Pharmacological and chemical features of Nepeta L. genus: its importance as a therapeutic agent. Phytother. Res. 32, 185–198 10.1002/ptr.5946 [DOI] [PubMed] [Google Scholar]
- 3.Zomorodian K., Saharkhiz M.J., Rahimi M.J., Shariatifard S., Pakshir K. and Khashei R. (2013) Chemical composition and antimicrobial activities of essential oil of Nepeta cataria L. against common causes of oral infections. J. Dent. (Tehran) 10, 329–337 [PMC free article] [PubMed] [Google Scholar]
- 4.Emami S.A., Asili J., Hossein Nia S., Yazdian-Robati R., Sahranavard M. and Tayarani-Najaran Z. (2016) Growth inhibition and apoptosis induction of essential oils and extracts of Nepeta cataria L. on human prostatic and breast cancer cell lines. Asian Pac. J. Cancer Prev. 17, 125–130 10.7314/APJCP.2016.17.S3.125 [DOI] [PubMed] [Google Scholar]
- 5.Fan J., Bao Y., Meng X., Wang S., Li T., Chang X. et al. (2017) Mechanism of modulation through PI3K-AKT pathway about Nepeta cataria L.’s extract in non-small cell lung cancer. Oncotarget 8, 31395–31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikravesh H., Khodayar M J., Mahdavinia M., Mansouri E., Zeidooni L. and Dehbashi F. (2018) Protective effect of gemfibrozil on hepatotoxicity induced by acetaminophen in mice: the importance of oxidative stress suppression. Adv. Pharm. Bull. 8, 331–339 10.15171/apb.2018.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H., Jiang Z., Chang X., Xue H., Yahefu W. and Zhang X. (2018) 4-hydroxyphenylacetic acid prevents acute APAP-induced liver injury by increasing Phase II and antioxidant enzymes in mice. Front. Pharmacol. 9, 653. 10.3389/fphar.2018.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancaster E.M., Hiatt J.R. and Zarrinpar A. (2015) Acetaminophen hepatotoxicity: an updated review. Arch. Toxicol. 89, 193–199 10.1007/s00204-014-1432-2 [DOI] [PubMed] [Google Scholar]
- 9.Du K., Ramachandran A. and Jaeschke H. (2016) Oxidative stress during acetaminophen hepatotoxicity: sources, pathophysiological role and therapeutic potential. Redox Biol. 10, 148–156 10.1016/j.redox.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Z., Chen C., Wang J., Xie W., Wang M., Li X. et al. (2016) Purple potato (Solanum tuberosum L.) anthocyanins attenuate alcohol-induced hepatic injury by enhancing antioxidant defense. J. Nat. Med. 70, 45–53 10.1007/s11418-015-0935-3 [DOI] [PubMed] [Google Scholar]
- 11.Yan M., Huo Y., Yin S. and Hu H. (2018) Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 17, 274–283 10.1016/j.redox.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W., Zeng C., Jia Q. and Yang X. (2018) Effects of the Kunlun snow chrysanthemum polysaccharides on acetaminophen-induced oxidative stress, inflammation and apoptosis using animal model. Pak. J. Pharm. Sci. 31, 985–990 [PubMed] [Google Scholar]
- 13.Wang D., Gao Q., Zhao G., Kan Z., Wang X., Wang H. et al. (2018) Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice. J. Agric. Food Chem. 25, 7674–7683 10.1021/acs.jafc.8b02293 [DOI] [PubMed] [Google Scholar]
- 14.Hou Y., Peng S., Li X., Yao J., Xu J. and Fang J. (2018) Honokiol alleviates oxidative stress-induced neurotoxicity via activation of Nrf2. ACS Chem. Neurosci. 9, 3108–3116 10.1021/acschemneuro.8b00290 [DOI] [PubMed] [Google Scholar]
- 15.Liu X.F., Zhou D.D., Xie T., Hao J.L., Malik T.H., Lu C.B. et al. (2018) The Nrf2 signaling in retinal ganglion cells under oxidative stress in ocular neurodegenerative diseases. Int. J. Biol. Sci. 14, 1090–1098 10.7150/ijbs.25996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud A.M., Germoush M.O., Al-Anazi K.M., Mahmoud A.H., Farah M.A. and Allam A.A. (2018) Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed. Pharmacother. 106, 499–509 10.1016/j.biopha.2018.06.171 [DOI] [PubMed] [Google Scholar]
- 17.Hu Y., Yu C., Yao M., Wang L., Liang B., Zhang B. et al. (2018) The PKCdelta-Nrf2-ARE signalling pathway may be involved in oxidative stress in arsenic-induced liver damage in rats. Environ. Toxicol. Pharmacol. 62, 79–87 10.1016/j.etap.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 18.Cao L., Kwara A. and Greenblatt D.J. (2017) Metabolic interactions between acetaminophen (paracetamol) and two flavonoids, luteolin and quercetin, through in vitro inhibition studies. J. Pharm. Pharmacol. 69, 1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon E., Babar A., Choudhary M., Kutner M. and Pyrsopoulos N. (2016) Acetaminophen-induced hepatotoxicity: a comprehensive update. J. Clin. Transl. Hepatol. 4, 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes J.D. and Dinkova-Kostova A.T. (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218 10.1016/j.tibs.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Loboda A., Damulewicz M., Pyza E., Jozkowicz A. and Dulak J. (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell. Mol. Life Sci. 73, 3221–3247 10.1007/s00018-016-2223-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrickson R.G. (2019) What is the most appropriate dose of N-acetylcysteine after massive acetaminophen overdose? Clin. Toxicol. (Phila.) 57, 686–691 10.1080/15563650.2019.1579914 [DOI] [PubMed] [Google Scholar]