To the Editor:
Cancer patients undergoing cytotoxic chemotherapy are at elevated risk of developing serious infections1. The risk of developing these infections increases when white blood cell (WBC) counts, particularly the absolute neutrophil counts (ANC), are reduced. This reduction in neutrophil counts, the most abundant white-blood-cell subtype, is referred to as neutropenia, with these infection episodes termed febrile neutropenia (FN). Patients have a high risk of developing FN during sustained severe neutropenia1 (ANC<500/µL), which is a common side effect of cytotoxic chemotherapies. FN occurs frequently, currently in approximately one in six of all chemotherapy patients2, and it is associated with a high rate of mortality3, where 11% of patients die after one or several hospitalizations4,5. In the US alone, the associated cost due to such hospitalizations accounts for $2.7B dollars annually2, contributing to up to 40% of the total cost of cancer treatments6.
Early detection of severe neutropenia can be key to preventing FN. Timely detection could enable preventive therapies, such as Granulocyte-Colony Stimulating Factors (GCSF)7 or prophylactic antibiotics, to be administered. Unfortunately, current neutrophil monitoring options are inadequate to monitor severe neutropenia with sufficient frequency to allow for early detection and therapy optimization. Their reliance on the extraction and analysis of blood samples, which requires trained medical oversight, typically limits this testing to clinical settings. Cost-effective high frequency monitoring of this parameter, therefore, requires a new method that can be used by outpatients with minimal risk.
To address this unmet need, we propose the first noninvasive technology that can automatically screen patients for severe neutropenia without requiring blood draws, thus enabling patients to have more frequent access to this test. Our proposed noninvasive device is a compact microscopy system that can acquire high-resolution, high-frame-rate videos of superficial capillaries (Figure S1)8, coupled with an automated software pipeline that can analyze those videos for a measurement of neutropenia. This design potentially opens the door to the use of such instrumentation in the patient’s home. We conducted a clinical study at two independent hospitals to assess the ability of our device and algorithm to detect severe neutropenia in chemotherapy patients undergoing standard therapy. In previously reported work8, we demonstrated an initial proof-of-concept of this principle using a small set of nailfold capillary video samples8, which were manually analyzed through visual inspection by human experts. Here, we further demonstrated and validated a fully automated software analysis pipeline and extended our results to a larger cohort of 44 patients.
The individuals enrolled in our study were selected from patients undergoing high-dose chemotherapy followed by Autologous Stem Cell Transplantation (ASCT) at the Massachusetts General Hospital (MGH) Boston, MA, USA, or at Hospital Universitario La Paz (HULP), Madrid, Spain. This specific patient population was selected due to its clinical relevance and highly standardized regimen which allows for predictable evolution of neutrophil count dynamics, ensuring a transition from baseline values (ANC>500/µL) to severe neutropenia (ANC<500/µL), as well as an opportunity to acquire multiple video samples at different time points (Figure S2). The protocol was approved by the corresponding IRB boards for a total of 44 patients to be included in this study (Supplementary Methods).
From the 44 enrolled subjects, video sessions containing at least one suitable capillary in their field of view were collected (Supplementary Methods) at different stages of the ASCT treatment (Figure S2). Specifically, a technical operator ensured that at least one capillary fulfilled the required quality criteria for analysis8. This resulted in a total of 115 imaging sessions from 42 subjects, with an average of three sessions per subject. Each imaging session is associated with a reference blood test performed by the gold-standard clinical-laboratory analyzer whose values, including the reference ANCs, are provided in the Supplementary Methods (Figure S3). To confirm the findings of our previous work correlating the number of capillaries analyzed with the classification accuracy8, we evaluated the diagnostic performance of our automatic pipeline on three distinct sets of imaging sessions: those that had at least one suitable capillary (all 115 imaging sessions from all 42 patients), at least two (100 sessions from 38 patients), or at least three (89 sessions from 35 patients).
Following the acquisition of these sessions using our clinical prototype8, an automated software pipeline composed of several processing steps (Supplementary Methods) was designed to analyze the raw videos. The analysis of each video session produced a separate data point consisting in a unitless “Leuko Index”, which was used to classify severe-neutropenic cases (ANC<500/µL) from the rest (ANC>500/µL). To produce this index, our pipeline first detected nailfold capillaries in the corresponding video session, then counted passing optical-absorption gaps —which as discussed in Bourquard et al 20188 can be considered as proxies of flowing neutrophils — inside these capillaries, and finally averaged the individual capillary counts into one single value. Results were then compared against the gold-standard ANC values to determine performance in separating severely neutropenic patients (ANC<500/µL) from the rest (ANC>500/µL), giving the opportunity to calculate the rate of correctly-classified severe neutropenia cases (true positives) against the rate of false alarms (false positives).
The classification performance for all imaging sessions, with at least three suitable capillaries detected, yielded an Area Under the Curve (AUC)9 of 0.95 (Figure 1). The use of all imaging sessions with at least one suitable capillary yielded an AUC of 0.91 (Figure S4), whereas those containing at least two suitable capillaries yielded an AUC of 0.94 (Figure S5). The intra-measurement agreement of our method when using only one, two or three capillaries per imaging session was shown to increase as more capillaries were used (87% agreement when using 1 vs. 2 capillaries and 91.4% agreement when using 2 vs. 3 capillaries), see Figure S6. Also, the classification performance improved with the amount of analyzed capillaries per session, with the percentage agreement increasing from 69.8% to 90.9% (Figure S6), which also corroborates previous results8.
Figure 1.
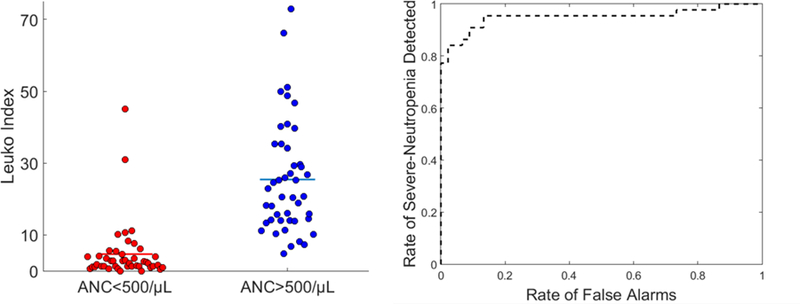
(Left) noninvasive imaging sessions (red and blue markers), acquired from chemotherapy patients in our study, were automatically analyzed by our software pipeline to yield a “Leukoindex” result which can be used to perform a classification with respect to the corresponding gold-standard ANC values obtained from blood tests. Each classification result is associated either with a severe-neutropenic reference state (ANC<500; red markers) or above (ANC>500; blue markers). Results corresponding to imaging sessions containing at least three suitable capillaries are shown. (Right) Receiver Operating Characteristic (ROC) curve associated with this classification (AUC = 0.95, 35 patients, and N =89 imaging sessions).
These results demonstrate, for the first time, that our noninvasive optical system (Figure S1), coupled with the proposed automated video analysis pipeline (Figure S7), is able to detect severe neutropenia with high accuracy in a patient cohort showing various degrees of ANC values (Figure S3), without the need for a blood draw. Future work includes the refinement of the device hardware to increase the number of suitable capillaries detected in a single session. The current analysis methods may also be adapted in the future to perform quantitative ANC measurements or differential detections of WBC subtypes.
Overall, this work demonstrates that patients can be screened for severe neutropenia automatically and noninvasively without the need to draw blood. Our noninvasive blood analysis method opens the door to frequent, precise and personalized management of patients at risk for febrile neutropenia.
Supplementary Material
Acknowledgments
The authors thank the Madrid-MIT M+Visión Consortium, the Catalyst program, and MIT linQ for their support and guidance in developing this project. They thank Martha L. Gray, Benjamin J. Vakoc, Timothy P. Padera, and all other MIT linQ faculty members who provided invaluable feedback and advice through regular working-group sessions and meetings. This work was supported by the NIH NCI SBIR Program (award no. 1R43CA228920-01A1); the Comunidad de Madrid through the Madrid-MIT M+ Visión Consortium; the Center For Future Technologies in Cancer Care through Grant NIH U54 (award no. 4U54EB015403-05); the Wallace H. Coulter Foundation at BU; the Deshpande Center for Technological Innovation; the MIT Sandbox Innovation Fund; and the M+Visión EU FP7-PEOPLE-2011-COFUND Program of the Fundación Madri+d (Comunidad de Madrid).
CCG, IB, AB, and ASF have an issued patent on the proposed technology under patent number US 9,984,277 B2 and title “Systems, Apparatus, and Methods for Analyzing Blood Cell Dynamics”. APT, IB, ASF, AB, and CCG report honoraria, financial support from, and current employment by Leuko Labs Inc. to conduct this research. IB, ASF, CCG, and AB hold equity in Leuko Labs Inc.
Footnotes
Disclosure of Conflict of Interest
All other authors have nothing to declare.
REFERENCES
- 1.Meza L, Baselga J, Holmes FA, Liang B, Breddy J, Group PS. Incidence of febrile neutropenia (FN) is directly related to duration of severe neutropenia (DSN) after myelosuppressive chemotherapy. Proc Am Soc Clin Oncol Volume 21 2002. p. 255b. [Google Scholar]
- 2.Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract 2017;13(6):e552–e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Cancer Netw JNCCN 2009. January;7(1):99–108. [DOI] [PubMed] [Google Scholar]
- 4.Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006. May 15;106(10):2258–2266. [DOI] [PubMed] [Google Scholar]
- 5.Lyman GH, Poniewierski MS, Crawford J, Dale DC, Culakova E. Cost of hospitalization in patients with cancer and febrile neutropenia and impact of comorbid conditions. Blood; 2015. 126(23):2089. [Google Scholar]
- 6.Schuette HL, Tucker TC, Brown ML, Potosky AL, Samuel T. The costs of cancer care in the United States: implications for action. Oncol Williston Park N 1995. November;9(11 Suppl):19–22. [PubMed] [Google Scholar]
- 7.Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 1991. July 18;325(3):164–170. [DOI] [PubMed] [Google Scholar]
- 8.Bourquard A, Pablo-Trinidad A, Butterworth I, Sánchez-Ferro Á, Cerrato C, Humala K, Fabra Urdiola M, Del Rio C, Valles B, Tucker-Schwartz JM, Lee ES, Vakoc BJ, Padera TP, Ledesma-Carbayo MJ, Chen Y-B, Hochberg EP, Gray ML, Castro-González C. Noninvasive detection of severe neutropenia in chemotherapy patients by optical imaging of nailfold microcirculation. Sci Rep 2018. March 28;8(1):5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lasko TA, Bhagwat JG, Zou KH, Ohno-Machado L. The use of receiver operating characteristic curves in biomedical informatics. Journal of biomedical informatics 2005. October 1;38(5):404–15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


