Abstract
Insects represent the most diverse and functionally important group of flying migratory animals around the globe, yet their small size makes tracking even large migratory species challenging. We attached miniaturized radio transmitters (less than 300 mg) to monarch butterflies (Danaus plexippus) and common green darner dragonflies (Anax junius) and tracked their autumn migratory movements through southern Ontario, Canada and into the United States using an automated array of over 100 telemetry towers. The farthest estimated distance a monarch travelled in a single day was 143 km at a wind-assisted groundspeed of 31 km h−1 (8.7 m s−1) and the farthest estimated distance a green darner travelled in a single day was 122 km with a wind-assisted groundspeed of up to 77 km h−1 (21.5 m s−1). For both species, increased temperature and wind assistance positively influenced the pace of migration, but there was no effect of precipitation. While limitations to tracking such small animals remain, our approach and results represent a fundamental advance in understanding the natural history of insect migration and environmental factors that govern their movements.
Keywords: Anax junius, common green darner, Danaus plexippus, monarch butterfly, Motus, radio-telemetry
1. Introduction
Billions of insects around the world undertake annual migrations. The occurrence of insect migration has been known for centuries and includes such well-known examples as desert locusts (Schistocerca gregaria) in Africa [1], painted lady butterflies (Vanessa cardui) in Europe [2] and monarch butterflies (Danaus plexippus) in North America [3]. While these migrations can have an enormous impact on the functioning of ecosystems [4,5], little is known about the proximate environmental factors that guide individual movements. Some aspects of insect migration may be similar to avian migration [6], but environmental conditions, such as wind and temperature, have a greater effect on the pace of insect migration [7]. The major challenge has been an inability to track individuals during flight, which means we have a limited understanding of how far or how fast insects can travel over successive days during migration.
Along with moths [8,9], dragonflies and butterflies are among the most charismatic migratory insects. Although their annual migrations are typically multi-generational [2,3,10], individuals of many species travel thousands of kilometres during a single migration [2,11]. Most butterflies and dragonflies migrate during the day and near the surface of the Earth where their self-propelled flight speed generally exceeds wind speed [12]. This allows these relatively large insects to, at least partially, correct for wind drift [13,14] and have greater control over their intended direction. Some species, such as painted lady and monarch butterflies, will also soar higher in the atmosphere [2,15,16] to take advantage of strong winds blowing in favourable directions [5,7,17]. In addition to wind, the temperature should have an effect on the pace of migration because a minimum ambient temperature is required for insect flight [18]. Precipitation may be disruptive to migration, but its effects are less certain [18]. Evaluating how these environmental factors influence the pace of individual migration is important for understanding the diverse migration strategies of insects.
In this study, we used an automated wildlife tracking system (Motus [19]) to radio-track individual monarch butterflies and common green darner dragonflies (Anax junius) as they migrated through southern Ontario, Canada in the autumn. The last summer generation of eastern North American monarch butterflies travels south to overwinter in central Mexico and then migrates north the following spring to produce the first breeding generation [3,20]. Common green darners also undertake an annual multi-generational migration from eastern North America to the southern United States, Mexico and the Caribbean and back [10,21]. For both species, we document individual trajectories at the beginning of autumn migration, describe the speed and daily distance of travel, and test the effects of temperature, wind and precipitation on the speed of migration.
2. Material and methods
(a). Field methods
Fieldwork occurred during September 2015 and 2016 in the Bruce Peninsula (ON, Canada; 45°13′ N, 81°37′ W), an area dominated by mixed-wood forests and exposed limestone cliffs. Insects were captured using butterfly nets at three main sites (meadow, residential gardens and alvar). Captured insects were sexed and weighed, and only insects weighing greater than 0.45 g were fitted with an NTQB-1 radio transmitter (Lotek Wireless Inc., Newmarket, ON, Canada). We used two different weights of transmitters; plastic coating (0.27 g) and clear finish (0.23 g). For both species, transmitters were adhered directly below the leg joints, on the ventral abdomen for monarchs, and the ventral thorax for green darners, using Instant Krazy Glue Advanced Gel (Elmers, OH, USA).
In total, 38 green darners (2015: n = 28; 2016: n = 10) were tagged, comprising 12 males and 26 females. For monarchs, 43 individuals were tagged (2015: n = 19; 2016: n = 24), comprising 27 males and 16 females. All tagged green darners were released within 1 h of capture. Most tagged monarchs could not sustain flight after release and were kept for one to two nights, fed honey–water solution and released in the morning. Movements of tagged insects were detected by the Motus Wildlife Tracking System (https://motus.org), an automated radio-telemetry network with over 100 towers [19]. In 2015, tagged insects were released at the base of one of two Motus towers on the Bruce Peninsula. However, in 2016, the nearest tower was relocated north of the capture sites. Tagged insects were released at the central capture location, approximately 42 km from the nearest southern tower, excluding one individual released at the new northern tower location.
(b). Data analysis
R v. 3.4.4 [22] was used for all statistical analyses. Motus movement data [19] were processed and filtered using the Motus package (https://motus.org/MotusRBook/). We filtered out false detections from series that had fewer than four consecutive bursts, were outside of the probable range of movement or came from towers that appeared to be having random radio noise interference. We confirmed that the remaining detection series had consecutive bursts at the tags' designated burst rate [23].
For subsequent analyses, we assumed that both species only migrated between certain hours during the day [6,24,25]. Activity hours were defined as 10 : 00–19 : 00 EST for monarchs and 07 : 00–19 : 00 EST for green darners (electronic supplementary material). Mean groundspeed between detections was estimated by dividing the straight line (loxodrome) distance between detections by the number of activity hours between detections. Airspeed was calculated by subtracting tailwinds from groundspeed. Crosswinds were not factored into the calculation. Circular statistics and a Rayleigh's test of uniformity were used to evaluate the mean direction of movements between detections (circular package [26]).
To understand how environmental variables influenced the estimated mean groundspeed of individuals between detections, we ran a general linear mixed model (lmerTest package [27]), with mean groundspeed (km h−1) as the response variable. Fixed effects included in the model were species, body mass (g), departure date (Julian), mean temperature (°C), total precipitation (mm), tailwind (m s−1), crosswind (m s−1) and individual ID (random effect). Results are reported as parameter estimates ± s.e. North American Regional Reanalysis (NARR) weather data were provided by the NOAA/OAR/ESRL PSD (Boulder, Colorado, USA; https://www.esrl.noaa.gov/psd/) at a grid resolution of 0.3°. See electronic supplementary material for analysis of weather data.
3. Results
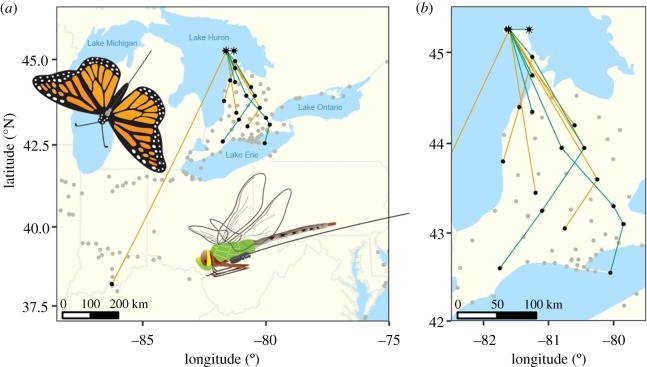
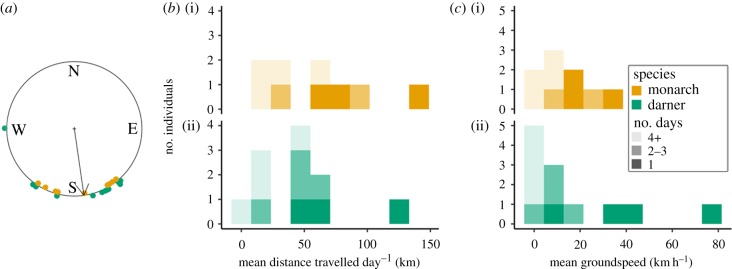
In total, 49% (21/43) of monarchs and 95% (36/38) of green darners were detected by the Motus tower closest to the release sites. Twenty-nine per cent (6/21) of monarchs (all males) and 17% (6/36) of green darners (all females) were detected away from the release sites, by up to four towers (figure 1). Most detections were in southern Ontario, but one monarch was detected in southern Indiana (figure 1a). The mean direction of movement was almost directly south (172°; Rayleigh test, r = 0.84, p < 0.001, n = 21 movements from 12 individuals; figure 2a).
Figure 1.
Sequential detections (black points) of radio-tagged monarch butterflies (orange lines) and common green darners (green lines) between Motus Wildlife Tracking System towers in (a) eastern North America and (b) zoomed in to southern Ontario, Canada. Grey points indicate active Motus towers in 2015 and asterisks (*) indicate the deployment sites. Lines connect individuals between Motus tower detections but do not indicate the actual path of travel between points. Map projection is Mercator.
Figure 2.
(a) Circular plot displaying the direction of movements between detections. Arrow shows the circular mean direction. (b) Histograms displaying the estimated mean distance of travel each day between detections and (c) the estimated mean groundspeed of travel between detections for radio-tagged monarchs (i) and common green darners (ii). Dark bars indicate distances and speeds calculated from detections between two towers within the same day, whereas lighter bars indicate calculations from detections between multiple (2–3 or 4+) days.
The average groundspeed between detections was 12 (±10 s.d.) km h−1 (3.3 m s−1) for monarchs and 16 (±23) km h−1 (4.5 m s−1) for green darners. The average daily distance travelled was 61 (±42) km for monarchs and 43 (±34) km for green darners (figure 2). The fastest green darner travelled at a groundspeed of 77 km h−1 (21.5 m s−1; figure 2) with 20 km h−1 (5.5 m s−1) tailwind assistance, resulting in an estimated airspeed of 58 km h−1 (16.0 m s−1). The fastest monarch travelled at a groundspeed of 31 km h−1 (8.7 m s−1) with 16 km h−1 (4.6 m s−1) tailwind assistance, resulting in an estimated airspeed of 15 km h−1 (4.1 m s−1). These fastest individuals also travelled the farthest within a single day, with the green darner travelling at least 122 km and the monarch at least 143 km between detections (figure 2). Groundspeeds and distances travelled per day would have been underestimated for individuals with multiple days in between detections because we could not determine if and for how long individuals rested during days when they were not detected. Averages would also be underestimated as a result.
The mean daytime temperature between detections ranged from 17.8 to 22.9°C, total precipitation ranged from 0 to 7.9 mm, and wind speed ranged from 3.6 to 28.9 km h−1. Groundspeed, as estimated from successive detections of the same individual, was positively influenced by the mean daytime temperature (β = 4.14 ± 1.45, d.f. = 12.80, t = 2.86, p = 0.01), tailwind assistance (β = 7.49 ± 0.95, d.f. = 11.98, t = 7.90, p < 0.001) and crosswind from the west (β = −3.16 ± 0.70, d.f. = 7.86, t = −4.53, p < 0.01). There was no significant effect of total precipitation on groundspeed (β = 0.59 ± 1.33, d.f. = 8.63, t = 0.45, p = 0.67). Complete results are reported in electronic supplementary material, table S1.
4. Discussion
Direct tracking of both monarch butterflies and common green darners revealed the capacity of these insects to travel long distances in a short period of time and how their migration was influenced by environmental conditions en route. The maximum daily distance a monarch butterfly covered (143 km) was close to a previous estimate for the species based on observed flight speeds (130 km day−1 [20]). We estimate the fastest monarch flew at an airspeed of 15 km h−1 (4.1 m s−1), close to airspeeds observed for ‘cruising’ monarchs (approx. 18 km h−1 or 4.9 m s−1 [28]) and matching the top speeds that have been measured for other Danaus species (15 km h−1 or 4.1 m s−1 [29]). If monarchs were to exploit stronger winds higher in the atmosphere [15,16], they theoretically could fly even faster [28], but this may not have been possible with the additional weight of the tags.
Consistent with previous observations that green darners move every few days [6], the number of days between some detections in this study suggests these green darners (and also monarchs) took rest days during migration. The farthest green darner travelled at least 122 km in a single day, within the range of a previous estimate for the species based on observed flight speeds (200 km day−1; [6,24]). However, the average daily distance estimated in this study (43 km day−1) was much farther than the average daily distance travelled by green darners in the only other direct tracking study to date (12 km day−1; [6]). Populations that emerge farther north, such as in this study, may migrate faster than mid-latitude populations because northern populations arrive at their over-wintering grounds first (by end of October; [10]). This may also explain why two green darners appeared to fly at very fast airspeeds; one at an airspeed of 32 km h−1 (8.9 m s−1), which is slightly faster than has been observed for another species of dragonfly (Pantala hymenaea at 26 km h−1 or 7.2 m s−1; [14]), and another at up to 58 km h−1 (16.0 m s−1). We likely underestimated the wind speed (overestimating airspeed) for the latter individual (e.g. if it flew higher in the atmosphere [11,30]) as this is much faster than has been observed in low altitude flight.
Our results show that groundspeed was influenced by both temperature and wind. Both green darners and monarchs moved faster at higher temperatures, likely because they require the ambient temperature to be above a certain threshold for flight [10]. At higher temperatures than were recorded in this study (greater than 23°C), green darners have been recorded flying slower with increasing temperature and are likely attempting to regulate their body temperature when it gets too hot [24]. Both species also had faster groundspeeds with increasing tailwind assistance. This suggests that they may not be adjusting their airspeed to wind conditions (i.e. they were not minimizing the energetic cost of migration [30]). If populations at the northern edge of their range have to travel farther to reach their over-wintering grounds, they may be more limited by time and unable to completely minimize the energetic cost of migration in favour of faster travel. Surprisingly, total precipitation did not appear to have an effect on flight speed. This may be because we did not track individuals during any heavy rain events and they had no difficulty with light rain, or because they were able to make up for lost time after the rain ceased.
Despite the advances made in understanding individual insect migration, several limitations remain. In this study, the tags weighed up to 49% of individual body weight and may have had a significant impact on the animals' ability to move (slowing them down), particularly for monarchs. Green darners appeared to handle the tags much better (see also [6]), but testing how transmitters affect migration should be a focus of future research (e.g. [31]). Those that did make significant movements could only be tracked at the start of migration because the batteries lasted for less than one month. Towers also have limited detection ranges and several were not functional during this study. Because of these limitations, we also had to group monarchs and green darners for statistical analysis, potentially losing our ability to differentiate species-specific effects. As various tracking devices become smaller, we will continue to advance our understanding of insect migration.
Supplementary Material
Acknowledgements
We thank Rachael Derbyshire and Carlene Gallant for their assistance in the field, and Laurie Adams for providing accommodations and access to field sites.
Data accessibility
Motus data are available at https://motus.org/data/downloads?projectID=55 and https://motus.org/data/downloads?projectID=93 to registered Motus users.
Authors' contributions
G.M.P., D.T.T.F. and D.R.N. designed the research. G.M.P. conducted fieldwork and S.M.K. analysed these data. All authors wrote the manuscript. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (D.R.N.), a Liber Ero Postdoctoral Fellowship (D.T.T.F.), a University of Guelph Research Chair (D.R.N.) and under the Operation Pollinator programme of Syngenta Canada Inc. (D.R.N., D.T.T.F.).
References
- 1.Kennedy JS. 1951. The migration of the desert locust (Schistocerca gregaria Forsk.). I. The behaviour of swarms. II. A theory of long-range migrations. Phil. Trans. R. Soc. Lond. B. 235, 163–298. ( 10.1098/rstb.1951.0003) [DOI] [PubMed] [Google Scholar]
- 2.Stefanescu C, et al. 2013. Multi-generational long-distance migration of insects: studying the painted lady butterfly in the Western Palaearctic. Ecography 36, 474–486. ( 10.1111/j.1600-0587.2012.07738.x) [DOI] [Google Scholar]
- 3.Flockhart DTT, Wassenaar LI, Martin TG, Hobson KA, Wunder MB, Norris DR. 2013. Tracking multi-generational colonization of the breeding grounds by monarch butterflies in eastern North America. Proc. R. Soc. B 280, 20131087 ( 10.1098/rspb.2013.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 5.Hu G, Lim KS, Horvitz N, Clark SJ, Reynolds DR, Sapir N, Chapman JW. 2016. Mass seasonal bioflows of high-flying insect migrants. Science 354, 1584–1587. ( 10.1126/science.aah4379) [DOI] [PubMed] [Google Scholar]
- 6.Wikelski M, Moskowitz D, Adelman JS, Cochran J, Wilcove DS, May ML. 2006. Simple rules guide dragonfly migration. Biol. Lett. 2, 325–329. ( 10.1098/rsbl.2006.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alerstam T, Chapman JW, Bäckman J, Smith AD, Karlsson H, Nilsson C, Reynolds DR, Klaassen RHG, Hill JK.. 2011. Convergent patterns of long-distance nocturnal migration in noctuid moths and passerine birds. Proc. R. Soc. B 278, 3074–3080. ( 10.1098/rspb.2011.0058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake VA, Reynolds DR. 2012. Radar entomology: observing insect flight and migration. Wallingford, UK: CABI. [Google Scholar]
- 9.Chapman JW, Reynolds DR, Mouritsen H, Hill JK, Riley JR, Sivell D, Smith AD, Woiwod IP. 2008. Wind selection and drift compensation optimize migratory pathways in a high-flying moth. Curr. Biol. 18, 514–518. ( 10.1016/j.cub.2008.02.080) [DOI] [PubMed] [Google Scholar]
- 10.Hallworth MT, Marra PP, McFarland KP, Zahendra S, Studds CE. 2019. Tracking dragons: stable isotopes reveal the annual cycle of a long-distance migratory insect. Biol. Lett. 14, 20180741 ( 10.1098/rsbl.2018.0741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RC. 2009. Do dragonflies migrate across the western Indian Ocean? J. Trop. Ecol. 25, 347–358. ( 10.1017/s0266467409006087) [DOI] [Google Scholar]
- 12.Chapman JW, Reynolds DR, Wilson K. 2015. Long-range seasonal migration in insects: mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 18, 287–302. ( 10.1111/ele.12407) [DOI] [PubMed] [Google Scholar]
- 13.Srygley RB, Oliveira EG, Dudley R. 1996. Wind drift compensation, flyways, and conservation of diurnal, migrant Neotropical Lepidoptera. Proc. R. Soc. Lond. Ser. B 263, 1351–1357. ( 10.1098/rspb.1996.0198) [DOI] [Google Scholar]
- 14.Srygley RB. 2003. Wind drift compensation in migrating dragonfiles. J. Insect Behav. 16, 217–232. ( 10.1023/A:1023915802067) [DOI] [Google Scholar]
- 15.Gibo DL, Pallett MJ. 1979. Soaring flight of monarch butterflies, Danaus plexippus (Lepidoptera: Danaidae), during the late summer migration in southern Ontario. Can. J. Zool. 57, 1393–1401. ( 10.1139/z79-180) [DOI] [Google Scholar]
- 16.Gibo DL. 1981. Altitudes attained by migrating monarch butterflies, Danaus p. plexippus (Lepidoptera: Danaidae), as reported by glider pilots. Can. J. Zool. 59, 571–572. ( 10.1139/z81-084) [DOI] [Google Scholar]
- 17.Hale R, Swearer SE. 2016. Ecological traps: current evidence and future directions. Proc. R. Soc. B 283, 1–8. ( 10.1098/rspb.2015.2647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds DR, Chapman JW, Drake VA. 2017. Riders on the wind: the aeroecology of insect migrants. In Aeroecology, pp. 145–178. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 19.Taylor PD, et al. 2017. The Motus Wildlife Tracking System: a collaborative research network. Avian Conserv. Ecol. 12, 8 ( 10.5751/ACE-00953-120108) [DOI] [Google Scholar]
- 20.Brower LP. 1995. Understanding and misunderstanding the migration of the Monarch butterfly (Nymphalidae) in North America: 1857–1995. J. Lepid. Soc. 49, 304–385. [Google Scholar]
- 21.Russell RW, May ML, Soltesz KL, Fitzpatrick JW. 1998. Massive swarm migrations of dragonflies (Odonata) in Eastern North America. Am. Midl. Nat. 140, 325–342. ( 10.1674/0003-0031(1998)140[0325:msmodo]2.0.co;2) [DOI] [Google Scholar]
- 22.R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 23.Gómez C, Bayly NJ, Norris DR, Mackenzie SA, Rosenberg KV, Taylor PD, Hobson KA, Daniel Cadena C. 2017. Fuel loads acquired at a stopover site influence the pace of intercontinental migration in a boreal songbird. Sci. Rep. 7, 3405 ( 10.1038/s41598-017-03503-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May ML. 1995. Dependence of flight behavior and heat production on air temperature in the green darner dragonfly Anax junius (Odonata: Aeshnidae). J. Exp. Biol. 198, 2385–2392. [DOI] [PubMed] [Google Scholar]
- 25.Perez SM, Taylor OR, Jander R. 1997. A sun compass in monarch butterflies. Nature 387, 292 ( 10.1038/387029a0)9153394 [DOI] [Google Scholar]
- 26.Agostinelli C, Lund U. 2011. R Package ‘Circular’: circular statistics (version 0.4-93). See https://r-forge.r-project.org/projects/circular/.
- 27.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1–26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 28.Urquhart FA. 1960. Part I General Discussion. In The monarch butterfly, pp. 39–62. Toronto, ON: University of Toronto Press. [Google Scholar]
- 29.Dudley R, Srygley RB. 1994. Flight physiology of neotropical butterflies: allometry of airspeeds during natural free flight. J. Exp. Biol. 191, 125–139. [DOI] [PubMed] [Google Scholar]
- 30.Srygley RB, Dudley R. 2008. Optimal strategies for insects migrating in the flight boundary layer: mechanisms and consequences. Integr. Comp. Biol. 48, 119–133. ( 10.1093/icb/icn011) [DOI] [PubMed] [Google Scholar]
- 31.Bowlin MS, Henningsson P, Muijres FT, Vleugels RHE, Liechti F, Hedenström A. 2010. The effects of geolocator drag and weight on the flight ranges of small migrants. Methods Ecol. Evol. 1, 398–402. ( 10.1111/j.2041-210X.2010.00043.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Motus data are available at https://motus.org/data/downloads?projectID=55 and https://motus.org/data/downloads?projectID=93 to registered Motus users.