Abstract
Invasive placentation with extended pregnancy is a shared derived characteristic unique to eutherian mammals that possess a highly effective system of haemostasis, platelets. These are found in all mammals but no other group of animals. We propose that platelets and megakaryocytes (large polyploid nucleated bone marrow cells that produce platelets) evolved from an ancestral 2 N thrombocyte by polyploidization and that the possession of platelets enabled the evolution of invasive placentation. This could explain why invasive placentation is limited to mammals.
Keywords: evolution, placenta, platelet, megakaryocyte, mammal
1. Introduction
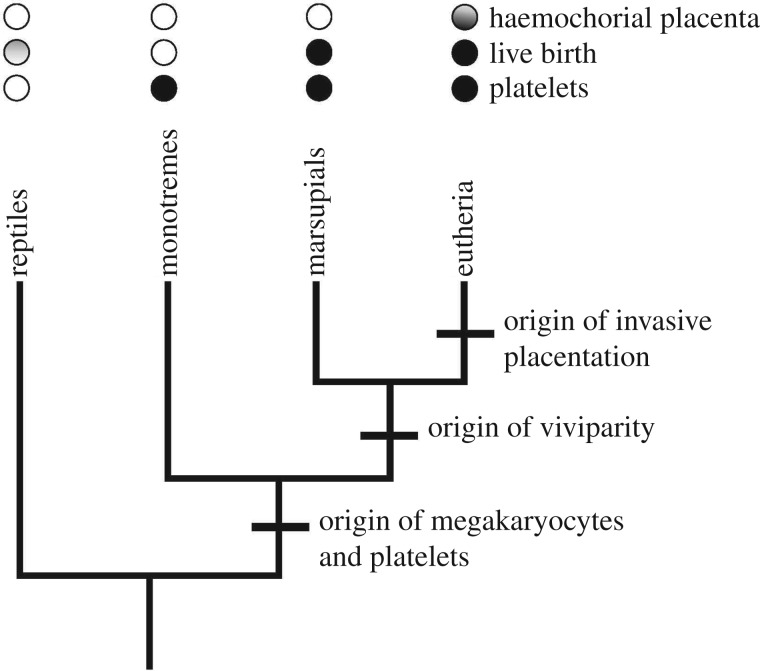
Mammals have many unique traits, two of which are: the megakaryocyte/platelet system (MK/P) and invasive (endothelio- and haemochorial) placentation. MK/P is not found in birds or reptiles [1]. Haemochorial placentation is only found in eutherian mammals [2–5] but not in marsupials and monotremes (figure 1). We propose that haemochorial placentation required MK/P for its evolution, thus explaining this nested distribution.
Figure 1.
Phylogenetic relationships of the major clades of mammals and the taxonomic distribution of haemostatic and reproductive characteristics. Platelets and megakaryocytes are found in all three clades of mammals but not in reptiles. Therians, i.e. eutherians and marsupials, share viviparity. In reptiles, the mode of reproduction is variable. Only eutherians have a haemochorial placenta. This condition is ancestral in eutherians, but there are some derived groups that have re-evolved non-invasive, epitheliochorial placentation: dots are shaded, with darker shading at the bottom, indicating an ancestral condition.
Giving birth to live neonates (viviparity) rather than laying eggs is widespread. It has evolved many times in fishes, frogs, salamanders, lizards, snakes and mammals [6,7]. Among vertebrates, viviparous lineages are only absent from the cyclostomes and the archosaurs including birds. Probably, viviparity has evolved more than 100 times in lizards and snakes alone [8–10]. Viviparity and placentation are also found in some invertebrates [11].
Surprisingly, only eutherian mammals have evolved invasive, haemochorial placentation even though many lineages have evolved various complex forms of placentation [8,12,13]. Although viviparity is simple to evolve [14], the evolution of haemochorial placentation is limited to animals with MK/P. We suggest that MK/P was an ‘exaptation’ sensu Gould & Vrba [15]: a trait that has a biological role in an organism, not originating for that function but acquiring its role by transfer of function. We argue that the preceding evolution of platelets was the exaptation necessary for the origin of invasive placentation.
2. The evolution of mammalian reproduction
There are four types of reproduction in mammals: egg-laying in monotremes, short embryo attachment in marsupials, deep placentation in ancestral placental mammals and reversion to non-invasive placentation as in horses and bovines [3–5]. The most ancestral form of mammalian reproduction is found in monotremes, egg-laying mammals (Platypus and the Echidna) [16,17] that already have some degree of oviparous matrotrophy through the eggshell [18]. Marsupial reproduction is characterized by a relatively long period of egg retention with ‘hatching’ from the egg within the uterus, then a brief period of attachment to the uterine mucosa—a step towards placental mammals. In non-macropod marsupials, embryo attachment is very brief, producing immature neonates [19,20]. There is longer gestation in macropods [21]. Molecular phylogeny studies of mammals [3–5] suggest that the ancestral fetal–maternal interface in eutherians was haemo- or at least endotheliochorial.
In marsupials, the very brief embryo attachment involves uterine inflammation followed by parturition [22,23]. In eutherian mammals, embryo implantation also involves inflammatory activation [24], followed by an anti-inflammatory state. Hence, the key event in the evolution of placental pregnancy was the ability to suppress the implantation-related inflammation allowing deep implantation with destruction of maternal blood vessels creating the haemochorial fetal–maternal interface [22,25]. This progression towards deeply invasive placentation in eutherians was only possible in animals that could handle the challenging haemostatic consequences of haemochorial implantation.
3. The evolution and function of megakaryocytes and platelets
Platelets are small enucleate secretory cells, produced from megakaryocytes [26]. They aggregate to occlude a site of bleeding, to initiate thrombus formation and secrete growth factors to repair blood vessels. Platelets have similar function and structure in all mammals including monotremes [27]. For haemostasis, reptiles and birds rely on the aggregation of circulating nucleated cells called thrombocytes [28], which are less efficient than platelets [29,30]. Thrombocyte-like cells occur in arthropods: coagulocytes in insects [31] and haemocytes in the limulus crab [32].
The physical and biological conditions of the pulmonary circulation support platelet production from megakaryocytes that have travelled in the venous circulation from the bone marrow [33–36]. Platelets are produced by physical fragmentation of megakaryocyte cytoplasm in the pulmonary circulation [37]. Megakaryocytes undergo true endomitosis: increase in nuclear DNA content within an intact nuclear membrane [38]. The unique step in the change from a 2 N thrombocyte to a large polyploid megakaryocyte would have been a late failure of cytokinesis giving incomplete mitosis aborted in anaphase, then repeated up to 128 N [38]. There is selective gene expression in higher ploidy cells [39,40].
Fragmentation of the polyploid nucleated cell to platelets would have given reproductive advantage owing to enhanced haemostasis after attack or injury. MK/P was a quantitative haemostatic advance as small size gave a large increase both in cellular surface area and speed of granule secretion. A further, qualitative, advantage over 2 N thrombocytes is that in response to bleeding megakaryocytes can increase their DNA content rapidly, up to 128 N, producing even more active platelets with increased receptor density, more organelles per unit cellular volume and increased capacity to produce prothrombotic proteins and to reduce bleeding time [41–49]. Platelet granules contain about a hundred cargo proteins produced by the megakaryocyte. Platelet-secreted proteins that are known to promote tumour growth (analogous to fetal growth) are VEGF, PDGF, EGF and TGF β.
4. The role of platelets in eutherian reproduction
In eutherian pregnancy, fertilization is associated with mild thrombocytopenia in mice [50] and women [51,52], owing to the secretion of embryo-derived platelet-activating factor (ePAF) [53], which also induces early pregnancy factor. Pretreatment of mice with PAF leaves them unresponsive to ePAF and is associated with reduced implantation rate [54]. Platelets are a major storage compartment of serotonin (5HT). Maternal 5HT is essential for early development of the mouse embryo [55,56]. 5HT in early gestation is entirely supplied by maternal platelets [57]. This is surprising, given a pre-neuronal role of 5HT in embryo development in the frog Xenopus [58] and sea urchins [59], which lack placentas.
After extravillous trophoblasts (EVTs) lose proliferative activity, they migrate towards uterine spiral arteries [60]. EVTs express the chemokine receptor CCR1 [61]. Platelets secrete MIPI-1α and MCP-3, which are CCR1 ligands [62]. Probably, these agents play a role in EVT migration and infiltration of the maternal arteries. Also, platelet α granule-secreted EGF, VEGF and PDGF enhance trophoblast invasion [63,64] and encourage trophoblasts to infiltrate arteries [65].
Safe disconnection of the placenta from the uterus is essential for the survival of the mother. Contraction of both the myometrium and the endometrium is important, as is cellular haemostasis. Haemostatic balance tilts towards hypercoagulability during human pregnancy [66]. Evidence that platelets are important comes from human mothers with Bernard–Soulier syndrome and Glanzmann's thrombasthenia, conditions manifesting a platelet dysfunction. Either primary or secondary haemorrhage occurs in 73% of pregnancies in patients with Bernard–Soulier syndrome [67], and in 50% of mothers giving birth with Glanzmann's thrombasthenia [68]. Knock-out (KO) experiments in mice show that maternal platelet defect is compatible with successful pregnancy [69].
The role of platelets in postpartum haemostasis alone is sufficient to support their role in the evolution of eutherian pregnancy. Other roles are rather specific to a subset of species and are thus likely derived, e.g. EVTs are a cell type limited to hominids. Fetal dependency on maternal 5HT in early development also has to be a derived condition, given that amphibian and sea urchin embryos can supply their own 5HT. A process with potential generality is platelet activation by ePAF and its role in early implantation. The role of platelets in implantation, however, is likely part of the inflammatory nature of implantation [24], which probably evolved from an inflammatory attachment reaction in the stem lineage of therians, i.e. before the most recent common ancestor of marsupials and eutherians [22,25].
5. An evolutionary scenario
The evolution of haemochorial, invasive placentation faced at least two obstacles: inflammation caused by embryo attachment to the uterine lining, and later, haemostasis. In marsupials, with the noted exception of Macropods (see above), fetal attachment to the uterine lining is followed quickly by various signs of inflammation, including neutrophil infiltration and parturition. By contrast, in eutherians, the attachment/implantation of the fetus is followed by an anti-inflammatory phase that allowed the extension of pregnancy beyond the limits of the length of the oestrus cycle [22]. The fact that inflammatory processes are involved in both marsupial and eutherian mammals, though with different outcomes, is correlated with the ‘generic’ aggressiveness of the therian blastocysts. In eutherians, it leads to implantation. Even in marsupials without implantation, the fetus is quite aggressive in attacking the luminal epithelium (LE) of the uterus; in the grey short-tailed opossum, Monodelphis domestica, at the end of gestation, cytoplasmic extensions of trophoblast cells can be seen to penetrate between the epithelial cells and breach the basal membrane of the LE [70,71] (GP Wagner 2015, personal observation), also in the Philander opossum [72] and bandicoots (Peramelidae) [73]. Differences in the invasiveness of the trophoblast between marsupials and eutherians are not differences in the fetus but rather in the way the maternal organism handles the situation. In marsupials, the partial invasion leads to expulsion (parturition) and in eutherians, the inflammatory reaction is attenuated and pregnancy extended.
The situation in reptiles is not as clear. In most cases of placental viviparous lizards, the placenta does not erode the LE but is in apposition with the LE and is held in place by uterine muscle contraction [74]. The lack of invasiveness could be explained by a lower aggressiveness of the fetus, as demonstrated in the case of an ectopic pregnancy in the southern grass skink (Pseudemoia entrecasteauxii [75]), which is a placentotrophic lizard. Any form of invasiveness is extremely rare in lizards, given the large number of viviparous lizards. In one, the African skink Trachylepis ivensi (Scincidae), a rare example of lizard ‘invasion’ does not lead to the establishment of a haemochorial placenta [76]. It is unclear whether this less invasive form may have been a way of lizards evolving a sustainable fetal–maternal relationship.
As soon as the mother had evolved a way of suppressing and managing the fetally induced inflammation, another problem arose: haemostasis. Haemochorial implantation leads to the partial destruction of the maternal blood vessels in the endometrium and thus raises the question of how the bleeding is limited to the area of placentation. The second problem arises at parturition, where the fetal–maternal interface is dissociated, leaving, in many species, a broad exposed lesion in the uterus. Fast and reliable haemostasis at the wound is essential for the survival of the mother. Mammalian neonates rely on lactation for survival and maternal demise thus also leads to neonatal demise. We argue that the fact that mammals have a much more effective system for haemostasis than other vertebrates (the MK/P system) may have been a key exaptation for the evolutionary establishment of haemochorial placentation.
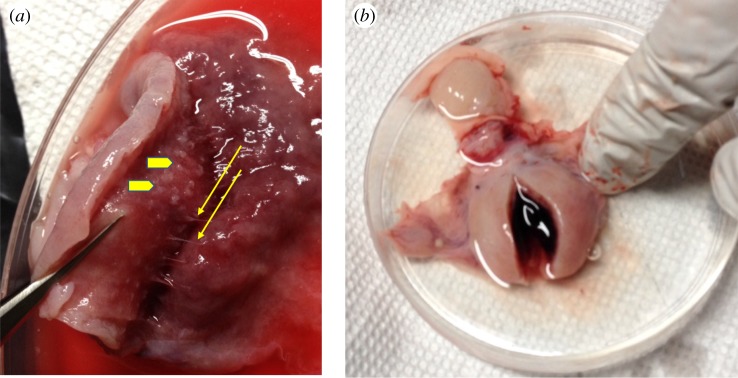
Eutherians vary greatly in how the haemochorial interface is organized, which may lead to different needs for haemostasis at parturition. One extreme example is that of the nine-banded armadillo, Dasypus novemcintus, whose placenta is technically haemochorial, in that the villi of the placenta are in direct contact with maternal blood [77,78]. However, this is achieved in a minimally invasive way. Single villi penetrate the endometrium and grow towards preformed maternal blood spaces and only expand and ramify once they have reached the varicosities (figure 2a). Hence, haemostasis during implantation and gestation is a minimal concern for armadillos, given that they have a well-contained space preformed into which placental extensions reach. Nevertheless, even the armadillo has to face the danger of a major haemorrhage at parturition (figure 2b). Another example is the massive postpartum bleeding in the African elephant, an animal with endotheliochorial placentation [79] and possibly also the dugong, also an afrotherian mammal [80], and the manatee [81]. Hence, we think the most important reason why haemochorial placenta is limited to eutherian mammals is that parturition of a haemochorial placenta leads to profuse bleeding in the uterus that needs to be arrested.
Figure 2.
The need for haemostasis in a minimally invasive haemochorial animal D-. novemcintus, which belongs to the eutherian clade most distantly related to humans. (a) The minimally invasive placenta of armadillo in third month gestation. The thin threads indicated by yellow arrows are the projections of the placenta entering the endometrium to the left. Arrow heads indicate penetration. The invasion through haemochorial is minimally destructive. (b) Postpartum uterus of armadillo, showing copious coagulated blood in the uterine cavity, indicating the need for effective haemostasis.
6. Conclusion
Deeply invasive haemochorial placentation is limited to the eutherian mammals. This is surprising given the large number of non-mammalian animals that have evolved viviparity and placentation. We argue that haemochorial placentation is limited to a clade of mammals, because mammals are the only vertebrate group that has evolved a highly effective and unique system of haemostasis: platelets. The effectiveness of haemostasis is essential at parturition where even minimally invasive placentae can haemorrhage.
All neonatal mammals, regardless of how developed they are at birth, rely on maternal lactation for their initial growth and survival after birth, and thus, the survival of the mother is critical. Consequently, the evolution of invasive placentation is most likely to succeed in a lineage that already has a highly effective system of haemostasis before the origin of deep placentation. From the standpoint of evolutionary theory, platelets are an exaptation, sensu Gould & Vrba [15], for the evolution of haemochorial placenta, i.e. a trait that has an important role but that evolved for another purpose prior to taking on this role. Platelets could be called a permissive exaptation as it may have permitted the evolution of a novel trait, haemochorial placentation, rather than acquiring a new function itself.
Platelet production from megakaryocytes is an important area for research in thrombosis. The ideas presented here may help stimulate new research into the powerful thrombotic forces associated with the evolution of the placenta but that also cause thrombosis of human arteries [41,82].
Data accessibility
This article has no additional data.
Authors' contributions
Both authors contributed equally.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Li JL, Zarbock A, Hidalgo A. 2017. Platelets as autonomous drones for hemostatic and immune surveillance. J. Exp. Med. 214, 2193–2204. ( 10.1084/jem.20170879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mossman HW. 1987. Vertebrate fetal membranes. New Brunswick, NJ: Rutgers University Press. [Google Scholar]
- 3.Wildman DE, Chen C, Erez O, Grossman LI, Goodman M, Romero R. 2006. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc. Natl Acad. Sci. USA 103, 3203–3208. ( 10.1073/pnas.0511344103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mess A, Carter AM. 2006. Evolutionary transformation of fetal membrane characters in Eutheria with special reference to Afrotheria. J. Exp. Zool. Part B (Mol. Dev. Evol.) 306B, 140–163. ( 10.1002/jez.b.21079) [DOI] [PubMed] [Google Scholar]
- 5.Elliot MG, Crespi BJ. 2009. Phylogenetic evidence for early hemochorial placentation in eutheria. Placenta 30, 949–967. ( 10.1016/j.placenta.2009.08.004) [DOI] [PubMed] [Google Scholar]
- 6.Wagner GP. 2018. Comparative placentation—mammals. In Encyclopedia of reproduction, vol. 2 (ed. Skinner MK.), pp. 455–461. New York, NY: Academic Press. [Google Scholar]
- 7.Blackburn DG. 2015. Evolution of vertebrate viviparity and specializations for fetal nutrition: a quantitative and qualitative analysis. J. Morphol. 276, 961–990. ( 10.1002/jmor.20272) [DOI] [PubMed] [Google Scholar]
- 8.Blackburn DG, Flemming AF. 2009. Morphology, development, and evolution of fetal membranes and placentation in squamate reptiles. J. Exp. Zool. B Mol. Dev. Evol. 312, 579–589. ( 10.1002/jez.b.21234) [DOI] [PubMed] [Google Scholar]
- 9.Blackburn DG. 2015. Evolution of viviparity in squamate reptiles: reversibility reconsidered. J. Exp. Zool. Part B Mol. Dev. Evol. 324, 473–486. ( 10.1002/jez.b.22625) [DOI] [PubMed] [Google Scholar]
- 10.Blackburn DG. 1999. Are viviparity and egg-guarding evolutionarily labile in squamates? Herpetologica 55, 556–573. [Google Scholar]
- 11.Anderson DT, Manton SM. 1972. Studies on Onychophora. 8. Relationship between embryos and oviduct in viviparous placental onychophorans Epiperipatus trinidadensis Bouvier and Macroperipatus torquatus (Kennel) from Trinidad . Phil. Trans. R. Soc. Lond. B 264, 161–189. ( 10.1098/rstb.1972.0011) [DOI] [Google Scholar]
- 12.Blackburn DG. 2006. Squamate reptiles as model organisms for the evolution of vivipary. Herpetol. Monogr. 20, 131–146. ( 10.1655/0733-1347(2007)20[131:SRAMOF]2.0.CO;2) [DOI] [Google Scholar]
- 13.Ostrovsky AN, Lidgard S, Gordon DP, Schwaha T, Genikhovich G, Ereskovsky AV. 2016. Matrotrophy and placentation in invertebrates: a new paradigm. Biol. Rev. 91, 673–711. ( 10.1111/brv.12189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinkle DW, Gibbons HW. 1977. The distribution and evolution of viviparity in reptiles, vol. 154 Museum of Zoology, Ann Arbor, MI: University of Michigan. [Google Scholar]
- 15.Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology 8, 4–15. ( 10.1017/S0094837300004310) [DOI] [Google Scholar]
- 16.Griffiths M. 1978. The biology and monotremes. New York, NY: Academic Press. [Google Scholar]
- 17.Renfree M, Shaw G. 2002. In Encyclopedia of life sciences, pp. 1–5. New York, NY: Wiley and Sons. [Google Scholar]
- 18.Hughes RL, Carrick FN. 1978. Reproduction in female monotremes. Aust. Zool. 20, 233–253. [Google Scholar]
- 19.Freyer C, Zeller U, Renfree MB. 2003. The marsupial placenta: a phylogenetic analysis. J. Exp. Zool. Part A 299A, 59–77. ( 10.1002/Jez.A.10291) [DOI] [PubMed] [Google Scholar]
- 20.Tyndale-Biscoe CH, Renfree MB. 1987. Reproductive physiology of marsupials. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 21.Freyer C, Zeller U, Renfree MB. 2007. Placental function in two distantly related marsupials. Placenta 28, 249–257. ( 10.1016/j.placenta.2006.03.007) [DOI] [PubMed] [Google Scholar]
- 22.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, Wagner GP. 2017. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc. Natl Acad. Sci. USA 114, E6566–E6575. ( 10.1073/pnas.1701129114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen VL, Faber LS, Salehpoor AA, Miller RD. 2017. A pronounced uterine pro-inflammatory response at parturition is an ancient feature in mammals. Proc. R. Soc. B 284, 20171694 ( 10.1098/rspb.2017.1694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mor G, Cardenas I, Abrahams V, Guller S. 2011. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann. NY Acad. Sci. 1221, 80–87. ( 10.1111/j.1749-6632.2010.05938.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavan AR, Griffith OW, Wagner GP. 2017. The inflammation paradox in the evolution of mammalian pregnancy: turning a foe into a friend. Curr. Opin. Genet. Dev. 47, 24–32. ( 10.1016/j.gde.2017.08.004) [DOI] [PubMed] [Google Scholar]
- 26.Stalker TJ, Newman DK, Ma P, Wannemacher KM, Brass LF. 2012. Platelet signaling. Handb. Exp. Pharmacol. 210, 59–85. ( 10.1007/978-3-642-29423-5_3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis JH, Phillips LL, Hann C. 1968. Coagulation and hematological studies in primitive Australian mammals. Comp. Biochem. Physiol. 25, 1129–1135. ( 10.1016/0010-406X(68)90601-4) [DOI] [PubMed] [Google Scholar]
- 28.Claver JA, Quaglia AIE. 2009. Comparative morphology, development, and function of blood cells in nonmammalian vertebrates. J. Exot. Pet. Med. 18, 87–97. ( 10.1053/j.jepm.2009.04.006) [DOI] [Google Scholar]
- 29.Schmaier AA, et al. 2011. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood 118, 3661–3669. ( 10.1182/blood-2011-02-338244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagasawa T Nakayasu C, Rieger AM, Barreda DR, Somamoto T, Nakao M. 2014. Phagocytosis by thrombocytes is a conserved innate immune mechanism in lower vertebrates. Front. Immunol. 5, 445 ( 10.3389/fimmu.2014.00445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theopold U, Schmidt O, Soderhall K, Dushay MS. 2004. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25, 289–294. ( 10.1016/j.it.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 32.Radomski MW, Martin JF, Moncada S. 1991. Synthesis of nitric oxide by the haemocytes of the American horseshoe crab (Limulus polyphemus). Phil. Trans. R. Soc. Lond. B 334, 129–133. ( 10.1098/rstb.1991.0102) [DOI] [Google Scholar]
- 33.Ouzegdouh Y, Capron C, Bauer T, Puymirat E, Diehl JL, Martin JF, Cramer-Bordé E. 2018. The physical and cellular conditions of the human pulmonary circulation enable thrombopoiesis. Exp. Hematol. 63, 22–27.e23. ( 10.1016/j.exphem.2018.04.001) [DOI] [PubMed] [Google Scholar]
- 34.Lefrançais E, et al. 2017. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109. ( 10.1038/nature21706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen NT. 1978. Occurrence of megakaryocytes in various vessels and their retention in the pulmonary capillaries in man. Scand. J. Haematol. 21, 369–375. ( 10.1111/j.1600-0609.1978.tb00381.x) [DOI] [PubMed] [Google Scholar]
- 36.Trowbridge EA, Martin JF, Slater DN. 1982. Evidence for a theory of physical fragmentation of megakaryocytes, implying that all platelets are produced in the pulmonary circulation. Thromb. Res. 28, 461–475. ( 10.1016/0049-3848(82)90163-3) [DOI] [PubMed] [Google Scholar]
- 37.Epstein B. 1948. Logarithmico-normal distribution in breakage of solids. Ind. Eng. Chem. 40, 2289–2291. ( 10.1021/ie50468a014) [DOI] [Google Scholar]
- 38.Mazzi S, Lordier L, Debili N, Raslova H, Vainchenker W. 2018. Megakaryocyte and polyploidization. Exp. Hematol. 57, 1–13. ( 10.1016/j.exphem.2017.10.001) [DOI] [PubMed] [Google Scholar]
- 39.Hancock V, Martin JF, Lelchuk R. 1993. The relationship between human megakaryocyte nuclear DNA content and gene expression. Br. J. Haematol. 85, 692–697. ( 10.1111/j.1365-2141.1993.tb03210.x) [DOI] [PubMed] [Google Scholar]
- 40.Podolak-Dawidziak M, Hancock V, Lelchuk R, Kotlarek-Haus S, Martin JF. 1995. The expression of mRNA for fibrinogen in megakaryocytes isolated from patients with T-cell lymphoma. Br. J. Haematol. 91, 362–366. ( 10.1111/j.1365-2141.1995.tb05304.x) [DOI] [PubMed] [Google Scholar]
- 41.Martin JF, Kristensen SD, Mathur A, Grove EL, Choudry FA. 2012. The causal role of megakaryocyte-platelet hyperactivity in acute coronary syndromes. Nat. Rev. Cardiol. 9, 658–670. ( 10.1038/nrcardio.2012.131) [DOI] [PubMed] [Google Scholar]
- 42.Martin JF, Trowbridge EA, Salmon G, Plumb J. 1983. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb. Res. 32, 443–460. ( 10.1016/0049-3848(83)90255-4) [DOI] [PubMed] [Google Scholar]
- 43.Martin JF, Daniel TD, Trowbridge EA. 1987. Acute and chronic changes in platelet volume and count after cardiopulmonary bypass induced thrombocytopenia in man. Thromb. Haemost. 57, 55–58. ( 10.1055/s-0038-1651061) [DOI] [PubMed] [Google Scholar]
- 44.Bessman JD. 1984. The relation of megakaryocyte ploidy to platelet volume. Am. J. Hematol. 16, 161–170. ( 10.1002/ajh.2830160208) [DOI] [PubMed] [Google Scholar]
- 45.Corash L, Levin J. 1990. The relationship between megakaryocyte ploidy and platelet volume in normal and thrombocytopenic C3H mice. Exp. Hematol. 18, 985–989. [PubMed] [Google Scholar]
- 46.Corash L, Chen HY, Levin J, Baker G, Lu H, Mok Y. 1987. Regulation of thrombopoiesis: effects of the degree of thrombocytopenia on megakaryocyte ploidy and platelet volume. Blood 70, 177–185. [PubMed] [Google Scholar]
- 47.Trowbridge EA, Martin JF. 1984. An analysis of the platelet and polyploid megakaryocyte response to acute thrombocytopenia and its biological implications. Clin. Phys. Physiol. Meas. 5, 263–277. ( 10.1088/0143-0815/5/4/008) [DOI] [PubMed] [Google Scholar]
- 48.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. 1982. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br. J. Haematol. 50, 509–519. ( 10.1111/j.1365-2141.1982.tb01947.x) [DOI] [PubMed] [Google Scholar]
- 49.Kristensen S, Bath P, Martin JF. 1988. The bleeding time is inversely related to megakaryocyte nuclear DNA content and size in man. Thromb. Haemost. 59, 357–359. ( 10.1055/s-0038-1647495) [DOI] [PubMed] [Google Scholar]
- 50.O'Neill C. 1985. Thrombocytopenia is an initial maternal response to fertilization in mice. J. Reprod. Fertil. 73, 559–566. ( 10.1530/jrf.0.0730559) [DOI] [PubMed] [Google Scholar]
- 51.O'Neill C, Gidley-Baird AA, Pike IL, Porter RN, Sinosich MJ, Saunders DM. 1985. Maternal blood platelet physiology and luteal-phase endocrinology as a means of monitoring pre- and postimplantation embryo viability following in vitro fertilization. J. In Vitro Fert. Embryo Transf. 2, 87–93. ( 10.1007/BF01139339) [DOI] [PubMed] [Google Scholar]
- 52.Roberts TK, Adamson LM, Smart YC, Stanger JD, Murdoch RN. 1987. An evaluation of peripheral blood platelet enumeration as a monitor of fertilization and early pregnancy. Fertil. Steril. 47, 848–854. ( 10.1016/S0015-0282(16)59177-8) [DOI] [PubMed] [Google Scholar]
- 53.O'Neill C. 1985. Partial characterization of the embryo-derived platelet-activating factor in mice. J. Reprod. Fertil. 75, 375–380. ( 10.1530/jrf.0.0750375) [DOI] [PubMed] [Google Scholar]
- 54.Podsiadly BT, Adamson LM, Stanger JD, Smart YC, Roberts TK. 1992. Prefertilization treatment of mice with platelet activating factor affects pregnancy. Mol. Reprod. Dev. 32, 363–368. ( 10.1002/mrd.1080320409) [DOI] [PubMed] [Google Scholar]
- 55.Cote F, Fligny C, Bayard E, Launay JM, Gershon MD, Mallet J, Vodjdani G. 2007. Maternal serotonin is crucial for murine embryonic development. Proc. Natl Acad. Sci. USA 104, 329–334. ( 10.1073/pnas.0606722104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaspar P, Cases O, Maroteaux L. 2003. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci. 4, 1002–1012. ( 10.1038/nrn1256) [DOI] [PubMed] [Google Scholar]
- 57.Kliman HJ, Quaratella SB, Setaro AC, Siegman EC, Subha ZT, Tal R, Milano KM, Steck TL. 2018. Pathway of maternal serotonin to the human embryo and fetus. Endocrinology 159, 1609–1629. ( 10.1210/en.2017-03025) [DOI] [PubMed] [Google Scholar]
- 58.Ori M, De Lucchini S, Marras G, Nardi I. 2013. Unraveling new roles for serotonin receptor 2B in development: key findings from Xenopus. Int. J. Dev. Biol. 57, 707–714. ( 10.1387/ijdb.130204mo) [DOI] [PubMed] [Google Scholar]
- 59.Buznikov GA. 2007. Preneural transmitters as regulators of embryogenesis. Current state of problem. Ontogenez 38, 262–270. ( 10.1134/S1062360407040042) [DOI] [PubMed] [Google Scholar]
- 60.Frank HG, Kaufmann P. 2000. Nonvillous parts and trophoblast invasion. In Pathology of the human placenta (eds Berniscke K, Kaufmann P), pp. 171–172. Berlin, Germany: Springer. [Google Scholar]
- 61.Sato Y, Higuchi T, Yoshioka S, Tatsumi K, Fujiwara H, Fujii S. 2003. Trophoblasts acquire a chemokine receptor, CCR1, as they differentiate towards invasive phenotype. Development 130, 5519–5532. ( 10.1242/dev.00729) [DOI] [PubMed] [Google Scholar]
- 62.Boehlen F, Clemetson KJ. 2001. Platelet chemokines and their receptors: what is their relevance to platelet storage and transfusion practice? Transfus. Med. 11, 403–417. ( 10.1046/j.1365-3148.2001.00340.x) [DOI] [PubMed] [Google Scholar]
- 63.Lash GE, Warren AY, Underwood S, Baker PN. 2003. Vascular endothelial growth factor is a chemoattractant for trophoblast cells. Placenta 24, 549–556. ( 10.1053/plac.2002.0923) [DOI] [PubMed] [Google Scholar]
- 64.Bass KE, Morrish D, Roth I, Bhardwaj D, Taylor R, Zhou Y, Fisher SJ. 1994. Human cytotrophoblast invasion is up-regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev. Biol. 164, 550–561. ( 10.1006/dbio.1994.1223) [DOI] [PubMed] [Google Scholar]
- 65.Sato Y, Fujiwara H, Zeng BX, Higuchi T, Yoshioka S, Fujii S. 2005. Platelet-derived soluble factors induce human extravillous trophoblast migration and differentiation: platelets are a possible regulator of trophoblast infiltration into maternal spiral arteries. Blood 106, 428–435. ( 10.1182/blood-2005-02-0491) [DOI] [PubMed] [Google Scholar]
- 66.Hellgren M. 2003. Hemostasis during normal pregnancy and puerperium. Semin. Thromb. Hemost. 29, 125–130. ( 10.1055/s-2003-38897) [DOI] [PubMed] [Google Scholar]
- 67.Peitsidis P, Datta T, Pafilis I, Otomewo O, Tuddenham EG, Kadir RA. 2010. Bernard Soulier syndrome in pregnancy: a systematic review. Haemophilia 16, 584–591. ( 10.1111/j.1365-2516.2009.02137.x) [DOI] [PubMed] [Google Scholar]
- 68.Civaschi E, et al. 2015. Analysis of 65 pregnancies in 34 women with five different forms of inherited platelet function disorders. Br. J. Haematol. 170, 559–563. ( 10.1111/bjh.13458) [DOI] [PubMed] [Google Scholar]
- 69.Sato Y, Fujiwara H, Konishi I. 2010. Role of platelets in placentation. Med. Mol. Morphol. 43, 129–133. ( 10.1007/s00795-010-0508-1) [DOI] [PubMed] [Google Scholar]
- 70.Harder JD, Stonerook MJ, Pondy J. 1993. Gestation and placentation in two New World opossums: Didelphis virginiana and Monodelphis domestica. J. Exp. Zool. 266, 463–479. ( 10.1002/jez.1402660511) [DOI] [PubMed] [Google Scholar]
- 71.Zeller U, Freyer C. 2001. Early ontogeny and placentation of the grey short-tailed opossum, Monodelphis domestica (Didelphidae : Marsupialia): contribution to the reconstruction of the marsupial morphotype. J. Zool. Syst. Evol. Res. 39, 137–158. ( 10.1046/j.1439-0469.2001.00167.x) [DOI] [Google Scholar]
- 72.Enders AC, Enders RK. 1969. The placenta of the four-eye opossum (Philander opossum). Anat. Rec. 165, 431–449. ( 10.1002/ar.1091650311) [DOI] [PubMed] [Google Scholar]
- 73.Padykula HA, Taylor JM. 1976. Ultrastructural evidence for loss of the trophoblastic layer in the chorioallantoic placenta of Australian bandicoots (Marsupialia: Peramelidae). Anat. Rec. 186, 357–385. ( 10.1002/ar.1091860303) [DOI] [PubMed] [Google Scholar]
- 74.Stewart JR, Blackburn DG. 2015. Viviparity and placentation in lizards. In Reproductive biology and phylogeny of lizards and tuatara (eds Rheubert JL, Siegel DS, Trauth SE), pp. 448–563. Boca Raton, FL: CRC Press. [Google Scholar]
- 75.Griffith OW, Van Dyke JU, Thompson MB. 2013. No implantation in an extra-uterine pregnancy of a placentotrophic reptile. Placenta 34, 510–511. ( 10.1016/j.placenta.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 76.Blackburn DG, Flemming AF. 2012. Invasive implantation and intimate placental associations in a placentotrophic African lizard, Trachylepis ivensi (scincidae). J. Morphol. 273, 137–159. ( 10.1002/jmor.11011) [DOI] [PubMed] [Google Scholar]
- 77.Enders AC. 1960. Development and structure of the villous haemochorial placenta of the nine-banded armadillo (Dasypus novemcinctus). J. Anat. 94, 34–45. [PMC free article] [PubMed] [Google Scholar]
- 78.Chavan AR, Wagner GP. 2016. The fetal–maternal interface of the nine-banded armadillo: endothelial cells of maternal sinus are partially replaced by trophoblast. Zool. Lett. 2, 11 ( 10.1186/s40851-016-0048-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Allen WR, Mathias S, Wooding FBP, van Aarde RJ. 2003. Placentation in the African elephant (Loxodonta africana): II—Morphological changes in the uterus and placenta throughout gestation. Placenta 24, 598–617. ( 10.1016/S0143-4004(03)00102-4) [DOI] [PubMed] [Google Scholar]
- 80.Marsh H, Heinsohn GE, Channells PW. 1984. Changes in the ovaries and uterus of the Dugong, Dugong-Dugon (Sirenia, Dugongidae), with age and reproductive activity. Aust. J. Zool. 32, 743–766. ( 10.1071/Zo9840743) [DOI] [Google Scholar]
- 81.Marmontel M. 1995. Age determination and population biology of the Florida Manatee, Trichechus manatus latirostris. PhD thesis, University of Florida. [Google Scholar]
- 82.Rocca B, Patrono C. 2015. Platelet progenitors: the hidden drug target. Eur. Heart J. 36, 3211–3213. ( 10.1093/eurheartj/ehv366) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.