Abstract
Neonicotinoid pesticides can impair bees' ability to learn and remember information about flowers, critical for effective foraging. Although these effects on cognition may contribute to broader effects on health and performance, to date they have largely been assayed in simplified protocols that consider learning in a single sensory modality, usually olfaction. Given that real flowers display a variety of potentially useful signals, we assessed the effects of acute neonicotinoid exposure on multimodal learning in free-flying bumblebees. We found that neonicotinoid consumption differentially impacted learning of floral stimuli, impairing scent, but not colour, learning. These findings raise questions about the mechanisms by which pesticides might differentially impair sensory systems, with implications for how neonicotinoids affect multiple aspects of bee ecology.
Keywords: bumblebee, Bombus impatiens, imidacloprid, cognition
1. Introduction
Honeybees (Apis) and bumblebees (Bombus) are ecologically and agriculturally critical pollinators that also serve as important model systems for the study of cognition [1]. As generalist foragers, individuals visit a variety of flower types and rapidly learn associations between floral cues and rewards such as nectar [2–5] and pollen [6–8]. This ability to learn associations is an important aspect of foraging performance, potentially linked to fitness ([9], but see [10]). Stressors such as pathogens, poor nutrition and pesticide exposure can have negative effects on bee learning [11–15], and thus have been highlighted as being potentially detrimental to foraging efficiency and colony growth [16].
Of these stressors, neonicotinoid pesticides have been an area of recent focus for their impacts on bee cognition. These pesticides are widely used (e.g. in the USA and China [17,18]), where crops can be seed- or spray-treated. As systemic pesticides, neonicotinoids are expressed in plants' nectar and pollen, where foraging bees can ingest them [19]. While many studies have found detrimental effects of these pesticides on learning (e.g. [16,20], reviewed in [14]), others have found impairment to other components of foraging behaviour such as motivation, but not learning per se [21,22].
Nearly all of the previous work on how neonicotinoids impact cognition addresses learning in a single sensory modality (usually olfaction: see Fig. 1 in [21]). However, these single-modality tests are not representative of natural foraging scenarios, since bees use multiple sensory modalities to locate and learn about flowers [23]. For example, components of multimodal floral displays can have interactive effects on bee learning [24], and floral colour can compensate in scenarios where scent is less useful [25]. The single-modality focus of pesticide research may explain discrepancies in recent reports of neonicotinoids’ impact on bee foraging [21]. Specifically, where studies have found neonicotinoid-induced learning impairment, they have generally addressed olfactory learning (e.g. [16,20,26–28]), while studies on visual learning have found either no effects [21,22], or effects consistent with other explanations [29]. However, these discrepancies could also be explained by differences in training protocol: olfactory learning is usually studied using harnessed bees in the proboscis extension response (PER) protocol [30,31], whereas visual learning is more commonly studied using free-flying bees. Thus, addressing whether impairment from neonicotinoids might be modality-specific requires learning be measured under the same conditions.
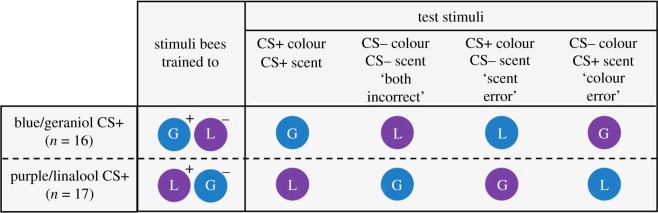
To explore whether bees’ olfactory and visual learning might be differentially impaired by a neonicotinoid, we assessed the effects of imidacloprid on multimodal learning in free-flying bumblebees, Bombus impatiens. We trained individuals to discriminate between flowers that differed in scent and colour cues before measuring performance in a test phase where flowers offered all possible combinations of the trained scents and colours, including flowers where these cues were put into conflict (figure 1). We expected that if scent and colour learning were differentially impaired by imidacloprid exposure, exposed bees would make more of one type of error (selecting the floral option with the incorrect scent or colour) compared with the control bees (figure 2b). Alternatively, if scent and colour learning were impaired equally, we expected that errors of each type would be equally common in control and imidacloprid-treated bees; this could also indicate an impairment to the ability to combine scent and colour stimuli (i.e. multisensory integration [23]).
Figure 1.
The training and test conditioned stimuli (CS) (circle colour indicates flower colour; G = geraniol-scented, L = linalool-scented; + = rewarding, − = unrewarding). Sample size indicates the bees that completed both training trials and performed in the test trial.
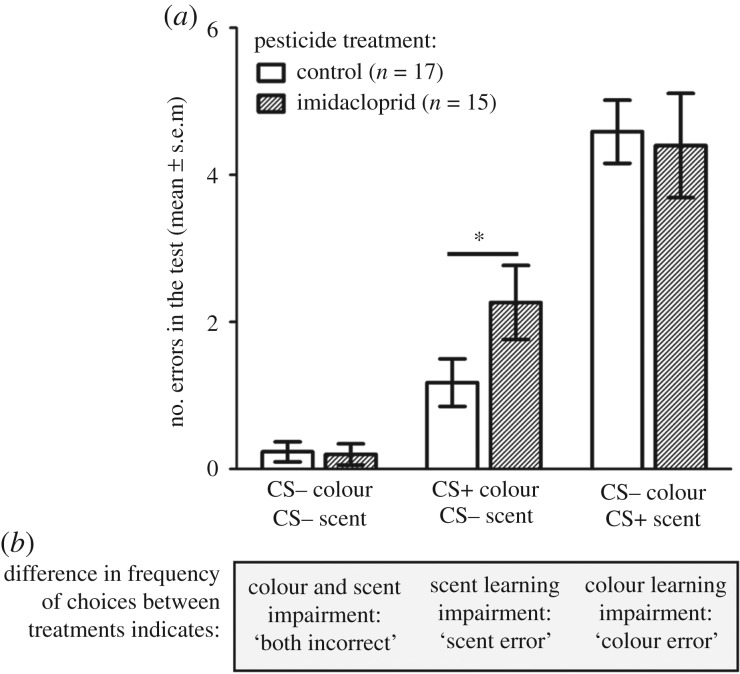
Figure 2.
(a) Number of visits (of the total of 20 flower visits) to the three incorrect flower types by treatment; (b) what differences between the pesticide versus control treatments would indicate; terminology used for the error type as it is referred to in the main text. Asterisk indicates significance at a level of p < 0.05).
2. Methods
(a). Subjects
We tested 60 Bombus impatiens individuals from three colonies (Koppert Biological Systems, USA; electronic supplementary material, table S1). Colonies were sequentially connected to a foraging arena (L ×W × H: 122 × 59 × 59 cm), via a gated passageway and trained to forage (during a ‘shaping’ phase) on a horizontal array of artificial flowers. We maintained colonies on 30% (w/w) sucrose pipetted directly into honeypots after each testing day, and approximately 500 mg honeybee-collected pollen (Koppert Biological Systems, USA) added to each colony every 2–3 days. We paint-marked foragers; details on shaping, arena, and artificial flowers are in the electronic supplementary material.
(b). Pesticide treatment
Imidacloprid solutions were only ever fed to individual bees before trial 1. We caught foragers as they left the colony by allowing them to walk into a dark holding container (electronic supplementary material, figure S1a) and fed them either 0.45 ng (22.5 ppb) imidacloprid-containing or control solutions, in 20 µl of 30% (w/w) sucrose (details in electronic supplementary material). This dose is in line with field-exposed plants’ nectar, which can range from 1 to 50 ppb, with maximum documented concentrations of 912 ppb [32,33]; see also Table S2 in Siviter et al. [14] for average and maximum estimates of neonicotinoid concentrations in nectar ingested for a given bumblebee foraging bout. Our dose is also in line with previous experimental work on bee behaviour [14]. All foragers consumed the solution, and were held in the container for 1 h before trial 1 to maximize pesticide absorption, in line with previous behavioural studies [20,21,34].
(c). Training and testing
We trained individual bees over two training trials (inter-trial interval approximately 5 min), followed by a test 10 min later. For each trial, we gave a bee access to a 48-flower array containing two artificial floral types: purple scented with linalool and blue scented with geraniol (electronic supplementary material, figure S1b,d,e). Each flower type contained either 4 µl of 50% (w/w) sucrose (rewarding flowers) or 4 µl of water (unrewarding flowers). Stimuli–reward pairings were counterbalanced across colonies and pesticide treatment (electronic supplementary material, table S1). We cleaned flower tops with 70% ethanol between trials and changed flower locations between each training trial and bee.
At the start of trial 1, we transferred the bee to a single white flower, identical to the ones used during the shaping phase, containing 4 µl of 50% (w/w) sucrose solution, and stimulated the bee's antennae with 50% (w/w) sucrose to induce proboscis extension. All bees consumed this sucrose, which was offered in order to motivate bees to initiate foraging after having been held in the dark. Bees then free-foraged on the array; we refilled visited flowers after bees visited three to six subsequent flowers. We ended trials when a bee returned to the colony via the connecting tube or left the array for more than 2 min (we then manually returned it to the colony). Trial 2 was run identically to trial 1. If bees did not return for trial 2, we transferred them to the arena and in most cases the bee then resumed foraging (electronic supplementary material, table S1). For the test trial, we presented bees with a 48-flower array with four flower types, all offering 4 µl of water (electronic supplementary material, figure S1c), representing the four possible combinations of the two colours and scents (figure 1), and allowed bees to make 20 visits.
(d). Behaviour coding and data analyses
We filmed training and test trials from above using an HD Sony camcorder (30 fps). From the videos we recorded the type of flower visits bees made (Solomon Coder; https://solomoncoder.com): ‘rewarding’ (consumes sucrose), ‘unrewarding’ (probes water), ‘empty’ (probes previously emptied well) or ‘does not drink’ (lands on the flower but does not probe the well). We analysed bees' learning and test performance, as well as their motivation (tendency to forage in trial 1 and to return to forage in trial 2) and foraging performance (see electronic supplementary material). All analyses were carried out in R v.3.4.3. [35].
3. Results
(a). Training trial behaviour
Regardless of pesticide treatment, bees learned across the two trials, performing better in trial 2 than trial 1 (z = 9.444, p < 0.0001; electronic supplementary material, figure S2a). Imidacloprid-treated bees performed worse than control bees (z = 2.504, p = 0.0123), with this effect being driven by the treatment where bees were trained that blue/geraniol flowers were rewarding (pesticide treatment × stimulus treatment: z = −1.970, p = 0.0488; electronic supplementary material, figure S2b,c). On trial 1, bees performed better when they were trained to purple/linalool flowers (trial × stimulus treatment: z = −2.454, p = 0.0141, stimulus treatment: z = 9.444; p = 0.00421). In line with previous findings [21], imidacloprid also impaired bees’ motivation to forage, and their ability to collect sucrose from flowers (electronic supplementary material).
(b). Test trial performance
Imidacloprid-treated bees made errors indicating impairment to olfactory, but not visual, learning. Specifically, these bees made more scent errors than control bees (error type × pesticide treatment: z = 2.302, p = 0.0213; pesticide treatment: z = −2.280, p = 0.023; figure 2a; electronic supplementary material, figure S3a), regardless of the stimulus combination they were trained to (Tukey post hoc tests: imidacloprid-treated bees made more scent errors both for blue/geraniol-trained bees: z = −5.659, p < 0.0001, and for purple/linalool-trained bees: z = −2.983, p = 0.0029). While both imidacloprid-treated and control bees made more colour than scent errors overall (error type: z = 3.847, p = 0.00012; figure 2a), they did not differ in the number of colour errors they made (Tukey post hoc comparison: z = −0.672, p = 0.502; figure 2a; electronic supplementary material, figure S3a). Overall, imidacloprid-treated bees did not differ from controls in the number of correct flower choices they made in the test (pesticide treatment: z = 1.063, p = 0.288; stimulus treatment: z = 0.573, p = 0.567). Regardless of pesticide treatment, bees that were trained to the purple/linalool stimulus made more scent errors than bees trained to blue/geraniol (error type × stimulus treatment: z = −2.650, p = 0.00804; electronic supplementary material, figure S3b). Although we cannot be certain because colour and scents were always paired in the current study, this result may be because purple was preferred over blue (supported by a previous finding with colour only [21] and consistent with our finding that 81% of bees went to purple/linalool flowers first on trial 1). Therefore, bees were more likely to visit purple test flowers even when they contained the incorrect scent.
4. Discussion
A growing body of research demonstrates that neonicotinoid pesticides have negative effects on bees [36], leading to recent restrictions in the EU [37]. In addition to impaired colony reproduction [19], neonicotinoids are associated with a number of sub-lethal behavioural effects [19], including impacts on bees’ ability to learn floral stimuli [14]. A missing piece of this emerging picture is the extent to which these impairments, documented nearly exclusively in relation to olfactory learning, transfer when bees encounter more sensorially realistic flowers and naturalistic foraging scenarios. We offered free-flying bumblebees flowers that varied in scent and colour, and found that olfactory learning, but not visual learning was impaired by acute exposure to the neonicotinoid imidacloprid: exposed bees made more errors visiting flowers of the incorrect scent than controls, for both combinations of stimuli that individuals were trained to. These results explain at least some of the discrepancies in previous findings, and raise further questions about the mechanism behind olfactory learning impairment, which could have implications for how these pesticides affect multiple aspects of bees' ecology.
How might neonicotinoids differentially impair olfactory learning? Neonicotinoids affect the insect brain by acting as agonists of nicotinic acetylcholine receptors (nAChRs), with different neonicotinoids likely differentially activating specific receptor subtypes [38–40]. It is possible that nAChR subtype expression differs in the olfactory and visual systems, leading to the differential effects of imidacloprid exposure observed here. In the honeybee, imidacloprid can impair odour coding in antennal lobes, the first odour processing centre of the bee brain [41], and may even impair detection of volatiles upstream in antennal sensilla through action on odorant-binding proteins [42]. To our knowledge, the effects of imidacloprid on the bee visual system at an analogous peripheral or central level have not been determined (but see [43]).
Beyond their effects on single sensory modalities, neonicotionids also impair other aspects of cognition, e.g. spatial working memory [34] and navigation [44]. These effects may be driven by neonicotinoids activating nAChRs in the Kenyon cells of the mushroom bodies [45,46], structures associated with cognition [47,48]. Given that these structures are also involved in multisensory integration [23], we might expect this also to be impaired by neonicotinoid exposure. While we did not find such evidence in the current study, this could be a useful avenue for future research.
Another clear result was that neonicotinoid-exposed bees were less motivated to forage (as in [21,49,50]). A lack of foraging motivation may explain some of the learning impairments found in previous studies: most have addressed pesticide effects on learning using the PER protocol [30,31] (Fig. 1 in [21]). While this protocol has proved extremely useful for studying learning in bees [51], because bees are immobilized, identifying specific effects on foraging behaviour can be difficult. For example, effects on motivation may be confounded with effects on learning. In the current study, all bees exhibited PER for sucrose when stimulated at the start of trial 1, but many were not motivated to subsequently forage. If this had been assessed in a PER protocol these bees may have been included as being ‘motivated’ to exhibit PER (when stimulated) but may not have been sufficiently motivated to exhibit PER for a conditioned stimulus alone. Since this is the measure of learning in PER assays, motivational deficits could be misinterpreted as learning deficits.
What consequences might the impairment to olfactory learning we found here have for bee performance more generally? Crucially, olfactory impairment was not always compensated for by the availability of visual cues: when blue/geraniol flowers were rewarding, imidacloprid-treated bees performed worse than control bees during the learning trials. That this effect was driven by the olfactory rather than the visual cue is supported both by the test results and by previous work where colour learning was unaffected by imidacloprid across a range of doses [21]. The lack of an effect during training for purple/linalool-trained bees is likely because this was the preferred flower type, and thus it might be more difficult to impair learning in relation to this stimulus. If not only learning, but also olfactory perception more generally is impaired [41,42], then neonicotinoids may have more serious holistic effects on behaviour than previously recognized. Bees rely upon chemoreception for a variety of foraging and non-foraging tasks [52], including pollen detection [53], nest recognition [54] and pheromonal communication within the colony [55], and thus any or all of these behaviours could also be disrupted.
While we did not find impairment to visual associative learning here, it is possible that it could be impaired under different scenarios, e.g. involving different neonicotinoids, chronic rather than acute exposure or when flowers offer different scents or colours. In the current study, the colour stimuli we chose were relatively difficult for bees to learn to discriminate compared with the scent stimuli, so if our effects simply reflected differences in stimulus discriminability, we would predict the opposite result (i.e. greater impairment to colour learning). However, a clear next step would be to test our finding over a broader range of stimuli. Given that generalist bees rely on a broad range of cognitive abilities [56], moving towards assays that measure cognition in more naturalistic settings is critical to fully understand these pesticides’ effects on bees.
Supplementary Material
Acknowledgements
We thank Sage Ellis for assistance with experiments. Thanks also to the Leonard Lab and Dennis Mathew for helpful feedback on earlier versions of the manuscript.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.87p3dd7 [57].
Authors' contributions
All authors contributed to the design of the experiment. F.M. collected the data, carried out the statistical analyses and wrote the initial version of the manuscript. All authors agree to be held accountable for the content and approve the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
Thanks to L'Oreal for Women in Science and USDA NIFA postdoctoral fellowships (F.M.) and USDA NIFA 2018-67014-27543 (A.S.L.).
References
- 1.Chittka L, Thomson JD. 2001. Cognitive ecology of pollination. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Chittka L, Thomson JD, Waser NM. 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377. ( 10.1007/s001140050636) [DOI] [Google Scholar]
- 3.Clarke D, Whitney H, Sutton G, Robert D. 2013. Detection and learning of floral electric fields by bumblebees. Science 340, 66–69. ( 10.1126/science.1230883) [DOI] [PubMed] [Google Scholar]
- 4.Dyer AG, Whitney HM, Arnold SEJ, Glover BJ, Chittka L. 2006. Bees associate warmth with floral colour. Nature 442, 525 ( 10.1038/442525a) [DOI] [PubMed] [Google Scholar]
- 5.Giurfa M. 2007. Behavioral and neural analysis of associative learning in the honeybee: a taste from the magic well. J. Comp. Physiol. A 193, 801–824. ( 10.1007/s00359-007-0235-9) [DOI] [PubMed] [Google Scholar]
- 6.Muth F, Papaj DR, Leonard AS. 2015. Colour learning when foraging for nectar and pollen: bees learn two colours at once. Biol. Lett. 11, 20150628 ( 10.1098/rsbl.2015.0628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muth F, Papaj DR, Leonard AS. 2017. Multiple rewards have asymmetric effects on learning in bumblebees. Anim. Behav. 126, 123–133. ( 10.1016/j.anbehav.2017.01.010) [DOI] [Google Scholar]
- 8.Muth F, Papaj DR, Leonard AS. 2016. Bees remember flowers for more than one reason: pollen mediates associative learning. Anim. Behav. 111, 93–100. ( 10.1016/j.anbehav.2015.09.029) [DOI] [Google Scholar]
- 9.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans LJ, Smith KE, Raine NE. 2017. Fast learning in free-foraging bumble bees is negatively correlated with lifetime resource collection. Sci. Rep. 7, 496 ( 10.1038/s41598-017-00389-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alghamdi A, Dalton L, Phillis A, Rosato E, Mallon E. 2008. Immune response impairs learning in free-flying bumble-bees. Biol. Lett. 4, 479–481. ( 10.1098/rsbl.2008.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gegear RJ, Otterstatter MC, Thomson JD. 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B 273, 1073–1078. ( 10.1098/rspb.2005.3423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iqbal J, Mueller U. 2007. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B. 274, 1517–1521. ( 10.1098/rspb.2007.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siviter H, Koricheva J, Brown MJF, Leadbeater E. 2018. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 55, 2812–2821. ( 10.1111/1365-2664.13193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arien Y, Dag A, Zarchin S, Masci T, Shafir S. 2015. Omega-3 deficiency impairs honey bee learning. Proc. Natl Acad. Sci. USA 112, 15 761–15 766. ( 10.1073/pnas.1517375112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley DA, Smith KE, Raine NE. 2015. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 5, 16508 ( 10.1038/srep16508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas MR, Tooker JF. 2015. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 49, 5088–5097. ( 10.1021/es506141g) [DOI] [PubMed] [Google Scholar]
- 18.Shao X, Liu Z, Xu X, Li Z, Qian X. 2013. Overall status of neonicotinoid insecticides in China: production, application and innovation. J. Pestic. Sci. 38, 1–9. ( 10.1584/jpestics.D12-037) [DOI] [Google Scholar]
- 19.Blacquière T, Smagghe G, van Gestel CAM, Mommaerts V. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21, 973–992. ( 10.1007/s10646-012-0863-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan K, Chen W, Dong S, Liu X, Wang Y, Nieh JC. 2015. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 5, 10989 ( 10.1038/srep10989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muth F, Leonard AS. 2019. A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci. Rep. 9, 1–13. ( 10.1038/s41598-019-39701-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lämsä J, Kuusela E, Tuomi J, Juntunen S, Watts PC. 2018. Low dose of neonicotinoid insecticide reduces foraging motivation of bumblebees. Proc. R. Soc. B 285, 20180506 ( 10.1098/rspb.2018.0506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard AS, Masek P. 2014. Multisensory integration of colors and scents: insights from bees and flowers. J. Comp. Physiol. A 200, 463–474. ( 10.1007/s00359-014-0904-4) [DOI] [PubMed] [Google Scholar]
- 24.Leonard AS, Dornhaus A, Papaj DR. 2011. Flowers help bees cope with uncertainty: signal detection and the function of floral complexity. J. Exp. Biol. 214, 113–121. ( 10.1242/jeb.047407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson DA, Whitney HM, Rands SA. 2017. Colour as a backup for scent in the presence of olfactory noise: testing the efficacy backup hypothesis using bumblebees (Bombus terrestris). R. Soc. Open Sci. 4, 170996 ( 10.1098/rsos.170996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decourtye A, Lacassie E, Pham-Delègue M-H. 2003. Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag. Sci. 59, 269–278. ( 10.1002/ps.631) [DOI] [PubMed] [Google Scholar]
- 27.Decourtye A, Devillers J, Cluzeau S, Charreton M, Pham-Delègue M-H. 2004. Effects of imidacloprid and deltamethrin on associative learning in honeybees under semi-field and laboratory conditions. Ecotoxicol. Environ. Safety 57, 410–419. ( 10.1016/j.ecoenv.2003.08.001) [DOI] [PubMed] [Google Scholar]
- 28.Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S, Gauthier M, Pham-Delègue M-H. 2004. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 78, 83–92. ( 10.1016/j.pestbp.2003.10.001) [DOI] [Google Scholar]
- 29.Phelps JD, Strang CG, Gbylik-Sikorska M, Sniegocki T, Posyniak A, Sherry DF. 2018. Imidacloprid slows the development of preference for rewarding food sources in bumblebees (Bombus impatiens). Ecotoxicology 27, 175–187. ( 10.1007/s10646-017-1883-3) [DOI] [PubMed] [Google Scholar]
- 30.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 31.Takeda K. 1961. Classical conditioned response in the honey bee. J. Insect Physiol. 6, 168–179. ( 10.1016/0022-1910(61)90060-9) [DOI] [Google Scholar]
- 32.Goulson D. 2013. Review: an overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 33.Bonmatin J-M, et al. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuelson EEW, Chen-Wishart ZP, Gill RJ, Leadbeater E. 2016. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6, 38957 ( 10.1038/srep38957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Team RC. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 36.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 37.European Commission. 2018 EU Press Release 27/4/18. See http://europa.eu/rapid/press-release_MEX-18-3583_en.htm.
- 38.Tomizawa M, Casida JE. 2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45, 247–268. ( 10.1146/annurev.pharmtox.45.120403.095930) [DOI] [PubMed] [Google Scholar]
- 39.Tomizawa M, Maltby D, Talley TT, Durkin KA, Medzihradszky KF, Burlingame AL, Taylor P, Casida JE. 2008. Atypical nicotinic agonist bound conformations conferring subtype selectivity. Proc. Natl Acad. Sci. USA 105, 1728–1732. ( 10.1073/pnas.0711724105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffat C, Buckland ST, Samson AJ, McArthur R, Chamosa Pino V, Bollan KA, Huang JT-J, Connolly CN. 2016. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 6, 24764 ( 10.1038/srep24764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrione M, Vallortigara G, Antolini R, Haase A. 2016. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 6, 38110 ( 10.1038/srep38110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Wu F, Zhao L, Tan J, Jiang H, Hu F. 2015. Neonicotinoid insecticide interact with honeybee odorant-binding protein: implication for olfactory dysfunction. Int. J. Biol. Macromol. 81, 624–630. ( 10.1016/j.ijbiomac.2015.08.055) [DOI] [PubMed] [Google Scholar]
- 43.Parkinson RH, Little JM, Gray JR. 2017. A sublethal dose of a neonicotinoid insecticide disrupts visual processing and collision avoidance behaviour in Locusta migratoria. Sci. Rep. 7, 936 ( 10.1038/s41598-017-01039-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer J, Müller T, Spatz A-K, Greggers U, Grünewald B, Menzel R. 2014. Neonicotinoids interfere with specific components of navigation in honeybees. PLoS ONE 9, e91364 ( 10.1371/journal.pone.0091364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Déglise P, Grünewald B, Gauthier M. 2002. The insecticide imidacloprid is a partial agonist of the nicotinic receptor of honeybee Kenyon cells. Neurosci. Lett. 321, 13–16. ( 10.1016/S0304-3940(01)02400-4) [DOI] [PubMed] [Google Scholar]
- 46.Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. 2013. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 ( 10.1038/ncomms2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. ( 10.1038/nrn1074) [DOI] [PubMed] [Google Scholar]
- 48.de Belle J, Heisenberg M. 1994. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science 263, 692–695. ( 10.1126/science.8303280) [DOI] [PubMed] [Google Scholar]
- 49.Cresswell JE, et al. 2012. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115, 365–371. ( 10.1016/j.zool.2012.05.003) [DOI] [PubMed] [Google Scholar]
- 50.Cresswell JE, Robert F-XL, Florance H, Smirnoff N. 2014. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris). Pest Manag. Sci. 70, 332–337. ( 10.1002/ps.3569) [DOI] [PubMed] [Google Scholar]
- 51.Giurfa M, Sandoz J-C. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54–66. ( 10.1101/lm.024711.111) [DOI] [PubMed] [Google Scholar]
- 52.Ayasse M, Jarau S. 2014. Chemical ecology of bumble bees. Annu. Rev. Entomol. 59, 299–319. ( 10.1146/annurev-ento-011613-161949) [DOI] [PubMed] [Google Scholar]
- 53.Dobson HEM, Bergström G. 2000. The ecology and evolution of pollen odors. Plant Syst. Evol. 222, 63–87. ( 10.1007/BF00984096) [DOI] [Google Scholar]
- 54.Rottler A-M, Schulz S, Ayasse M. 2013. Wax lipids signal nest identity in bumblebee colonies. J. Chem. Ecol. 39, 67–75. ( 10.1007/s10886-012-0229-0) [DOI] [PubMed] [Google Scholar]
- 55.Granero AM, Sanz JMG, Gonzalez FJE, Vidal JLM, Dornhaus A, Ghani J, Serrano AR, Chittka L. 2005. Chemical compounds of the foraging recruitment pheromone in bumblebees. Naturwissenschaften 92, 371–374. ( 10.1007/s00114-005-0002-0) [DOI] [PubMed] [Google Scholar]
- 56.Chittka L. 2017. Bee cognition. Curr. Biol. 27, R1049–R1053. ( 10.1016/j.cub.2017.08.008) [DOI] [PubMed] [Google Scholar]
- 57.Muth F, Francis JS, Leonard AS. 2019. Data from: Modality-specific impairment of learning by a neonicotinoid pesticide Dryad Digital Repository. ( 10.5061/dryad.87p3dd7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Muth F, Francis JS, Leonard AS. 2019. Data from: Modality-specific impairment of learning by a neonicotinoid pesticide Dryad Digital Repository. ( 10.5061/dryad.87p3dd7) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.87p3dd7 [57].