Abstract
The Earth is getting brighter at night, as artificial light at night (ALAN) continues to increase and extend its reach. Despite recent recognition of the damaging impacts of ALAN on terrestrial ecosystems, research on ALAN in marine systems is comparatively lacking. To further our understanding of the impacts of ALAN on marine organisms, this study examines how the reproductive fitness of the common clownfish Amphiprion ocellaris is influenced by the presence of ALAN. We assessed how exposure to low levels of ALAN affects (i) frequency of spawning, (ii) egg fertilization success, and (iii) hatching success of A. ocellaris under control (12 : 12 day–night) and treatment (12 : 12 day–ALAN) light regimes. While we found exposure to ALAN had no impact on the frequency of spawning or fertilization success, ALAN had dramatic effects on hatching. Amphiprion ocellaris eggs incubated in the presence of ALAN simply did not hatch, resulting in zero survivorship of offspring. These findings suggest ALAN can significantly reduce reproductive fitness in a benthic-spawning reef fish. Further research in this field is necessary to fully understand the extent of this impact on population and community dynamics in the wild.
Keywords: Amphiprion ocellaris, light pollution, anthropogenic disturbance, reproductive fitness, spawning
1. Background
For most of evolutionary history, organisms have experienced invariant cycles of light days and dark nights, that shifted predictably with lunar and seasonal phases [1]. This reliable day–night cycle has led to biological clocks, circadian rhythms, that coordinate and regulate daily behaviours and physiology for most organisms on Earth. Changes in light–dark rhythms are often closely linked to the timing of significant events such as courtship, reproduction and migration [2]. Day–night cycles also influence species interactions (predation, competition) and habitat use, by regulating the duration and time in which organisms conduct key activities [2]. The correct functioning of natural systems fundamentally relies on light days and dark nights. However, the relatively recent invention and proliferation of artificial lights have altered the night environment over a substantial portion of the Earth's surface [3], resulting in a mismatch between evolutionary adaptations and the present environmental conditions many animals now face.
The Earth is getting artificially brighter, at a rate of 2.2% per year [4]. As a result of these brighter nights, the impacts of artificial light at night (ALAN) have become an increasing focus in terrestrial ecology. Research has shown that ALAN is a major form of anthropogenic pollution that can affect a wide range of biotic processes, including physiology [5,6], behaviour [7,8], animal movements [9,10], species interactions [11], community structure [12,13]) and reproduction [14–16].
Despite recent recognition of the damaging and far-reaching impacts of ALAN in terrestrial ecosystems, research on the impacts of ALAN in subtidal marine ecosystems is less studied ([17], but see [18–20]). The marine environment is not exempt from light pollution and just as on land, light pollution is increasingly spreading to even remote marine habitats through growing urbanized coastlines and increasing marine infrastructure emitting ALAN (e.g. offshore oil platforms, piers, cruise ships and hotels constructed directly above reefs; [17,21]). There is, therefore, a critical need to understand the extent and ecological impacts of ALAN in marine systems [1,13].
To further our understanding of the impacts of ALAN on marine organisms, we examined whether ALAN impacts the reproductive fitness of coral reef fishes. Using the common clownfish Amphiprion ocellaris (Pomacentridae), we explored how ALAN influenced (i) frequency of spawning, (ii) egg fertilization success, and (iii) hatching success of A. ocellaris under control 12 : 12 day–night, and treatment 12 : 12 day–ALAN light regimes. As both A. ocellaris parents and eggs are site attached, often on shallow reefs, the presence of light pollution in their natural habitat could mean direct and chronic exposure to ALAN. Furthermore, many pomacentrids are known to spawn near dawn [22], and embryos of some species are known to hatch immediately after dusk (e.g. [23,24]), suggesting light pollution has the potential to disrupt the natural light cues used for timing reproductive processes.
2. Material and methods
This study used 10 breeding pairs of the common clownfish A. ocellaris, housed at Flinders University's Animal Housing facilities. Each of 10 aquaria was fitted with an overhead LED light programmed to emit near white light of approximately 5000 K, to imitate commercially available and widely used white LED lights (full details in electronic supplementary material, S1). Five breeding pairs were randomly assigned to the control treatment, and were on a 12 h light (mean (±s.e.) lx = 2482.4 ± 41.8) and 12 h dark (no detectable light with light meter (0.1 lx resolution)) cycle, and five breeding pairs were given an ALAN treatment, with a light cycle of 12 h light during the day (same as the control treatment), and 12 h of dim light during the night (mean (±s.e.) lx = 26.5 ± 1.5). Light measurements were taken by point measurements at the water's surface of each aquaria, during the day and night at two time points throughout the experiment. Lux was determined using an Extech LT40 LED light meter (FLIR Commercial Systems Inc., USA), by taking one reading 6 cm beneath each light, directly above the water in each aquarium. This measurement represents the light reaching the surface of the water, but does not account for the light attenuation and the reduced light intensity that would have reached the bottom of the aquaria (estimated to be 10–15 lx). These lux levels were comparable to levels measured in near shore light-polluted areas (e.g. 5–21.6 lx [13]; 25–68 lx [25]), and were conservative relative to locations illuminated at night by marine infrastructure (e.g. approx. 150–210 lx [18]). Light treatments were separated by black plastic sheeting to block all light at night from control treatments.
Aquaria were checked each morning for the presence of eggs. Fish consistently laid eggs on a terracotta pot placed in each aquarium. Each new egg clutch was photographed with an underwater camera (Olympus Tough TG4), and the date of spawning recorded. Egg clutches were photographed each day of development to record fertilization success. Egg clutches were reared with their parents until the evening of the eighth day—the evening of hatching—at which time each egg clutch was photographed, and the pot with attached eggs were transferred to a 7 l hatching tank. Hatching tanks were exposed to identical conditions as their parent tank, including the same light treatment. Photographs were later viewed in ImageJ [26], and the total number of eggs, the number of healthy eggs, unfertilized or dead (white) eggs, and missing eggs, were recorded for each clutch (electronic supplementary material, S1 and figure S3). Photographs of remaining unhatched eggs on day 9 (post-hatch), along with counts of hatched larvae in the hatching tank, were used to calculate hatching success rate for each clutch. ‘Hatch success’ was calculated as the proportion of eggs that hatched successfully, out of the total number of healthy, developed eggs remaining at the end of the embryonic period (day 8). After 60 days, the experiment was terminated, and the ALAN treatment lights re-set to 12 h light–12 h dark cycle (same as the control treatment). The frequency of spawning, egg fertilization success and hatching success were monitored for another 60 days to record recovery from the extended light exposure.
Differences in spawning frequency, egg fertilization and hatching success between ALAN exposed and control treatments, and between the experimental and recovery periods, were analysed by fitting generalized linear mixed models with model selection using Akaike information criterion for small sample sizes (AICc). See electronic supplementary material, S1 for full details of statistical analysis.
3. Results
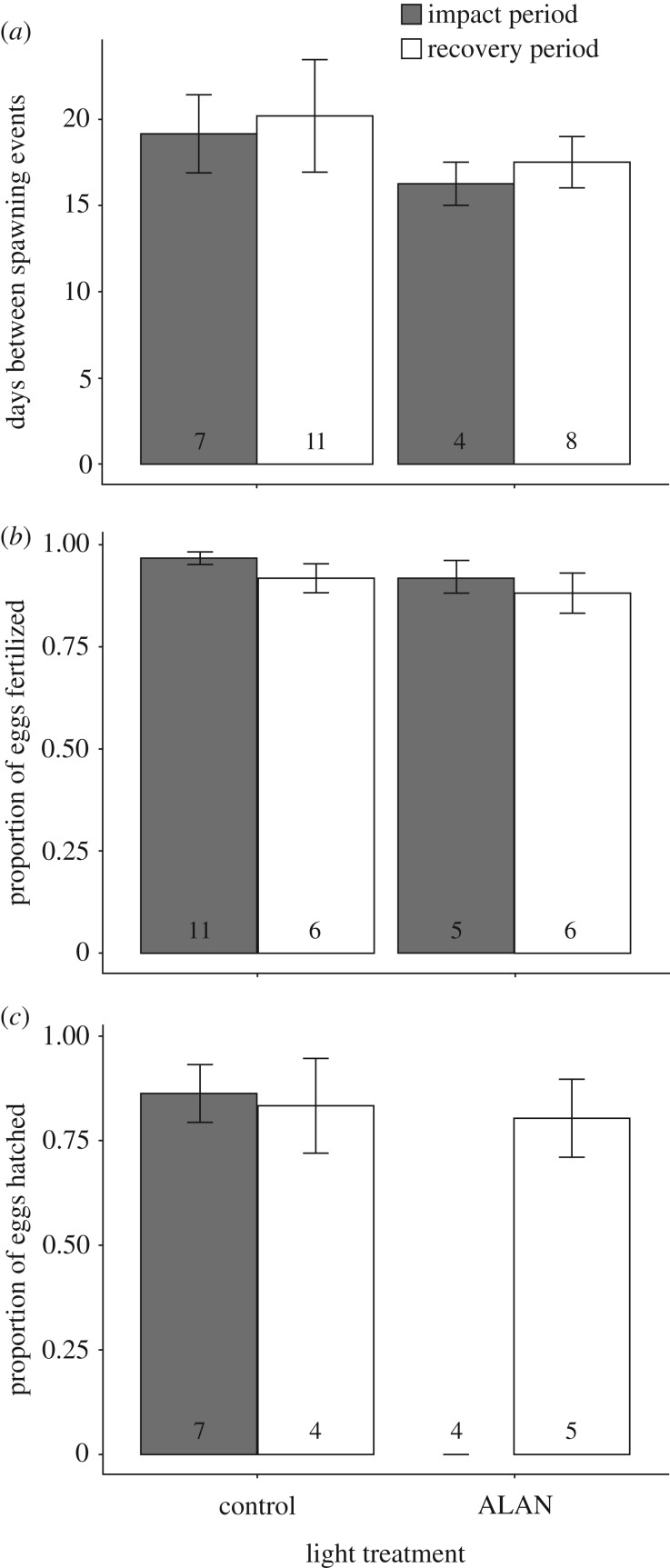
There was no significant difference in spawning frequency between control and ALAN treatment pairs of A. ocellaris, nor between impact and recovery periods (figure 1a), as the best fit model retained only breeding pair as a random factor (electronic supplementary material, S2 and table S2). However, it should be noted the ΔAICc value for the model retaining treatment as an explanatory variable was 2, suggesting similar support for this model. Spawning was slightly less frequent in the control (19 ± 2.26 day interval) compared to the ALAN treatment (16.25 ± 1.25 day interval); however, this relationship was not strong.
Figure 1.
Comparison of mean (±s.e.) (a) spawning frequency, (b) fertilization success and (c) successful hatch rate from control and ALAN treatment groups, during the experimental ‘impact’ period and during ‘recovery’ following removal of ALAN treatment. Numbers represent the sample size for each group.
The best fit model for fertilization success retained experimental period as an explanatory variable, as fertilization success decreased slightly in the recovery period (figure 1b). However, similar support was found (i.e. ΔAICc ≤ 2) for all other candidate models excluding the full model, with the null model ranked a close second best with a ΔAICc value of 0.1 (electronic supplementary material, S2 and table S3).
The best fit model for hatching success was the full model, which retained the interaction term (treatment×experimental period) as explanatory variables (electronic supplementary material, S2 and table S4). Hatching success was significantly lower in clutches that developed under ALAN compared to clutches from the control group. In fact, eggs from clutches reared under ALAN conditions during the impact period had a hatch rate of 0%. This result differs significantly from eggs from clutches reared under ambient day–night lighting conditions during the same experimental period, for which a mean of 86% (±7%) of the eggs successfully hatched (figure 1c). Furthermore, there was no difference in the proportion of eggs hatched between ALAN and control groups during the recovery period (mean ± s.e. ALAN = 0.80 ± 0.09; mean ± s.e. control = 0.83 ± 0.11), indicating that this negative effect of ALAN on hatch rate disappeared as soon as the impact period ended, and light regimes were restored to ambient conditions.
4. Discussion
The overwhelming finding from this study is that light pollution can have a devastating effect on reproductive success of marine organisms. Amphiprion ocellaris eggs incubated in the presence of ALAN simply did not hatch, resulting in zero survivorship of offspring. Amphiprion ocellaris embryos have evolved to hatch after dusk to avoid the threat of diurnal predators [22,24]. Newly hatched A. ocellaris, like most reef fishes, are small in size (approx. 5 mm) and transparent, which likely makes them less visible in darkness, providing protection from nocturnal predators as they emerge from their eggs after dusk [24]. Our results show that the presence of ALAN, even at relatively low levels (approx. 26 lx), masks the environmental cue that triggers hatching in A. ocellaris.
Through inhibition of hatching, light pollution, therefore, has the potential to significantly reduce the reproductive fitness of site-attached reef fish who settle to near shore habitat exposed to chronic ALAN conditions. This inhibition of hatching by ALAN can also have significant impacts on metapopulation dynamics through creation of a population sink—where within-habitat reproduction is insufficient to balance local mortality [27]. As reef fish populations are most often replenished by larvae recruiting from distant reefs, a single source of light pollution has the potential to impact more distant reef population dynamics by reducing larval supply. Furthermore, it is well known that many coral reef fishes are attracted to light in their larval phase (e.g. use of light traps for sampling larval fish [28]). If larval reef fish are attracted to and preferentially settle to ALAN exposed reefs where their reproductive fitness is compromised, light pollution would be creating an ecological trap for recruiting reef fishes, and this could have significant and broad-scale consequences in areas with extensive ALAN pollution. Further research is clearly needed to understand the extent of the impacts of ALAN on habitat selection at settlement and subsequent population persistence.
These findings likely extend to other reef fish in the family Pomacentridae, as many species have been shown to share similar reproductive behaviours, including the timing of hatching during early evening [23,29]. However, ALAN may not have the same impact on reproductive success in fish species with different reproductive strategies. For example, light pollution may not have the same impact on marine pelagic spawners, whose eggs are released into the water column and are carried offshore, as embryos from these species are unlikely to experience chronic exposure to coastal light pollution. Furthermore, species whose embryos hatch during the day (e.g. Oryzias latipes [30], Salmo salar [31]) will not be entrained to hatch with the onset of darkness, and therefore, hatching is unlikely to be inhibited by light pollution. However, the presence of ALAN may create the opposite problem for these species; by masking the normal day–night cycle, embryos may hatch at the wrong time of day or night and optimal timing of the hatching event can be essential for the survival of embryos and subsequent larvae. Thus, the impacts of ALAN are dependent on species-specific life histories, and given the great diversity of life-history strategies among marine taxa, much more research is needed to fully understand the extent of the impacts of light pollution in marine systems.
5. Conclusion and future directions
Despite growing evidence of the physiological and behavioural effects of light pollution, we still have little understanding of the ecological consequences, particularly in the marine environment. This study has demonstrated the negative impacts of ALAN on one measure of fitness—reproductive success—in one species of coral reef fish. Given that ALAN has the potential to disrupt the natural cycles of light and dark that are used ubiquitously to initiate or regulate circadian, lunar and annual rhythms, more research is needed on other aspects of fitness and other organisms to understand the species-specific impacts of light pollution in the marine environment. Furthermore, ALAN is an anthropogenic disturbance that can simultaneously, directly affect multiple trophic levels through, for example, modifying behaviour [32] and physiology [33], attraction of predator and prey species (e.g. [34,35]), and shifting temporal niches [18,36]. Therefore, future research on light pollution should include in situ studies to include realistic costs (e.g. increased predation) and benefits (e.g. increased prey resources) associated with ALAN. Lastly, in order to develop strategies to mitigate the impacts of light pollution in the marine environment, we need a broader understanding of the impacts of different types and different spectrums of light on a wide range of marine organisms. Understanding the extent of the impacts of ALAN on marine systems is critical for developing solutions and complementing current coral reef management strategies to minimize the additional effects of light pollution on these already globally stressed and vulnerable ecosystems.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Paris Bates, Kristen Schubert, Cassie Hoepner and Corey Patten for their help with data collection, and the Flinders University Animal Facilities staff for help with monitoring the A. ocellaris breeding population.
Ethics
This work was carried out in accordance with Flinders University Animal Welfare Committee, project E470/18.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1rm2290 [37].
Authors' contributions
E.K.F. and S.E.S. developed the original idea; E.K.F. and K.B.d.S. collected the data, E.K.F. analysed the data and wrote the manuscript; S.E.S. and K.B.d.S. provided significant editorial advice. All authors approve the final version of this manuscript and agree to be held accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by an Endeavour Research Fellowship and a Fisheries Society of the British Isles Small Research grant awarded to E.K.F.
References
- 1.Gaston KJ, Bennie J. 2014. Demographic effects of artificial nighttime lighting on animal populations. Environ. Rev. 22, 323–330. ( 10.1139/er-2014-0005) [DOI] [Google Scholar]
- 2.Gaston KJ, Davies TW, Nedelec SL, Holt LA. 2017. Impacts of artificial light at night on biological timings. Annu. Rev. Ecol. Evol. Syst. 48, 49–68. ( 10.1146/annurev-ecolsys-110316-022745) [DOI] [Google Scholar]
- 3.Longcore T, Rich C. 2004. Ecological light pollution. Front. Ecol. Environ. 2, 191–198. ( 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2) [DOI] [Google Scholar]
- 4.Kyba CC, et al. 2017. Artificially lit surface of Earth at night increasing in radiance and extent. Science Adv. 3, e1701528 ( 10.1126/sciadv.1701528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215–224. ( 10.1111/j.1600-079X.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 6.Bruning A, Holker F, Franke S, Kleiner W, Kloas W. 2018. Influence of light intensity and spectral composition of artificial light at night on melatonin rhythm and mRNA expression of gonadotropins in roach Rutilus rutilus. Fish Physiol. Biochem. 44, 1–12. ( 10.1007/s10695-017-0408-6) [DOI] [PubMed] [Google Scholar]
- 7.Buchanan BW. 1993. Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim. Behav. 45, 893–899. ( 10.1006/anbe.1993.1109) [DOI] [Google Scholar]
- 8.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 9.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 10.Van Grunsven RH, Creemers R, Joosten K, Donners M, Veenendaal EM. 2017. Behaviour of migrating toads under artificial lights differs from other phases of their life cycle. Amphibia-Reptilia 38, 49–55. ( 10.1163/15685381-00003081) [DOI] [Google Scholar]
- 11.Rotics S, Dayan T, Kronfeld-Schor N. 2011. Effect of artificial night lighting on temporally partitioned spiny mice. J. Mammal. 92, 159–168. ( 10.1644/10-MAMM-A-112.1) [DOI] [Google Scholar]
- 12.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764–767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies TW, Coleman M, Griffith KM, Jenkins SR. 2015. Night-time lighting alters the composition of marine epifaunal communities. Biol. Lett. 11, 20150080 ( 10.1098/rsbl.2015.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker B, Richardson J. 2006. The effect of artificial light on male breeding-season behaviour in green frogs, Rana clamitans melanota. Can. J. Zool. 84, 1528–1532. ( 10.1139/z06-142) [DOI] [Google Scholar]
- 15.Bruning A, Holker F, Franke S, Kleiner W, Kloas W. 2016. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total Environ. 543, 214–222. ( 10.1016/j.scitotenv.2015.11.023) [DOI] [PubMed] [Google Scholar]
- 16.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 17.Davies TW, Duffy JP, Bennie J, Gaston KJ. 2014. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 12, 347–355. ( 10.1890/130281) [DOI] [Google Scholar]
- 18.Bolton D, Mayer-Pinto M, Clark G, Dafforn K, Brassil W, Becker A, Johnston E. 2017. Coastal urban lighting has ecological consequences for multiple trophic levels under the sea. Sci. Total Environ. 576, 1–9. ( 10.1016/j.scitotenv.2016.10.037) [DOI] [PubMed] [Google Scholar]
- 19.Manriquez PH, Jara ME, Diaz MI, Quijon PA, Widdicombe S, Pulgar J, Manriquez K, Quintanilla-Ahumada D, Duarte C. 2019. Artificial light pollution influences behavioral and physiological traits in a keystone predator species, Concholepas concholepas. Sci. Total Environ. 661, 543–552. ( 10.1016/j.scitotenv.2019.01.157) [DOI] [PubMed] [Google Scholar]
- 20.Pulgar J, et al. 2019. Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ. Pollut 244, 361–366. ( 10.1016/j.envpol.2018.10.063) [DOI] [PubMed] [Google Scholar]
- 21.Davies TW, Duffy JP, Bennie J, Gaston KJ. 2016. Stemming the tide of light pollution encroaching into marine protected areas. Conserv. Lett. 9, 164–171. ( 10.1111/conl.12191) [DOI] [Google Scholar]
- 22.Johannes RE. 1978. Reproductive strategies of coastal marine fishes in the tropics. Environ. Biol. Fishes 3, 65–84. ( 10.1007/BF00006309) [DOI] [Google Scholar]
- 23.Robertson DR, Petersen CW, Brawn JD. 1990. Lunar reproductive cycles of benthic-brooding reef fishes: reflections of larval biology or adult biology. Ecol. Monogr. 60, 311–329. ( 10.2307/1943060) [DOI] [Google Scholar]
- 24.McAlary FA, McFarland WN. 1993. The effect of light and darkness on hatching in the pomacentrid Abudefduf saxatilis. Environ. Biol. Fishes 37, 237–244. ( 10.1007/bf00004631) [DOI] [Google Scholar]
- 25.Szekeres P, Wilson AD, Haak CR, Danylchuk AJ, Brownscombe JW, Elvidge CK, Shultz AD, Birnie-Gauvin K, Cooke SJ. 2017. Does coastal light pollution alter the nocturnal behavior and blood physiology of juvenile bonefish (Albula vulpes)? Bull. Mar. Sci. 93, 491–505. ( 10.5343/bms.2016.1061) [DOI] [Google Scholar]
- 26.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. 2017. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 18, 529 ( 10.1186/s12859-017-1934-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulliam HR. 1988. Sources, sinks, and population regulation. Am. Nat. 132, 652–661. ( 10.1086/284880) [DOI] [Google Scholar]
- 28.Fisher R, Bellwood D. 2002. A light trap design for stratum-specific sampling of reef fish larvae. J. Exp. Mar. Biol. Ecol. 269, 27–37. ( 10.1016/S0022-0981(01)00384-7) [DOI] [Google Scholar]
- 29.Kohda M. 1988. Diurnal periodicity of spawning activity of permanently territorial damselfishes (Teleostei: Pomacentridae). Environ. Biol. Fishes 21, 91–100. ( 10.1007/BF00004845) [DOI] [Google Scholar]
- 30.Schoots AFM, Meijer RC, Denucé JM. 1983. Dopaminergic regulation of hatching in fish embryos. Dev. Biol. 100, 59–63. ( 10.1016/0012-1606(83)90200-2) [DOI] [PubMed] [Google Scholar]
- 31.Brännäs E. 1987. Influence of photoperiod and temperature on hatching and emergence of Baltic salmon (Salmo salar L). Can. J. Zool. 65, 1503–1508. ( 10.1139/z87-232) [DOI] [Google Scholar]
- 32.Kurvers R, Dragestein J, Holker F, Jechow A, Krause J, Bierbach D. 2018. Artificial light at night affects emergence from a refuge and space use in guppies. Sci. Rep. 8, 14131 ( 10.1038/s41598-018-32466-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimovski AM, Robert KA. 2018. Artificial light pollution: shifting spectral wavelengths to mitigate physiological and health consequences in a nocturnal marsupial mammal. J. Exp. Zool. Part A Ecol. Integr. Physiol. 329, 497–505. ( 10.1002/jez.2163) [DOI] [PubMed] [Google Scholar]
- 34.Nightingale B, Longcore T, Simenstad CA. 2006. Artificial night lighting and fishes. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 257–276. Washington DC: Island Press. [Google Scholar]
- 35.Becker A, Whitfield AK, Cowley PD, Järnegren J, Næsje TF. 2013. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 50, 43–50. ( 10.1111/1365-2664.12024) [DOI] [Google Scholar]
- 36.Ryer CH, Olla BL. 1999. Light-induced changes in the prey consumption and behavior of two juvenile planktivorous fish. Mar. Ecol. Prog. Ser. 181, 41–51. ( 10.3354/meps181041) [DOI] [Google Scholar]
- 37.Fobert E, Burke Da Silva K, Swearer S. 2019. Data from: Artificial light at night causes reproductive failure in clownfish Dryad Digital Repository. ( 10.5061/dryad.1rm2290) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fobert E, Burke Da Silva K, Swearer S. 2019. Data from: Artificial light at night causes reproductive failure in clownfish Dryad Digital Repository. ( 10.5061/dryad.1rm2290) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.1rm2290 [37].