Abstract
Juvenile animals generally disperse from their birthplace to their future breeding territories. In fragmented landscapes, habitat-specialist species must disperse through the anthropogenic matrix where remnant habitats are embedded. Here, we test the hypothesis that dispersing juvenile frugivores leave a footprint in the form of seed deposition through the matrix of fragmented landscapes. We focused on the Sardinian warbler (Sylvia melanocephala), a resident frugivorous passerine. We used data from field sampling of bird-dispersed seeds in the forest and matrix of a fragmented landscape, subsequent disperser identification through DNA-barcoding analysis, and data from a national bird-ringing programme. Seed dispersal by Sardinian warblers was confined to the forest most of the year, but warblers contributed a peak of seed-dispersal events in the matrix between July and October, mainly attributable to dispersing juveniles. Our study uniquely connects animal and plant dispersal, demonstrating that juveniles of habitat-specialist frugivores can provide mobile-link functions transiently, but in a seasonally predictable way.
Keywords: cross-habitat spillover, DNA barcoding, intraspecific variability, natal dispersal, mobile links
1. Introduction
Highly mobile animals that actively move in fragmented landscapes and connect habitats through ecological processes—i.e. by transporting propagules, nutrients or diseases—are termed ‘mobile links’ (sensu [1]; e.g. [2,3]). Frugivores play a major role as mobile links by dispersing seeds in the increasingly fragmented and deforested landscapes of the planet [4,5], fostering regeneration of disturbed habitats and functional connectivity among patches of remnant vegetation [3,6–8]. Such mobile-link services can be unevenly distributed among species within local frugivore assemblages [3] because distinct frugivore species may vary in their response to landscape alteration [9]. Yet, we know virtually nothing about within-species differences in mobile-link services provided by consistent groups of individuals (e.g. different genders or age groups), despite the recently recognized relevance of intraspecific variability in other functional components of frugivore-mediated seed dispersal [10,11].
Every year, millions of juvenile animals perform a dispersal event that occurs only once in their lives, from their birthplace to their future breeding territories, known as natal dispersal [12,13]. In fragmented landscapes, natal dispersal means that many juveniles of habitat-specialist species must leave natural habitats to travel across the low-quality habitats of the matrix [14,15]. Juvenile frugivores could therefore mediate distinctive seed-dispersal services through this ‘cross-habitat spillover’ [16], operating as mobile links transiently but in a seasonally predictable way.
Here, we test the hypothesis that dispersing juvenile frugivores leave a landscape-scale footprint in the form of seed deposition through the matrix of fragmented landscapes. We focused on a resident avian frugivore—the Sardinian warbler (Sylvia melanocephala, Sylviidae)—as a study species because it is a resident species that breeds locally, produces dispersing juveniles and occurs in the landscape throughout the year, allowing comparisons between seasons. We used an exceptional dataset resulting from (i) a 2-year field sampling of bird-dispersed seeds in forest and matrix of a fragmented landscape, and (ii) subsequent DNA-barcoding analysis that unveiled the contribution of the Sardinian warbler to seed deposition patterns. Finally, we combined this information with a national bird-ringing dataset in order to link seasonal patterns of seed dispersal in forest and matrix with seasonal captures of adult and juvenile warblers in analogous habitats.
2. Material and methods
(a). The frugivore species
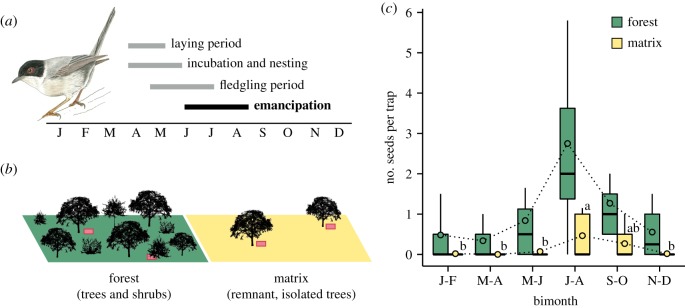
The Sardinian warbler is an approximately 11-g passerine that is resident in the southern Iberian Peninsula [17] and shows a marked territorial behaviour and site-fidelity [18]. This warbler plays an important role throughout the Mediterranean Basin as a seed disperser of many fleshy-fruited species [19]. Although it can occur in a wide variety of habitats, it typically prefers open forests with abundant understory [17]. Juvenile dispersal in our study landscape is expected to begin between late June and early July (i.e. after emancipation), according to the laying, incubation, nesting and fledgling phenology in warm Iberian regions (figure 1a) [17]. Sexual maturity is reached during the first year of life [17].
Figure 1.
(a) Breeding phenology of the Sardinian warbler in the Iberian Peninsula (based on [14]); drawing: Daniel García. (b) Study design: we placed seed traps (red rectangles) beneath trees and shrubs in a Mediterranean forest (n = 28) and beneath isolated trees in an adjacent agricultural matrix (n = 28). (c) Boxplot (median, quartiles and 10th/90th percentiles; circles: mean values) showing the bimonthly number of seeds dispersed by Sardinian warblers (identified through DNA barcoding) per seed trap in forest and matrix, during two sampling years. Different letters denote significant differences among bimonths in the matrix (Tukey post hoc tests: adjusted p-values < 0.05). (Online version in colour.)
(b). Study landscape
The study was conducted in a lowland landscape (40–60 m a.s.l.) located in southern Spain (Cádiz province; 36° 39′ N, 5° 57′ W). The study plot has a size of ca 900 × 700 m and includes a Mediterranean forest remnant surrounded by an agricultural matrix (approx. 65% of landscape cover) (electronic supplementary material, figure S1). A detailed description is provided in [3]. Briefly, the forest has a tree layer composed of large Mediterranean oaks (Quercus spp.) and an understory of treelets and shrubs dominated by fleshy-fruited species. The matrix is mostly devoted to cereal crops but preserves some isolated trees, mainly oaks (approx. 80%) and wild olive trees (Olea europaea var. sylvestris, approx. 20%); hence, the wild olive is virtually the only fruit species in the matrix [3]. The avian frugivore assemblage in this landscape is dominated by migratory species [20], the Sardinian warbler being the main resident species inhabiting the forest.
(c). Seed-dispersal data
We sampled community-wide seed dispersal mediated by birds in the forest and matrix of the study landscape (figure 1b). We conducted periodic sampling surveys (fortnightly) for 2 years, from November 2013 to October 2015; thus, including the entire fruiting periods of local fleshy-fruited species. We placed seed traps beneath tree and shrub canopies to quantify the magnitude of bird-mediated seed rain in each habitat type. Seed traps were 0.22 m2 plastic trays covered with wire mesh to prevent post-dispersal seed predation. We monitored a total of 56 seed traps, 28 in the forest, beneath the canopy of different oak trees and shrubs, and 28 in the matrix, beneath distinct isolated oaks at distances ranging from 5 to 190 m from the forest edge (electronic supplementary material, figure S1).
In each sampling survey, we recorded the number of bird-dispersed seeds per seed trap and sampled individual seeds or droppings with seeds for DNA-barcoding analysis. We did so by putting each sample with a minimum of handling into a 1.5- or 2.0-ml sterile tube. Tubes were labelled and stored in a freezer at −20°C until DNA extraction. We additionally sampled bird-dispersed seeds visually detected, particularly beneath isolated oaks in the matrix, where we expected a very low contribution by Sardinian warblers. With this, we aimed at increasing sample sizes because DNA-barcoding identification generally fails (PCR failure) in 5–10% of samples [3,20,21]. Conversely, we generally collected just a subsample of the seeds in the forest when seed traps received many seeds of certain plant species [20]. A detailed description of this section is available in the electronic supplementary material.
(d). Seed disperser identification through DNA barcoding
DNA of animal origin can be extracted from the surface of defecated or regurgitated seeds, allowing the identification of the frugivore species responsible for dispersal events [3,20,21]. This approach allowed us to know the contribution (%) of Sardinian warblers to the magnitude of seed deposition, for different seed species and periods of the year, quantified in the seed traps we placed in the forest and matrix of our study landscape. Detailed laboratory protocols for DNA extraction, PCR and sequencing can be found in [3,21]. Resulting sequences (length: median = 414 bp; range = 80–417 bp) were aligned and edited using SEQUENCHER 4.9, and then identified at the species level using the ‘BARCODE OF LIFE DATA’ identification system (BOLD: http://www.boldsystems.org; [22]). We successfully identified the disperser species in 1669 bird droppings (90.2% of 1851 analysed) containing a total of 1907 seeds. We classified species-level identifications as dispersal events mediated by either ‘Sardinian warblers’ (447 samples with 472 seeds) or ‘other species’ (1222 samples with 1435 seeds); the identity of the ‘other species’ can be found in [3,20].
(e). Bird-ringing data
We used a ringing database provided by SEO/Birdlife of Sardinian warblers captured (n = 126 891) in Spain between 1975 and 2018 [23] in order to link seasonal patterns of seed deposition with seasonal captures of juvenile individuals in analogous habitats. We filtered the original database by classifying bird age categories (EURING classification) as ‘juveniles’ or ‘adults’ and excluding birds with unknown age. We clustered the original habitat classification of each ringing capture into ‘woodland’ and ‘open habitats’, analogous to the forest and matrix in our study landscape. Finally, we selected a subset of the warmer Spanish provinces, dominated by the thermo- and meso-Mediterranean bioclimate, aiming to use ringing data from localities with the climatic conditions as close as possible with those of our study landscape (thermo-Mediterranean). The final subset included 33 457 ringing captures, including 22 536 juveniles and 10 921 adults. A detailed description of data filtering is available in the electronic supplementary material.
(f). Data analysis
All data analyses were performed using R 3.5.2 (R Development Core Team 2018). We used bimonths, a six-level factor (January–February, March–April, etc.), as the temporal scale of data aggregation per seed trap. Bimonths provided a proper temporal resolution while preventing a dominance of zeros in our dataset. We used DNA-barcoding identifications in different bimonths of each sampling year, habitats, microhabitats and 50-m distance classes from the forest edge in the matrix, to calculate the relative contribution of Sardinian warblers to seed rain of different plant species in those spatio-temporal combinations (fwarbler = nwarbler/ntotal). We then estimated the number of seeds dispersed by Sardinian warblers in each seed trap as SRwarbler = SRtotal × fwarbler; where SRtotal is the total number of seeds of a plant species found in each seed trap in different bimonths of each year (see a similar procedure in [20]). These estimates were rounded and converted into integers. Finally, we aggregated the accumulated number of seeds dispersed by Sardinian warblers per seed trap and bimonth (seed species pooled), which resulted in 672 data points; i.e. 28 seed traps × 2 habitats × 6 bimonths × 2 years. We then fitted a negative binomial generalized linear mixed-model using the package lme4 (v. 1.1-19) [24] to analyse, through a two-way ANOVA design (Habitat × Bimonth), bimonthly differences in the magnitude of seed rain (i.e. number of seeds per trap) dispersed by Sardinian warblers in forest and matrix. We included the seed-trap identity as a random factor to account for the repeated measures per trap. The significance of fixed effects (p-values of Wald χ2 tests) was computed using the ‘Anova’ function of the package car (v. 2.1-6) [25].
Finally, we used non-parametric Spearman's rank correlation tests to test for a positive relationship (i.e. one-tailed test) between the average bimonthly seed rain dispersed by Sardinian warblers in the forest and matrix of our study landscape and the accumulated number of ringing captures of juvenile individuals in analogous habitats (n = 6 bimonths). We did not expect this relationship for adults. A detailed description of data analysis is available in the electronic supplementary material.
3. Results
A total of 1651 bird-dispersed seeds of 10 fleshy-fruited species fell in the seed traps during the study. Sardinian warblers were identified as the disperser of 396 seeds (24%) belonging to four plant species: Rhamnus alaternus (209), Pistacia lentiscus (166), Rhamnus lycioides (20) and Rubus ulmifolius (1). Spatially, warblers dispersed 349 seeds (88%) in the forest and 47 (12%) in the matrix. Seasonally, there was a marked seed-dispersal peak in both habitats between July–August (highest peak) and September–October (figure 1c). Apart from this peak, seed dispersal in the matrix was negligible the rest of the year (figure 1c). According to these patterns, Habitat (Wald , p < 10–15) and Bimonth (Wald , p < 10–13), but not their interaction (Wald , p = 0.344), had highly significant effects on warbler-mediated seed rain.
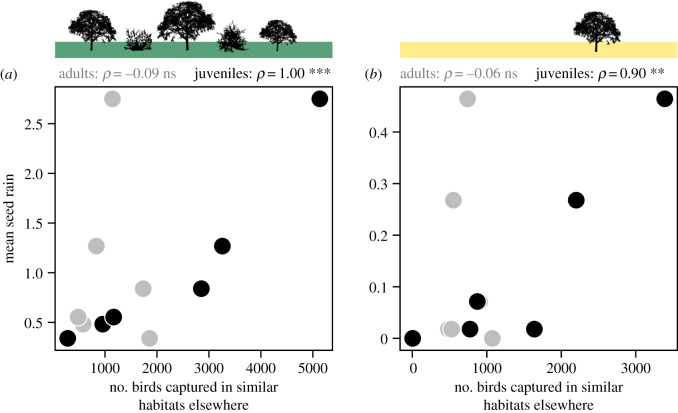
Notably, the bimonthly mean seed rain dispersed by Sardinian warblers in the forest and matrix of our study landscape was strongly correlated with the accumulated number of juvenile warblers captured bimonthly in similar habitats within warm Spanish regions, while unrelated to the number of adults (figure 2). This correlation held with the percentage of juvenile individuals per bimonth, which is independent of any seasonal difference in the mist-netting effort (electronic supplementary material, figure S2).
Figure 2.
Bimonthly mean seed rain (nseeds per seed trap) recorded in (a) the forest and (b) the matrix of our study landscape in relation to the accumulated number of adult (grey dots) and juvenile (black dots) Sardinian warblers captured bimonthly between 1975 and 2018 in similar habitats, in a subset of warm Spanish provinces. Spearman's (ρ) rank correlation tests are shown above the panels for juveniles and adults (ns: p > 0.5; **p = 0.007; ***p = 0.001). (Online version in colour.)
4. Discussion
Our results strongly support our hypothesis that dispersing juvenile frugivores leave a landscape-scale footprint in the form of seed deposition through the matrix of fragmented landscapes. We found a marked peak in the seed-dispersal function mediated by Sardinian warblers in our study landscape between July and October (figure 1). This period matches the expected period of natal dispersal, which may occur several weeks after emancipation [17]. Importantly, this period neither coincides with the seed-dispersal peaks mediated by other frugivore species (electronic supplementary material, figure S3) nor with the peak in fruit availability at the study site (October–February; J. P. González-Varo 2018, unpublished data).
The seed-dispersal peak in the forest clearly reflected the increase in the population density of Sardinian warblers after the recruitment of new juveniles, whereas the peak in the matrix reflected the spillover of dispersing juvenile warblers from the forest. Both ideas are supported by the strong correlation between the bimonthly seed-rain magnitude in the forest and matrix of our study landscape and the number of juvenile warblers captured bimonthly by bird ringers in analogous habitats (figure 2). The spillover from the forest was also supported by a clear distance-decay pattern in warbler-mediated seed rain in the matrix (from 0 to 200 m from the forest edge; electronic supplementary material, figure S4).
Our study uniquely connects animal and plant dispersal, showing that habitat-specialist frugivores can operate as mobile links in a seasonally predictable way, during their juvenile dispersal through the matrix of fragmented landscapes. Importantly, such transient and distinctive seed-dispersal services can foster regeneration in disturbed habitats and plant population connectivity [3]. As natal dispersal is a common ecological process within vertebrates [12,26], the main frugivores and seed dispersers of fleshy-fruited plants [27], our findings might be broadly generalizable across biomes and plant–frugivore assemblages.
Supplementary Material
Acknowledgements
The ‘Molecular Ecology Laboratory’ (Estación Biológica de Doñana, LEM–EBD–CSIC; ISO9001:2015 and ISO14001:2015 certifications) provided logistical support. SEO/BirdLife (Oficina de Anillamiento) provided the ringing data. Benno Simmons kindly checked the English grammar and style.
Ethics
‘Servicio de Cría Caballar de las Fuerzas Armadas' provided permission to work at the study site.
Data accessibility
Data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.97q0n06 [28].
Authors' contributions
J.P.G.-V. conceived the study. J.P.G.-V. and P.J. planned the sampling design and collected the field data. J.M.A. performed laboratory work. J.P.G.-V. and S.D.-G. analysed the data and drafted the article with inputs from P.J. All authors revised and approved the manuscript. All authors agree to be held accountable for the content of this paper.
Competing interests
The authors have no competing interests.
Funding
Spanish MINECO (CGL2017-82847-P) and Junta de Andalucía Excellence Projects (RNM–5731) awarded to P.J., Severo Ochoa Award for Centres of Excellence in R + D + I (SEV-2012-0262) and a postdoctoral fellowship from the Severo Ochoa Program awarded to J.P.G.-V. (SEV-2012-0262). While writing this paper, J.P.G.-V. was funded by a Spanish ‘Ramon y Cajal’ fellowship (RYC-2017-22095).
References
- 1.Lundberg J, Moberg F. 2003. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6, 87–98. ( 10.1007/s10021-002-0150-4) [DOI] [Google Scholar]
- 2.Kremen C, et al. 2007. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol. Lett. 10, 299–314. ( 10.1111/j.1461-0248.2007.01018.x) [DOI] [PubMed] [Google Scholar]
- 3.González-Varo JP, Carvalho CS, Arroyo JM, Jordano P. 2017. Unravelling seed dispersal through fragmented landscapes: frugivore species operate unevenly as mobile links. Mol. Ecol. 26, 4309–4321. ( 10.1111/mec.14181) [DOI] [PubMed] [Google Scholar]
- 4.Ellis EC, Klein Goldewijk K, Siebert S, Lightman D, Ramankutty N. 2010. Anthropogenic transformation of the biomes, 1700 to 2000. Glob. Ecol. Biogeogr. 19, 589–606. ( 10.1111/j.1466-8238.2010.00540.x) [DOI] [Google Scholar]
- 5.Haddad NM, et al. 2015. Habitat fragmentation and its lasting impact on Earth's ecosystems. Sci. Adv. 1, e1500052 ( 10.1126/sciadv.1500052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timóteo S, Correia M, Rodríguez-Echeverría S, Freitas H, Heleno R. 2018. Multilayer networks reveal the spatial structure of seed-dispersal interactions across the Great Rift landscapes. Nat. Commun. 9, 140 ( 10.1038/s41467-017-02658-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Castro A, Yang S, Carlo TA. 2019. How does avian seed dispersal shape the structure of early successional tropical forests? Funct. Ecol. 33, 229–238. ( 10.1111/1365-2435.13250) [DOI] [Google Scholar]
- 8.Auffret AG, et al. 2017. Plant functional connectivity—integrating landscape structure and effective dispersal. J. Ecol. 105, 1648–1656. ( 10.1111/1365-2745.12742) [DOI] [Google Scholar]
- 9.Schleuning M, Fründ J, García D. 2015. Predicting ecosystem functions from biodiversity and mutualistic networks: an extension of trait-based concepts to plant–animal interactions. Ecography 38, 380–392. ( 10.1111/ecog.00983) [DOI] [Google Scholar]
- 10.Zwolak R. 2018. How intraspecific variation in seed-dispersing animals matters for plants. Biol. Rev. 93, 897–913. ( 10.1111/brv.12377) [DOI] [PubMed] [Google Scholar]
- 11.Snell RS, et al. 2019. Consequences of intraspecific variation in seed dispersal for plant demography, communities, evolution, and global change. AoB Plants plz016 ( 10.1093/aobpla/plz016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwood P, Harvey P. 1982. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 13, 1–21. ( 10.1146/annurev.es.13.110182.000245) [DOI] [Google Scholar]
- 13.Paradis E, Baillie SR, Sutherland WJ, Gregory RD. 1998. Patterns of natal and breeding dispersal in birds. J. Anim. Ecol. 67, 518–536. ( 10.1046/j.1365-2656.1998.00215.x) [DOI] [Google Scholar]
- 14.Anders A, Faaborg J, Thompson F III. 1998. Postfledging dispersal, habitat use, and home-range size of juvenile wood thrushes. The Auk 115, 349–358. ( 10.2307/4089193) [DOI] [Google Scholar]
- 15.Matthysen E, Adriaensen F, Dhondt AA. 1995. Dispersal distances of nuthatches, Sitta europaea, in a highly fragmented forest habitat. Oikos 72, 375–381. ( 10.2307/3546123) [DOI] [Google Scholar]
- 16.Blitzer EJ, Dormann CF, Holzschuh A, Klein A-M, Rand TA, Tscharntke T. 2012. Spillover of functionally important organisms between managed and natural habitats. Agric. Ecosyst. Environ. 146, 34–43. ( 10.1016/j.agee.2011.09.005). [DOI] [Google Scholar]
- 17.Aparicio R. 2016. Curruca cabecinegra – Sylvia melanocephala (Gmelin, 1789). In Enciclopedia virtual de los vertebrados españoles (eds Salvador A, Morales M). Madrid, Spain: Museo Nacional de Ciencias Naturales. [Google Scholar]
- 18.Bas JM, Pons P, Gómez C. 2005. Home range and territory of the Sardinian warbler Sylvia melanocephala in Mediterranean shrubland. Bird Study 52, 137–144. ( 10.1080/00063650509461383) [DOI] [Google Scholar]
- 19.Herrera CM. 1984. A study of avian frugivores, bird-dispersed plants, and their interaction in Mediterranean scrublands. Ecol. Monogr. 54, 2–23. ( 10.2307/1942454) [DOI] [Google Scholar]
- 20.González-Varo JP, Arroyo JM, Jordano P. 2019. The timing of frugivore-mediated seed dispersal effectiveness. Mol. Ecol. 28, 219–231. ( 10.1111/mec.14850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Varo JP, Arroyo JM, Jordano P. 2014. Who dispersed the seeds? The use of DNA barcoding in frugivory and seed dispersal studies. Methods Ecol. Evol. 5, 806–814. ( 10.1111/2041-210X.12212) [DOI] [Google Scholar]
- 22.Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org) Mol. Ecol. Notes 7, 355–364. ( 10.1111/j.1471-8286.2007.01678.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anónimo. 2018. Banco de datos de anillamiento del remite ICONA – Ministerio de Medio Ambiente. Datos de anillamiento y recuperaciones en España. (Madrid, Ministerio de Agricultura, Alimentación y Medio Ambiente, SEO/BirdLife, ICO, EBD-CSIC y GOB.
- 24.Bates D, Maechler M, Bolker B. 2013. lme4: linear mixed-effects models using S4 classes.
- 25.Fox J, Weisberg S. 2011. An R companion to applied regression, 2nd edn Beverley Hills, CA: SAGE Publishing. [Google Scholar]
- 26.Clobert J, Baguette M, Benton TG, Bullock JM. 2012. Dispersal ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 27.Herrera CM. 2002. Seed dispersal by vertebrates. In Plant–animal interactions: an evolutionary approach (eds Herrera CM, Pellmyr O), pp. 185–208. Oxford, UK: Blackwell Science. [Google Scholar]
- 28.González-Varo JP, Díaz-García S, Arroyo JM, Jordano P. 2019. Data from: Seed dispersal by dispersing juvenile animals: a source of functional connectivity in fragmented landscapes Dryad Digital Repository. ( 10.5061/dryad.97q0n06.2) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- González-Varo JP, Díaz-García S, Arroyo JM, Jordano P. 2019. Data from: Seed dispersal by dispersing juvenile animals: a source of functional connectivity in fragmented landscapes Dryad Digital Repository. ( 10.5061/dryad.97q0n06.2) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository at: https://doi.org/10.5061/dryad.97q0n06 [28].