Abstract
As a colonizing species expands its range, individuals at the invasion front experience different evolutionary pressures than do those at the range-core. For example, low densities at the edge of the range mean that males should rarely experience intense sperm competition from rivals; and investment into reproduction may trade-off with adaptations for more rapid dispersal. Both of these processes are predicted to favour a reduction in testis size at the invasion front. To explore effects of invasion stage in Australian cane toads (Rhinella marina), we collected and dissected 214 adult males from three regions: one in the species' range-core (northeastern Australia), and two from invasion fronts (one in northwestern Australia and one in southeastern Australia). Despite the brief duration of separation between toads in these areas (approx. 85 years), testis masses averaged greater than 30% higher (as a proportion of body mass) in range-core males than in conspecifics sampled from either vanguard of the invasion. Previous work has documented low reproductive frequencies in female cane toads at the invasion front also, consistent with the hypothesis that evolutionary and ecological pressures unleashed by an invasion can favour relatively low resource allocation to reproduction in both sexes.
Keywords: Amphibia, Anura, Bufo marinus, mating system, sexual selection
1. Introduction
As a colonizing species expands its range into new territory, it is subject to an array of evolutionary forces that differ from those experienced in the range-core. As a result, invasive plants and animals exhibit distinctive morphological, physiological and behavioural traits at the leading edge of their range expansion [1]. Some of those novel traits reflect natural selection and/or spatial sorting for higher rates of dispersal [2], whereas others reflect adaptations to local environments [3] or to low abundance in newly invaded areas [4]. Decreased intraspecific competition for resources may favour the evolution of ‘r-selected’ life-history traits [4]; and also, low population densities might decrease the intensity of male–-male rivalry for mating opportunities.
In high-density aggregations of reproductive animals (as at the range-core), adult males are under intense selection to outcompete other males via an array of tactics [5]. Although displays and combat bouts are the most obvious forms of male–male rivalry, the competition to fertilize eggs may also be important. For example, multi-male groups around spawning female anurans (frogs and toads) reduce the proportion of the female's clutch sired by any single male and have resulted in the evolution of larger testes because fertilization success is enhanced by the production of large amounts of sperm [6,7]. More generally, the intensity of sperm competition predicts testis size in diverse lineages of organisms, e.g. mammals [8–10], birds [11,12], reptiles [13], anurans [14–17]. The inference of a functional relationship between these traits is strongly supported by experimental studies that manipulate the intensity of sexual selection in laboratory colonies of insects, generating rapid shifts in relative testis mass [18,19].
There are three reasons to expect lower testis mass at an invasion front than in the range-core. First, sperm competition is reduced at the invasion front because spawning aggregations are less likely to include rival males (because of low population densities). Second, opportunities for remating likely will be rare (because of low availability of mates), so that an inability to rapidly replenish sperm stores is unlikely to curtail male reproductive success [6,20]. Third, dispersal-enhancing traits accumulate at expanding range fronts, favouring reallocation of resources from other functions (such as reproduction) into traits that enhance dispersal [21]. In summary, we predict low testis mass at an invasion front because a scarcity of rivals reduce the importance of high sperm counts, and because of trade-offs between investment into reproduction versus dispersal. By contrast, we predict high testis mass in individuals from range-core areas.
The invasion of cane toads (Rhinella marina, formerly Bufo marinus) across Australia provides an ideal model system with which to test this prediction. Released in northeastern Australia in 1935, toads have now spread thousands of kilometres westwards across the continent, and southwards down the eastern coast [22]. In the course of their invasion, cane toads have evolved a suite of traits that enhance the rate of dispersal and that adapt them to novel abiotic changes within the invaded range [3,23,24]. Consistent with a trade-off between reproduction versus dispersal, female cane toads from the invasion front have low reproductive frequencies even under standardized conditions of food supply [25].
2. Material and methods
We collected adult male cane toads (greater than 85 mm snout–vent length, = SVL [26]) from three areas: Townsville and Richmond, Queensland (19°14′ S, 146°47′ E and 20°44′ S, 143°09′ E, respectively, both within the range-core; toads present for greater than 80 years), Kununurra, Western Australia (15°48′ S, 128°43′ E, western invasion front; toads present for less than 8 years) and Brooms Head, New South Wales (29°38′ S, 153°18′ E, southern invasion front; toads present for less than 15 years). Each area was sampled three times, to encompass seasonal variation in the reproductive state in April 2017, September 2017 and April 2018.
Toads were hand-collected at night and returned to local field laboratories where they were maintained for 10 days before use in experiments on sperm–egg interactions. The animals were kept under a reversed day–night cycle to facilitate experimental work. They were kept in enclosures (597 × 362 × 381 mm: L × W × H) with fresh water and fed crickets ad libitum four times per week. To ensure that all toads were at similar (and maximal) levels of testicular activity [27], we injected each animal with 0.25 ml synthetic gonadotrophin diluted 1 : 20 with saline (Lucrin; Abbott Australasia), approximately 8–12 h before humanely killing them (by double-pithing), and then immediately dissecting them. Testes mass was recorded to the closest 0.001 g, body mass to 0.1 g and SVL to 1 mm.
Researchers have used a variety of indices to quantify testis mass relative to body size. For example, some authors calculate the Gonosomatic Index (GSI) as ‘mass of testis divided by body mass' whereas others use ‘mass of testis divided by body length’ [28]. Given statistical issues associated with ratio measurements, it may be preferable to quantify relative testis mass as the residual scores from the linear regression of testis mass (combined left plus right testes) against body mass, fitted to the entire dataset [28,29]. This procedure yields scores with a mean of zero; individuals with testes heavier than expected from their body mass will have positive residual scores, and individuals with lighter-than-expected testes will have negative scores. In practice, all of the indices of relative testis mass are highly correlated with each other, and thus will usually generate similar conclusions. In our dataset, the index of relative testis mass based on the residual score was highly correlated with the GSI regardless of whether the denominator was toad body mass (n = 213, r = 0.90, p < 0.0001) or toad body length (n = 213, r = 0.83, p < 0.0001). The two GSI indices were also highly correlated with each other (n = 213, r = 0.91, p < 0.0001). Analyses based on all three indices generated identical conclusions. We thus report analyses based on the residual score and use GSI calculations only to show effect sizes.
We used JMP 14.0 to analyse our data, after checking assumptions of normality and variance homogeneity. We used ANOVA with area and season as factors (plus their interaction) to look for differences in male body sizes, body condition and relative testis mass. Non-significant interaction terms were deleted, and the ANOVA recalculated to estimate main effects. To quantify body condition and relative testis mass, we regressed these variables against male SVL and used the residual scores as our indices. We used Pearson correlations to evaluate the strength of association between variables.
3. Results
Mean body lengths of adult males did not differ significantly among the three areas or the three seasons, nor did these factors interact significantly (main effect of area, F2,210 = 0.82, p = 0.44; main effect of season, F2,210 = 2.45, p = 0.09; interaction area × season, F4,206 = 1.99, p = 0.10; table 1). Body condition of males was lower in September 2017 than in either of the other two sampling periods (Tukey post hoc test p < 0.05; main effect of season, F2,205 = 15.32, p < 0.0001) and this was true for all three areas (main effect of area, F2,205 = 1.34, p = 0.26; interaction, F4,205 = 0.44, p = 0.78).
Table 1.
Sample sizes and morphological traits of male cane toads (Rhinella marina) collected from three areas, each sampled on three occasions. The ‘residual testis mass' is the residual score from the general linear regression of testis mass against toad body mass. Table shows mean values ± s.e.
| location | season | sample size | body mass (g) | snout–vent length (mm) | testis mass (mg) | residual testis mass |
|---|---|---|---|---|---|---|
| range-core | Apr 2017 | 25 | 118.26 ± 6.64 | 106.64 ± 1.81 | 374.84 ± 39.41 | 0.13 ± 28.59 |
| Sep 2017 | 54 | 112.48 ± 3.72 | 109.69 ± 1.13 | 488.46 ± 23.61 | 127.57 ± 18.12 | |
| Apr 2018 | 17 | 110.89 ± 5.84 | 106.76 ± 1.79 | 411.65 ± 28.83 | 59.07 ± 28.68 | |
| western front | Apr 2017 | 16 | 130.14 ± 5.23 | 110.50 ± 1.37 | 305.19 ± 15.53 | −105.26 ± 16.92 |
| Sep 2017 | 28 | 107.71 ± 4.90 | 107.64 ± 1.80 | 353.39 ± 27.61 | 10.38 ± 18.16 | |
| Apr 2018 | 17 | 132.35 ± 6.19 | 111.88 ± 1.60 | 398.94 ± 30.12 | −18.13 ± 30.13 | |
| southern front | Apr 2017 | 15 | 114.39 ± 4.21 | 104.40 ± 1.38 | 230.27 ± 17.37 | −132.83 ± 18.80 |
| Sep 2017 | 26 | 112.28 ± 4.26 | 108.38 ± 1.67 | 263.92 ± 13.96 | −92.81 ± 13.09 | |
| Apr 2018 | 17 | 112.18 ± 6.96 | 104.71 ± 2.12 | 253.24 ± 20.35 | −103.20 ± 11.56 |
The average mass of the testis relative to male body mass was lower in April 2017 than in either of the other two seasons (Tukey post hoc test p < 0.05; main effect of season, F2,204 = 16.17, p < 0.0001) and lower at the invasion fronts than the range-core (Tukey post hoc tests show range-core > western front > southern front; main effect of area, F2,204 = 52.36, p < 0.0001; interaction, F4,204 = 1.22, p = 0.29; figure 1). Using the mass-based GSI to express effect sizes, the mean ratio of testis mass to body mass was 3.89 mg g−1 in animals from the range-core, 2.38 mg g−1 at the southern invasion front and 2.95 mg g−1 at the western invasion front. Thus, this GSI index averaged 63% higher at the range-core than the southern invasion front and 32% higher at the range-core than the western invasion front.
Figure 1.
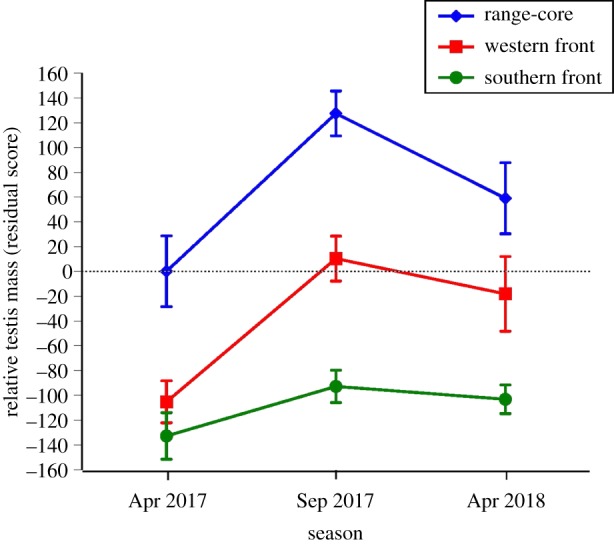
Relative mass of the testis (quantified as residual scores from the general linear regression of testis mass versus body mass) of adult male cane toads (Rhinella marina) from the range-core and two invasion-front regions within Australia. The graph shows mean values and associated standard errors. (Online version in colour.)
4. Discussion
Relative to male body mass, the testes of cane toads were larger in some sampling periods than others. This result is unsurprising, because most anurans display seasonal reproduction, e.g. cane toads in northeastern Australia [30]. Seasonal fluctuations in testis mass followed the same pattern across all regions. The more interesting result involves a geographical divergence in relative testis sizes: cane toads from the range-core (northeastern Australia) had testes greater than 30% heavier (relative to body mass) than did conspecifics from newly invaded regions of northwestern and southeastern Australia. Although each of these three regions is thousands of kilometres from the others, the toad invasion of Australia is relatively recent (beginning in 1935) and hence, those divergences have arisen over a brief period. Although intraspecific geographical variation in relative testis sizes has been reported in other amphibians, those changes presumably have occurred over much longer periods [31–33].
Our sampling design does not unequivocally identify invasion stage as the causal factor for shifts in gonad size. The three areas that we sampled differ in many respects, including in climatic conditions [3], and it remains possible that conditions in both southeastern and northwestern Australia favour (or generate) smaller testes. However, we know of no obvious shared factor between those very different locations. The shifts in testis size might be due to evolutionary processes (i.e. to be heritable) or might be driven by phenotypic plasticity [34]. Again, we cannot distinguish the relative importance of those two processes from our samples. Nonetheless, we note that food is likely to be super-abundant at the invasion front (because of lowered intraspecific competition [35]) and hence, we might expect toads at these sites to be better-fed and thus, to be able to invest more into testis growth [36,37]. However, toads at the invasion front had smaller not larger testes.
As noted in the Introduction, we might expect smaller testes at the invasion front for several reasons. A trade-off between investment into reproduction versus the rate of dispersal is plausible, especially given that low rates of reproduction also typify female cane toads at the invasion front [25]. The actual energy investment into large versus small testes is trivial overall (less than 0.7% of body mass), but a larger testis may produce more testosterone, translating into a greater investment of time and energy into reproductive activities. Such activities could be far riskier, more energetically expensive and less compatible with rapid dispersal, than the simple allocation of nutrients to enlargement of the gonads. Opposing the ‘dispersal versus reproduction’ trade-off idea, however, is the fact that dispersal rates are high at the northwestern front only; toads on the southeastern front may not disperse any faster than do range-core individuals [22].
Could higher densities of toads in the range-core impose selection for larger testes? The possibility that increased opportunities for matings in rapid succession favour larger testes (to avoid sperm depletion [38]) seems unlikely. Kruse & Mounce [39] reported that male American toads (Bufo americanus) rarely mate more than once per season but are capable of fertilizing five clutches in successive days. Larger testes in range-core toads also cannot be adaptive to larger clutch sizes (as posited by Emerson [40] for interspecific comparisons within anurans) because in Australian cane toads, clutches are smaller in the range-core (J DeVore, S Ducatez and M Crossland 2017, personal communication). A final and more plausible possibility is that (as in other anurans [7]), high population densities of toads in range-core areas increase the intensity of sperm competition [41,42], which in turn selects for males that can produce more sperm.
In summary, our data strongly support the prediction that evolutionary pressures at an expanding range edge will result in smaller testes than in range-core regions, but we are unable to unambiguously tease apart the evolutionary forces at work in this situation. Neither can we identify the direction of change (i.e. have Australian cane toads exhibited a reduction in testis size at the range edge, or an increase in testis size at the range-core, or both of these changes?) because we lack data on the ancestral condition (testis size in native-range cane toads). Future work could usefully document the situation in other parts of the cane toad's extensive geographical range. It would also be of great interest to document mating systems of cane toads across their Australian range, to clarify the degree to which larger testes (and potentially, the behavioural as well as sperm-production consequences of that increased testicular volume) might influence male reproductive success—and perhaps, do so differently in different parts of the continent.
Acknowledgements
We thank G. Clarke, M. Crossland, M. Greenlees, J. DeVore, S. Ducatez, S. McCann, L. Pettit and C. Whittington for help with animal collection, and C. Bezzina and M. Elphick for help with laboratory and husbandry work.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.nt8705p [43]
Authors' contributions
C.R.F. and R.S. both contributed to the conception of the study, and analysis and interpretation of data; C.R.F. gathered the data, C.R.F. and R.S. drafted the article. Both authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
The work was supported by Australian Research Council grant no. FL120100074 to R.S.
References
- 1.Berggren H, Tinnert J, Forsman A. 2012. Spatial sorting may explain evolutionary dynamics of wing polymorphism in pygmy grasshoppers. J. Evol. Biol. 25, 2126–2138. ( 10.1111/j.1420-9101.2012.02592.x) [DOI] [PubMed] [Google Scholar]
- 2.Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proc. Natl Acad Sci. USA 108, 5708–5711. ( 10.1073/pnas.1018989108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosmala G, Brown GP, Christian K, Hudson CM, Shine R. 2018. The thermal dependency of locomotor performance evolves rapidly within an invasive species. Ecol. Evol. 8, 4403–4408. ( 10.1002/ece3.3996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips BL. 2009. The evolution of growth rates on an expanding range edge. Biol. Lett. 5, 802–804. ( 10.1098/rsbl.2009.0367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 6.Jennions MD, Passmore NI. 1993. Sperm competition in frogs: testis size and a ‘sterile male’ experiment on Chiromantis xerampelina (Rhacophoridae). Biol. J. Linn. Soc. 50, 211–220. ( 10.1111/j.1095-8312.1993.tb00927.x) [DOI] [Google Scholar]
- 7.Byrne PG, Roberts JD, Simmon LW. 2002. Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 15, 347–355. ( 10.1046/j.1420-9101.2002.00409.x) [DOI] [Google Scholar]
- 8.Harcourt AH, Harvey PH, Larson SG, Short RV. 1981. Testis weight, body weight and breeding system in primates. Nature 293, 55–57. [DOI] [PubMed] [Google Scholar]
- 9.Harcourt AH, Purvis A, Liles L. 1995. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct. Ecol. 9, 468–476. ( 10.2307/2390011) [DOI] [Google Scholar]
- 10.Heske EJ, Ostfeld RS. 1990. Sexual dimorphism in size, relative size of testes, and mating systems in North American voles. J. Mammal. 71, 510–519. ( 10.2307/1381789) [DOI] [Google Scholar]
- 11.Møller AP. 1991. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 137, 882–906. [Google Scholar]
- 12.Møller AP, Briskie JV. 1995. Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav. Ecol. Sociobiol. 36, 357–365. ( 10.1007/BF00167797) [DOI] [Google Scholar]
- 13.Kahrl AF, Cox CL, Cox RM. 2016. Correlated evolution between targets of pre- and postcopulatory sexual selection across squamate reptiles. Ecol. Evol. 6, 6452–6459. ( 10.1002/ece3.2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao WB, Mi ZP, Zhou CQ, Jin L, Han X, Lou SL, Ma J. 2011. Relative testis size and mating systems in anurans: large testis in multiple-male mating in foam-nesting frogs. Anim. Biol. 61, 225–238. ( 10.1163/157075511X570312) [DOI] [Google Scholar]
- 15.Roberts JD, Byrne PG. 2011. Polyandry, sperm competition, and the evolution of anuran amphibians. In Advances in the study of behaviour, vol. 43 (eds Brockmann HJ, Roper TJ, Naguib M, Mitani JC, Simmons LW), pp. 1–53. Oxford, UK: Elsevier. [Google Scholar]
- 16.Stockley P, Gage MJG, Parker GA, Møller AP. 1997. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 149, 933–954. ( 10.1086/286031) [DOI] [PubMed] [Google Scholar]
- 17.Awata S, Takeyama T, Makino Y, Kitamura Y, Kohda M. 2008. Cooperatively breeding cichlid fish adjust their testis size but not sperm traits in relation to sperm competition risk. Behav. Ecol. Sociobiol. 62, 1701 ( 10.1007/s00265-008-0598-0) [DOI] [Google Scholar]
- 18.Hosken DJ, Ward PI. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13. ( 10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 19.Simmons LW, García-González F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591. ( 10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 20.Cartar RV. 1985. Testis size in sandpipers: the fertilization frequency hypothesis. Naturwissenschaften 72, 157–158. ( 10.1007/BF00490407) [DOI] [Google Scholar]
- 21.Burton OJ, Phillips BL, Travis JM. 2010. Trade-offs and the evolution of life-histories during range expansion. Ecol. Lett. 13, 1210–1220. ( 10.1111/j.1461-0248.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 22.Urban MC, Phillips BL, Skelly DK, Shine R. 2008. A toad more traveled: the heterogeneous invasion dynamics of cane toads in Australia. Am. Nat. 171, E134–E148. ( 10.1086/527494) [DOI] [PubMed] [Google Scholar]
- 23.Hudson CM, McCurry MR, Lundgren P, McHenry CR, Shine R. 2016. Constructing an invasion machine: the rapid evolution of a dispersal-enhancing phenotype during the cane toad invasion of Australia. PLoS ONE 11, e0156950 ( 10.1371/journal.pone.0156950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCann SM, Kosmala GK, Greenlees MJ, Shine R. 2018. Physiological plasticity in a successful invader: rapid acclimation to cold occurs only in cool-climate populations of cane toads (Rhinella marina). Conserv. Physiol. 6, cox072 ( 10.1093/conphys/cox072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson CM, Phillips BL, Brown GP, Shine R. 2015. Virgins in the vanguard: low reproductive frequency in invasion-front cane toads. Biol. J. Linn. Soc. 116, 743–747. ( 10.1111/bij.12618) [DOI] [Google Scholar]
- 26.Gonzalez-Bernal E, Greenlees MJ, Brown GP, Shine R. 2016. Toads in the backyard: why do invasive cane toads (Rhinella marina) prefer buildings to bushland? Popul. Ecol. 58, 293–302. ( 10.1007/s10144-016-0539-0) [DOI] [Google Scholar]
- 27.Kouba AJ, Vance CK. 2009. Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod. Fert. Dev. 21, 719–737. ( 10.1071/RD09038) [DOI] [PubMed] [Google Scholar]
- 28.Devlaming V, Grossman G, Chapman F. 1982. On the use of the gonosomatic index. Comp. Biochem. Physiol. A 73, 31–39. ( 10.1016/0300-9629(82)90088-3) [DOI] [Google Scholar]
- 29.Packard GC, Boardman TJ. 1988. The misuse of ratios, indices, and percentages in ecophysiological research. Physiol. Zool. 61, 1–9. [Google Scholar]
- 30.Yasumiba K, Alford RA, Schwarzkopf L. 2016. Seasonal reproductive cycles of cane toads and their implications for control. Herpetologica 72, 288–292. ( 10.1655/Herpetologica-D-15-00048.1) [DOI] [Google Scholar]
- 31.Hettyey A, Laurila A, Herczeg G, Jönsson KI, Kovács T, Merilä J. 2005. Does testis weight decline towards the Subarctic? A case study on the common frog, Rana temporaria. Naturwissenschaften 92, 188–192. ( 10.1007/s00114-005-0607-3) [DOI] [PubMed] [Google Scholar]
- 32.Chen W, Pike DA, He D, Wang Y, Ren L, Wang X, Fan X, Lu X. 2014. Altitude decreases testis weight of a frog (Rana kukunoris) on the Tibetan plateau. Herpetol. J. 24, 183–188. [Google Scholar]
- 33.Jin L, Yang SN, Liao WB, Lüpold S. 2016. Altitude underlies variation in the mating system, somatic condition, and investment in reproductive traits in male Asian grass frogs (Fejervarya limnocharis). Behav. Ecol. Sociobiol. 70, 1197–1208. ( 10.1007/s00265-016-2128-9) [DOI] [Google Scholar]
- 34.Stuart KC, Shine R, Brown GP. 2018. Proximate mechanisms underlying the rapid modification of phenotypic traits in cane toads (Rhinella marina) across their invasive range within Australia. Biol. J. Linn. Soc. 126, 68–79. ( 10.1093/biolinnean/bly150) [DOI] [Google Scholar]
- 35.Brown GP, Kelehear C, Shine R. 2013. The early toad gets the worm: cane toads at an invasion front benefit from higher prey availability. J. Anim. Ecol. 82, 854–862. ( 10.1111/1365-2656.12048) [DOI] [PubMed] [Google Scholar]
- 36.Ghorbankhani F, Souri M, Moeini MM, Mirmahmoudi R. 2015. Effect of nutritional state on semen characteristics, testicular size and serum testosterone concentration in Sanjabi ram lambs during the natural breeding season. Anim. Reprod. Sci. 153, 22–28. ( 10.1016/j.anireprosci.2014) [DOI] [PubMed] [Google Scholar]
- 37.Vega-Trejo R, Jennions MD, Head ML. 2016. Are sexually selected traits affected by a poor environment early in life? BMC Evol. Biol. 16, 263 ( 10.1186/s12862-016-0838-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hettyey A, Vagi B, Hevizi G, Toeroek J. 2009. Changes in sperm stores, ejaculate size, fertilization success, and sexual motivation over repeated matings in the common toad, Bufo bufo (Anura: Bufonidae). Biol. J. Linn. Soc. 96, 361–371. ( 10.1111/j.1095-8312.2008.01126.x) [DOI] [Google Scholar]
- 39.Kruse KC, Mounce M. 1982. The effects of multiple mating on fertilization capability in male American toads (Bufo americanus). J. Herpetol. 16, 410–412. [Google Scholar]
- 40.Emerson SB. 1997. Testis size variation in frogs: testing the alternatives. Behav. Ecol. Sociobiol. 41, 227–235. ( 10.1007/s002650050383) [DOI] [Google Scholar]
- 41.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 42.Parker G. 2016. The evolution of expenditure on testes. J. Zool. 298, 3–19. [Google Scholar]
- 43.Friesen CR, Shine R. 2019. Data from: At the invasion front, male cane toads (Rhinella marina) have smaller testes Dryad Digital Repository. ( 10.5061/dryad.nt8705p) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Friesen CR, Shine R. 2019. Data from: At the invasion front, male cane toads (Rhinella marina) have smaller testes Dryad Digital Repository. ( 10.5061/dryad.nt8705p) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.nt8705p [43]


