Short abstract
Synovitis contributes to temporomandibular joint (TMJ) pain, nevertheless, the detailed nociceptive mechanism remains unclear. In this study, a rat model of TMJ synovitis was induced by intra-articular injection with complete Freund’s adjuvant (CFA). After CFA-induced synovitis, pain behaviors were observed. Then, TMJ, trigeminal ganglion, and trigeminal nucleus caudalis (TNC) tissues were collected, and immunohistochemistry was used to detect the expression of substance P (SP) and protein gene product 9.5 (PGP9.5) in the synovium tissue. Furthermore, the gene expression level of SP and PGP9.5 in synovium was detected by reverse transcription-polymerase chain reaction (RT-PCR). Afterwards, the expression of SP in the trigeminal ganglion and TNC and c-fos in the TNC was detected by immunohistochemistry. Compared with the control group, the expression of SP and PGP9.5 nerve fibers density and gene levels of them in the synovium tissue were significantly increased in CFA-induced TMJ synovitis rats. Similarly, SP expression in the trigeminal ganglion and TNC, and c-fos expression in the TNC were also obviously increased in CFA-induced TMJ synovitis rats. Collectively, CFA-induced rat TMJ synovitis resulted in obvious pain. This nociceptive reaction could be attributed to the augmented quantity of SP and PGP9.5 positive-stained nerve fibers distributed in the inflammatory synovium as well as enhanced SP expression in the trigeminal ganglion and TNC tissue. c-fos expression in the rat TNC illustrates CFA-induced TMJ synovitis can evoke the acute pain.
Keywords: synovitis, trigeminal ganglion, trigeminal nucleus caudalis, substance P, c-fos, complete Freund’s adjuvant
Introduction
Clinical symptoms of temporomandibular disorders (TMD) are characterized by joint pain, joint noise, and jaw movement disorder. Among them, pain is frequently the predominant reason for patients’ visits. Pain may originate from temporomandibular joint (TMJ) tissues rich in peripheral nerves, such as muscle, ligaments, synovium, and subchondral bone.1–3 Recently, inflammatory reaction in synovium tissues of patients with TMJ pain is detected through arthroscopic and histopathological investigations. Tissue inflammation is able to change the properties of sensory pathways and lead to hyperalgesia.4 To simulate the painful circumstance of human TMJ to investigate the specific nociceptive molecules, an animal model of synovitis induced by complete Freund’s adjuvant (CFA) injection has been recommended, as it has unique advantages in repeatability and accessibility. Among these CFA-induced TMJ inflammation studies, the inflammatory mediators such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and inducible nitric oxide synthase (iNOS) are detected in the synovium.5 However, based on our knowledge, there have been no studies demonstrating the relationship between nociceptive behaviors and TMJ synovitis induced by CFA injection.
As a member of tachykinin family, the neuropeptide substance P (SP) plays an important role in the modulation and transmission of neuronal pathways related to pain sensation, promoting wound healing and accelerating vasodilation.6 Under the environment of rheumatic arthritis, peripheral nerve fibers in synovium are able to synthesize and release SP, which causes local inflammatory pain.7 Concerning the potential role of SP in nociceptive tissues, it is intriguing to explore the expression and distribution of SP in inflamed TMJ synovium, although it has not been detected yet.
Meanwhile, nociceptive stimulation signals of peripheral nerve fibers distributed in the synovium are transmitted to the primary afferent neurons in the trigeminal ganglion (TG) and conveyed to the trigeminal nucleus caudalis (TNC) of brainstem. TG mainly receives pain signals from the mouth and maxillofacial region as peripheral nociceptors activated by chemical and mechanical stimuli.8 SP can be synthesized and released from primary afferent neurons in TG.9 Especially in the spinal dorsal horn, SP is able to produce a persistent spontaneous pain and hyperalgesia.10,11 Nevertheless, whether TMJ synovitis can affect SP expression in primary afferent neurons of TG remains largely unknown. Similarly, as an important part of the central nervous system, TNC plays a key role in the transmission of pain signals. However, the expression of SP in TNC during TMJ synovitis is still unclear.
Therefore, this study is first to demonstrate the relationship between nociceptive behaviors and rat TMJ synovitis induced by CFA injection. Next, it is aimed to investigate the expression of SP in synovium, TG, and TNC. Besides, c-fos immunoreactivity was widely used as a sign of neuronal activity of acute pain in the TNC.12,13 This study is also to detect whether c-fos can be expressed in TNC in this inflamed model.
Materials and Methods
Male Sprague-Dawley rats (eight-week-old) obtained from the Experimental Animal Centre of Hubei Province were used. All animal experimental procedures were approved by the Ethics Committee for Animal Research, Wuhan University, China.
Induction of TMJ Inflammation
Ninety-six Sprague-Dawley rats were randomly assigned into control and the experimental groups, including 8-h, 16-h, 24-h, 48-h, one-week, two-week, three-week, and four-week groups. Initially, rats were anesthetized with light isoflurane. In the experimental groups, inflammation was induced by bilateral TMJ intra-articular administration with CFA (1:1 oil:saline emulsion, 50 µl; Sigma-Aldrich, St. Louis, MO, USA.), and the control group was received bilateral injections with 50 µl saline. According to Kameoka’s and Xu’s method,14,15 the anterosuperior portion of the zygomatic arch root was identified as the insertion point. The needle was percutaneously injected into the anterosuperior compartment of the TMJ and then advanced posteriorly to contact the edge of the anterolateral condyle.
Measurement of food intake
Previous study showed that food intake could be used as an indicator for TMJ pain.16,17 Food intake was measured daily after TMJ injection of each rat. As previously described, each rat was isolated in a cage with no food but only water for 15 h. To eliminate drinking influence, the rat was merely fed with food lasted 2 h, and the amount of eaten food was calculated as food intake.
Nociceptive behaviors assessment
As TMJ injection accomplished, the rats recovered from the anesthesia until fully awake, and then they were returned to a test chamber for nociceptive behaviors observation during a 45-min period which was consistent with previous studies.18,19 Video record was used to assess the spontaneous nociceptive behaviors. The recording time was divided into 15 blocks of 3 min, and a pain score (seconds) was quantified for each block by measuring the duration of time spent in rubbing the orofacial region, flinching with the head and shaking of the head. The quantitative measurement of CFA-induced TMJ nociception was evaluated together by their sum.
Measurement of head withdrawal threshold
The head withdrawal threshold was closely related with hyperalgesia of the orofacial region. The skin around the TMJ was stimulated by an electronic pain threshold detector von Frey filament (IITC Life Science, CA, USA), which was used to assess the minimum threshold force causing suddenly head withdrawal behavior. The head withdrawal threshold was counted at least five tests per joint for each rat at an interval of a few seconds. The lowest threshold force could lead to head withdrawal behavior indicative of nociceptive response. The average of these five values was identified as the withdrawal threshold.
Tissue harvest and processing
Rats from two groups were euthanized with overdosed isoflurane (n = 6). After 8-h, 16-h, 24-h, 48-h, one-week, two-week, three-week, and four-week postinjection, the TNC of brainstems was removed and then immediately fixed in 4% paraformaldehyde for 24 h at 4°C, following washed, gradient-dehydrated, and embedded in paraffin. At one week, TMJs were selected unilaterally for hematoxylin–eosin assessment. At week one, two, three, or four, TMJs were selected unilaterally for immunohistochemistry (IHC) assessment, and the contralateral TMJ synovium tissues were harvested for real-time quantitative PCR. The integrated TMJ tissues including synovial membrane, articular disc, cartilage, and mandible condyle were dissected and fixed in 4% paraformaldehyde and then demineralized in 10% EDTA. After decalcification, the TMJ tissues were gradient-dehydrated and embedded in paraffin. Meanwhile, after one-week, two-week, three-week, and four-week postinjection, the TG was removed and immediately fixed in 4% paraformaldehyde and then embedded in paraffin. Continuous sections at 4 µm thickness of all paraffin-embedded specimens were performed.
Immunohistochemistry
Immunohistochemical staining for CD34, c-fos, SP, and protein gene product 9.5 (PGP9.5) was performed individually and experimental method was the same as previous study.20 After dewaxed, rehydrated, and washed, the sections were then treated with pepsin (DIG-3009, Maixin, Fuzhou, China) to retrieve antigen at 37°C for 30 min and incubated with 0.3% hydrogen peroxide for 20 min, following handled with serum (Zhongshan Biotechnology, Beijing, China) for 30 min. The sections were then treated with rabbit anti-CD34 (1:3000; ab81289, Abcam, MA, USA), rabbit anti-c-fos (1:200; SC-52, Santa Cruz Biotechnology, CA, USA), mouse anti-SP (1:500; ab14184, Abcam, MA, USA), and rabbit anti-PGP9.5 (1:500; AB1761-1, Millipore, CA, USA) primary antibody overnight at 4°C. The sections were then washed thoroughly with PBS and incubated with immunohistochemical kit (Zhongshan Biotechnology, Beijing, China). The bound immunocomplex was visualized under a microscope by 3, 3-diaminobenzidine (Maixin, Fuzhou, China). Finally, the sections were counterstained with hematoxylin.
For measurement, five magnified visual fields (×400) of the brainstem, TMJ, and TG sections were randomly selected under an Olympus DP72 microscope by two inspectors. The means of the five measurements were considered as the value of each section. Five sections were randomly selected from each group.
Real-time polymerase chain reaction for the detection of SP and PGP9.5 in synovium
Total messenger RNA (mRNA) was extracted from the TMJ synovium tissues using a Trizol Kit (Invitrogen, CA, USA) according to the manufacturer’s instructions.21 First Strand cDNA Synthesis Kit (Thermo Scientific, Rockford, IL, USA) was applied to reversely transcribe total RNA into cDNA. For mRNA measurement, the primer sequences designed and synthesized by Biotech Company (Shanghai, China) of SP and PGP9.5 were as follows: GAPDH: Fwd 5′ AGA CAG CCG CAT CTT CTT GT, Rev 5′ TGA TGG CAA CAA TGT CCA CT; SP: Fwd 5′ TGG TCA GAT CTC TCA CAA AGG, Rev 5′ TGC ATT GCG CTT CTT TCA TA; PGP9.5: Fwd 5′ GCT TCG CCG ACG TGC TAG GG, Rev 5′ TTT TCA TGC TGG GCC GTG AGG G. Polymerase chain reaction was performed using a TaKaRa SYBR Premix ExTaq kit (TakaRa, Dalian, China) in accordance with the instruction of the manufacturer. The levels of SP and PGP9.5 mRNA were determined by calculating the density ratio of SP and PGP9.5 mRNA/GAPDH mRNA.
Statistical analysis
All data were presented in the form of the mean ± standard error of the mean. Data were calculated and visualized by using GraphPad Prism 6.0. Statistical analysis was carried out through one-way analysis of variance. A Student–Newman–Keuls posttest was performed and statistical significance was considered at p < 0.05.
Results
TMJ inflammation, nociceptive behaviors, and mechanical sensitivity assessment
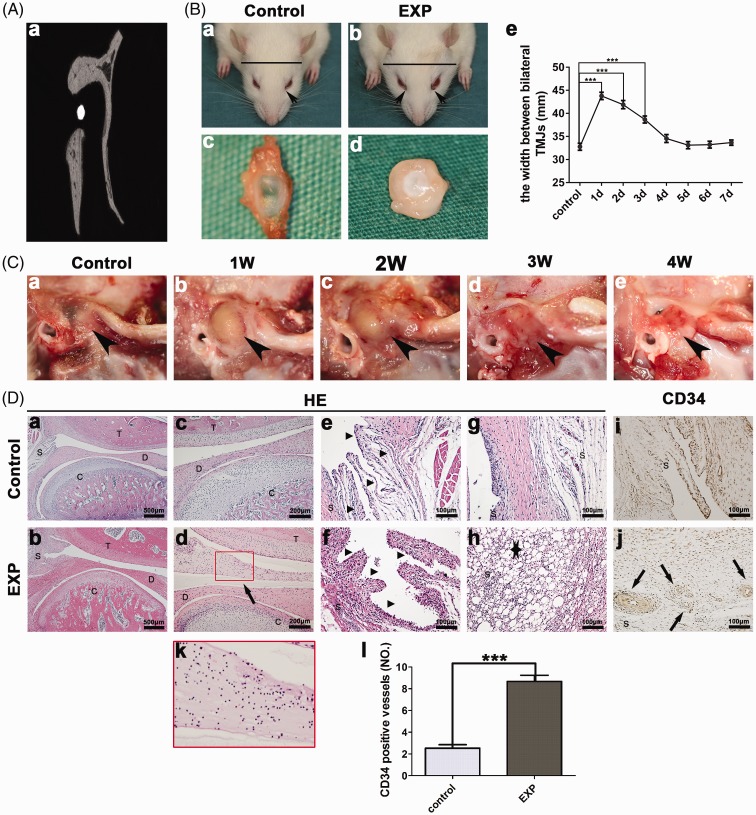
As shown in Figure 1(Aa), the needle was located between the condyle and zygomatic arch root. Moreover, 24 h after CFA injection, external sign of chromodacryorrhea appeared around eyelid and the linear head width between bilateral TMJs displayed remarkable increases (Figure 1(Bb)), but not in the control group (Figure 1(Ba)). As time went on, the width gradually reduced within seven days (*p < 0.05, **p < 0.01, ***p < 0.001) (Figure 1(Be)). In the control group, a normal TMJ disc was highly transparent and thin (Figure 1(Bc)), while an inflamed TMJ disc was thickened and malformed (Figure 1(Bd)). Compared with the control group, severe swelling of TMJ region was observed at one and two weeks after CFA injection and then gradually faded away at three and four weeks (Figure 1(C)). Among the four experimental groups, CFA-induced TMJ inflammation peaked at one week. Therefore, we selected the one-week experimental group for histology analyses. At one week after CFA injection, the TMJ articular upper cavity was full of inflammatory secretions (Figure 1(Dd)). Synovial lining cells were apparently hyperplasic and abundant mononucleated cells infiltrated into the synovium (Figure 1(Df)). A mass of lipid droplets (Figure 1(Dh)) and proliferative blood vessels stained by CD34 (Figure 1(Dj)) were also present in the inflamed synovium. In the control group, no obvious inflammation was observed (Figure 1(Da), (Dc), (De), (Dg), (Di)).
Figure 1.
Anterior superior puncture technique and inflammation of TMJ. (A) Image showed the needle tip in the TMJ cavity (a). (B) Compared with the control group (a and c), obvious chromodacryorrhea around the eyelid was appeared (b) (arrow) and TMJ disc was thickened (d) in the experiment group. The linear head width between bilateral TMJs displayed remarkable increases at 1d (e). (C) Severe swelling of TMJ region was detected at one and two weeks after CFA injection (b and c) (black arrow). (D) In contrast with the control group (c, e, g, and i), upper articular cavity was filled with inflammatory secretions (d), synovial cells apparent hyperplasia (f), lipid droplets (h) (star), and proliferative blood vessels stained by CD34 (j) in the experiment group. The red squares in (d) are magnified in (k). Comparison in the CD34 positive vessels between the experimental and control groups (l) (*p < 0.05, **p < 0.01, ***p < 0.001). EXP: one-week experiment group; T: temporal bone; D: articular disc; C: condyle; S: synovium.
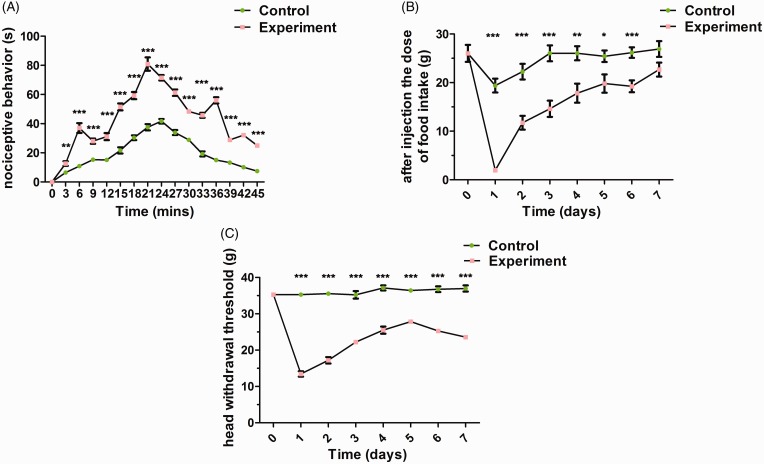
To examine whether CFA could induce TMJ inflammatory pain, nociceptive behaviors and mechanical sensitivity were analyzed. Compared with the control group, repetitious nociceptive behaviors such as rubbing the orofacial region, flinching with the head and shaking of the head occurred after CFA administration during a 45-min period. The sum of nociceptive behaviors was used as an index of TMJ pain and peaked at 21-min post-TMJ CFA injection (*p < 0.05, **p < 0.01, ***p < 0.001) (Figure 2(A)). Mechanical sensitivity was analyzed by measuring food intake (Figure 2(B)) and head withdrawal threshold (Figure 2(C)). Before TMJ injection, there were no differences among all groups. However, one day after CFA injection, food intake and head withdrawal threshold significantly decreased in CFA-induced TMJ synovitis rats, and then gradually increased within seven days, but not in the control group (*p < 0.05, **p < 0.01, ***p < 0.001).
Figure 2.
Nociceptive behaviors and mechanical sensitivity assessment. (A) Compared with the control group, higher frequency of nociceptive behaviors was exhibited after CFA administration into rat TMJs during a 45-min period. Mechanical sensitivity was analyzed by measuring food intake (B) and head withdrawal threshold (C). Compared with the control group, food intake and head withdrawal threshold started significantly decreases at one day after CFA injection (*p < 0.05, **p < 0.01, ***p < 0.001).
Nerve fibers of SP and PGP 9.5 expression in the synovium
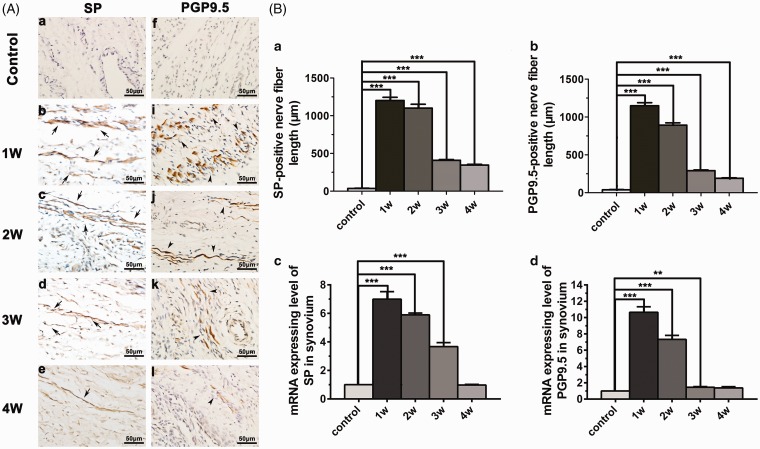
SP immunoreactive nerve fibers were detected in the TMJ synovium. Nerve fibers remained only at low levels in the control group. In contrast, an abundant sprouting of SP positive nerve fibers was detected in the synovium of inflamed TMJ (Figure 3(A)). SP positive nerve fibers expression levels increased in one- and two-week experimental group and then gradually decreased at three and four weeks. Consistently, the SP mRNA expression was significantly increased in the inflamed TMJ compared with control group (Figure 3(B)).
Figure 3.
Nerve fibers of SP and PGP 9.5 expression in the synovium. (A) Compared with the control group, an abundant sprouting of SP and PGP 9.5 nerve fibers was detected in the synovium in the experimental group. (B) SP and PGP 9.5 nerve fibers expression levels increased in the one- and two-week experimental group and then gradually decreased at three and four weeks. The gene levels of SP and PGP 9.5 mRNA expression were consistent with those of immunohistochemistry (*p < 0.05, **p < 0.01, ***p < 0.001). SP: substance P; mRNA: messenger RNA; PGP 9.5: protein gene product 9.5.
PGP 9.5, a unique marker of nerve fibers, was primary protein component in the neuronal cytoplasm.22,23 Compared with the control group, an abundant sprouting of PGP 9.5 positive nerve fibers was detected in the synovium of inflamed TMJ (Figure 3(A)). The distribution of PGP 9.5 positive nerve fibers and gene level were similar to those of SP in the synovium (Figure 3(B)).
SP expression in the TG
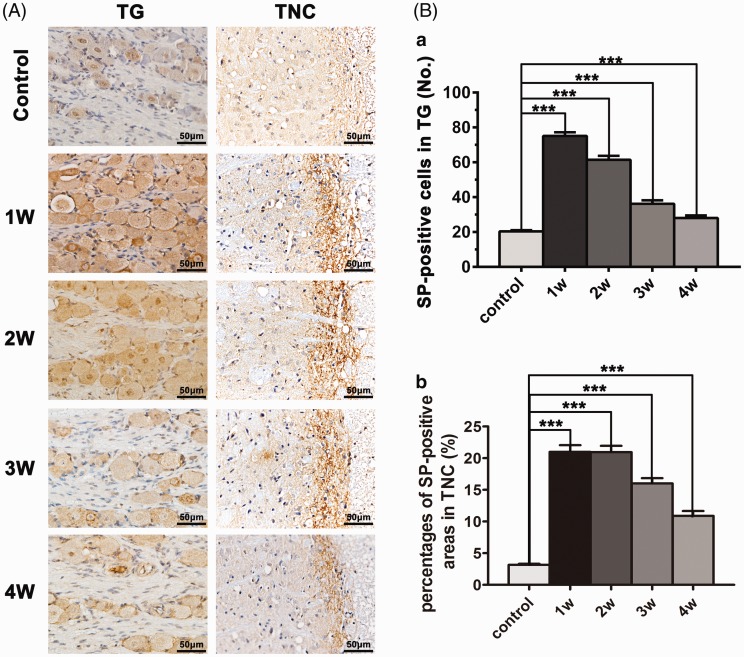
SP expression of the TG was significantly elevated in CFA-induced TMJ synovitis rats compared with that in the control group (Figurer 4(A)). In CFA-induced TMJ synovitis rats, SP expression in the TG increased and peaked at one week and then gradually decreased at three and four weeks (Figure 4(Ba)).
Figure 4.
SP expression in the TG and TNC. (A) Compared with the control group, SP expression in the TG and TNC was upregulated in the experimental group. (B) In the experimental group, SP expression in the TG and TNC increased and peaked at one week and then gradually decreased at three and four weeks (*p < 0.05, **p < 0.01, ***p < 0.001). TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis.
SP and c-fos expression in the TNC
SP protein expression located in the spinal trigeminal tract nerve fibers and the gelatinous layer was examined and widely observed in endochylema of the TNC (Figure 4(A)). In CFA-induced TMJ synovitis rats, the density of SP-immunoreactive fibers in the TNC was obviously higher especially at one and two weeks than that in the control group (Figure 4(Bb)).
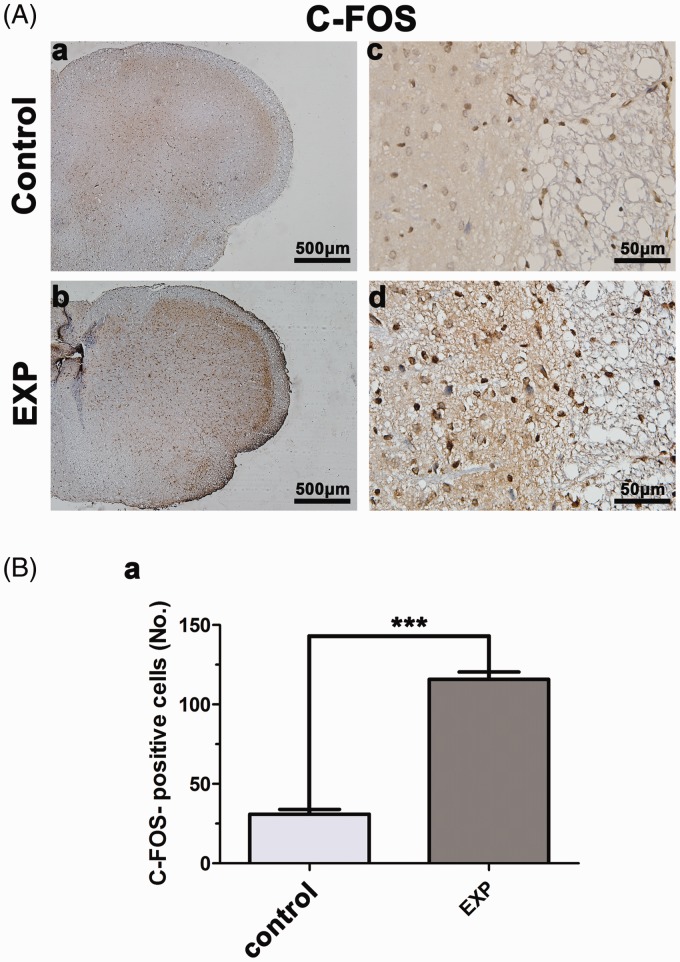
c-fos protein expression in the TNC has been regarded as a sign of neuronal activity of acute pain.24,25 There was a distinct difference in the number of c-fos positive neuronal nuclei in the TNC between CFA-induced TMJ synovitis rats and the control group (Figure 5(A)). In the control group, c-fos positive cells were barely detected in the TNC. On the contrary, an increased quantity of c-fos positive cells were observed in the TNC when CFA was injected in the rat TMJ at 24 h, but not at 8 h, 16 h and 48 h (data not shown) (Figure 5(B)).
Figure 5.
c-fos expression in the trigeminal nucleus caudalis. (A and B) In the control group, few c-fos positive cells were detected in the TNC. In contrast, 24 h after CFA injection, an increased quantity of c-fos positive cells was observed in the TNC. EXP: experiment (*p < 0.05, **p < 0.01, ***p < 0.001).
Discussion
Intra-articular injection of CFA was a simple and reproducible method to induce joint inflammation.5 Inflammation was believed to be an important risk factor in TMJ pain, because the concomitant pro-inflammatory factors, including IL-1β, TNF-α, and prostaglandin E2 were upregulated in inflammation and could lead to hyperalgesia.26,27 Furthermore, inflammatory mediators such as IL-1β and TNF-α were present at high levels in the synovium tissues of rats or patients with TMJ pain and hyperalgesia.28,29 In this study, obvious synovitis such as inflammatory mononuclear cells, synovial cells hyperplasia, abundant lipid droplets, and vascular proliferation presented in the CFA-induced TMJ synovitis rats, consistent with previous study.30 Moreover, 24 h after CFA injection, external sign of chromodacryorrhea appeared around eyelid and the swelling of TMJ region was observed. Previous study showed that food intake was related with TMJ pain and could be used as an indicator for TMJ pain,16,17 and head withdrawal threshold was closely related with hyperalgesia of the orofacial region. As shown in this study, the low food intake and head withdrawal threshold indicated that a severe inflammatory pain was produced by CFA injection into rat TMJ.
SP has been considered as a significant nociceptive marker of peripheral sensory nerve fibers.31–33 In this study, the total length density of SP immunoreactivity nerve fibers increased in the TMJ synovium of arthritic rats, compared with that in the control group. Meanwhile, previous study demonstrated that in the inflammatory joint, nerves innervated in the synovium tissues were activated and sensitized by SP released from inflammatory and immune cells.34,35 In this study, an abundant sprouting of PGP 9.5 positive nerve fibers was detected in the synovium of inflamed TMJ compared with the control group, indicating that SP could lead to the nerve fibers infiltration in the synovitis tissues. Certainly, we cannot exclude the possibility that other nociceptive factors could induce nerve fibers infiltration. Researchers have demonstrated that nerve fibers infiltration is a key factor that leads to local tissues pain.2,3 Therefore, these findings suggest that SP release in the synovium tissues involved in the generation and maintenance of TMJ synovitis pain.
As nociceptive stimulation signals of peripheral nerve fibers distributed in the synovium were transmitted from TG to TNC.36,37 Hence, it is intriguing to explore whether TMJ synovitis can affect the SP expression in the TG and TNC as well. Our results showed that CFA-induced TMJ synovitis results in upregulation of SP both in TG and TNC of rats. Likewise, in an occlusal trauma rat model, increased SP expression was detected in the TNC.38 This increased SP expression in TG and TNC was accompanied with the lower head withdrawal threshold, indicating that the influence of nociceptive stimulus on both peripheral and central nervous system activation.
c-fos protein was a well-established marker for neuronal activation of acute pain in the central neural system.24,25 In response to various acute pain stress, c-fos protein can be rapidly induced in several brain regions, such as the hypothalamus, limbic forebrain, and central amygdala.39 Under acute pain stress, hypothalamus showed a peak in c-fos expression at 4 h after a formalin injection into the rat hind paw.40 In this study, the quantity of c-fos positive neurons significantly increased in the TNC after CFA injection into rat TMJs at 24 h, whereas few was detected in the control group, indicating that acute pain occurs when rat TMJ synovitis is induced by CFA injection. It was worth noting that for CFA-induced rat TMJ synovitis, c-fos expression was detected in TNC at 24 h, but not at 8 h, 16 h, and 48 h. Other studies showed that c-fos expression could be rapidly increased in the dorsal horn at as early as 1.5 h, which was induced by capsaicin, formalin, or CFA injection into the rat hind paw.41 This discrepancy may be resulted from the different induction medium and dosage used.
Collectively, CFA-induced rat TMJ synovitis resulted in obvious pain. This nociceptive reaction could be attributed to the augmented quantity of SP and PGP9.5 positive-stained nerve fibers distributed in the inflammatory synovium as well as enhanced SP expression in the TG and TNC tissue. c-fos expression in the rat TNC illustrates that CFA-induced TMJ synovitis could evoke the acute pain. These present results suggest that peripheral and central SP expression may be involved in the generation and maintenance of TMJ synovitis pain induced by CFA. To date, few studies of the TMJ pain have been performed in inflamed TMJ models. Improved understanding of inflammation and TMJ pain will enrich our knowledge of pathogenesis of TMJ pain and help design clinically relevant strategies for TMJ pain. The mechanisms of inflammation in the TMJ pain should further be clarified in the following studies.
Author Contributions
Xing Long and Jin Ke had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Xing Long and Jin Ke contributed to study design. Liqin Xu, Henghua Jiang, Yaping Feng, and Pinyin Cao contributed to acquisition of data. Xing Long, Jin Ke, and Liqin Xu were involved in manuscript preparation. Liqin Xu, Henghua Jiang, and Jin Ke contributed to statistical analysis.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants (Nos. 81600889, 8177041189, and 81870789) from the National Science Foundation of China.
References
- 1.Suri S, Gill SE, de Camin SM, Wilson D, McWilliams DF, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis 2007; 66: 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez-Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther 2012; 14: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grassel S. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res Ther 2014; 16: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeda M, Takahashi M, Matsumoto S. Suppression of neurokinin-1 receptor in trigeminal ganglia attenuates central sensitization following inflammation. J Peripher Nerv Syst 2012; 17: 169–181. [DOI] [PubMed] [Google Scholar]
- 5.Wang XD, Kou XX, Mao JJ, Gan YH, Zhou YH. Sustained inflammation induces degeneration of the temporomandibular joint. J Dent Res 2012; 91: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao P, Waxman SG, Hains BC. Sodium channel expression in the ventral posterolateral nucleus of the thalamus after peripheral nerve injury. Mol Pain 2006; 2: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu GY, Chen L, Fei H, Su YC, Zhu GX, Chen YJ. Psychological stress may contribute to temporomandibular joint disorder in rats. J Surg Res 2013; 183: 223–229. [DOI] [PubMed] [Google Scholar]
- 8.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol 2011; 97: 179–206. [DOI] [PubMed] [Google Scholar]
- 9.Igawa K, Funahashi H, Miyahara Y, Naono-Nakayama R, Matsuo H, Yamashita Y, Sakoda S, Nishimori T, Ishida Y. Distribution of hemokinin-1 in the rat trigeminal ganglion and trigeminal sensory nuclear complex. Arch Oral Biol 2017; 79: 62–69. [DOI] [PubMed] [Google Scholar]
- 10.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 2004; 361: 184–187. [DOI] [PubMed] [Google Scholar]
- 11.Caudle RM, King C, Nolan TA, Suckow SK, Vierck CJ, Neubert JK. Central sensitization in the trigeminal nucleus caudalis produced by a conjugate of substance P and the A subunit of cholera toxin. J Pain 2010; 11: 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knyihar-Csillik E, Toldi J, Krisztin-Peva B, Chadaide Z, Nemeth H, Fenyo R, Vecsei L. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci Lett 2007; 418: 122–126. [DOI] [PubMed] [Google Scholar]
- 13.Knyihar-Csillik E, Toldi J, Mihaly A, Krisztin-Peva B, Chadaide Z, Nemeth H, Fenyo R, Vecsei L. Kynurenine in combination with probenecid mitigates the stimulation-induced increase of c-fos immunoreactivity of the rat caudal trigeminal nucleus in an experimental migraine model. J Neural Transm 2007; 114: 417–421. [DOI] [PubMed] [Google Scholar]
- 14.Kameoka S, Matsumoto K, Kai Y, Yonehara Y, Arai Y, Honda K. Establishment of temporomandibular joint puncture technique in rats using in vivo micro-computed tomography (R_mCT (R)). Dentomaxillofac Rad 2010; 39: 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu LQ, Guo HL, Li C, Xu J, Fang W, Long X. A time-dependent degeneration manner of condyle in rat CFA-induced inflamed TMJ. Am J Transl Res 2016; 8: 556–567. [PMC free article] [PubMed] [Google Scholar]
- 16.Harper RP, Kerins CA, Talwar R, Spears R, Hutchins B, Carlson DS, Mclntosh JE, Bellinger LL. Meal pattern analysis in response to temporomandibular joint inflammation in the rat. J Dent Res 2000; 79: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 17.Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH, Ma XC. 17-beta-estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci 2010; 30: 8710–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roveroni RC, Parada CA, Cecilia M, Veiga FA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the TMJ formalin test. Pain 2001; 94: 185–191. [DOI] [PubMed] [Google Scholar]
- 19.Desidera AC, Nascimento GC, Gerlach RF, Leite-Panissi C. Laser therapy reduces gelatinolytic activity in the rat trigeminal ganglion during temporomandibular joint inflammation. Oral Dis 2015; 21: 652–658. [DOI] [PubMed] [Google Scholar]
- 20.Cao P, Feng Y, Deng M, Li J, Cai H, Meng Q, Fang W, Li Y, Ke J, Long X. MiR-15b is a key regulator of proliferation and apoptosis of chondrocytes from patients with condylar hyperplasia by targeting IGF1, IGF1R and BCL2. Osteoarthr Cartilage 2019; 27: 336–346. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Liu Y, Deng M, Li J, Cai H, Meng Q, Fang W, Long X, Ke J. MicroRNA221-3p modulates Ets-1 expression in synovial fibroblasts from patients with osteoarthritis of temporomandibular joint. Osteoarthritis Cartilage 2016; 24: 2003–2011. [DOI] [PubMed] [Google Scholar]
- 22.Jochmann E, Boettger MK, Anand P, Schaible HG. Antigen-induced arthritis in rats is associated with increased growth-associated protein 43-positive intraepidermal nerve fibres remote from the joint. Arthritis Res Ther 2015; 17: 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galosi E, La Cesa S, Di Stefano G, Karlsson P, Fasolino A, Leone C, Biasiotta A, Cruccu G, Truini A. A pain in the skin. Regenerating nerve sprouts are distinctly associated with ongoing burning pain in patients with diabetes. Eur J Pain 2018; 22: 1727–1734. [DOI] [PubMed] [Google Scholar]
- 24.Vincent K, Wang SF, Laferriere A, Kumar N, Coderre TJ. Spinal intracellular metabotropic glutamate receptor 5 (mGluR5) contributes to pain and c-fos expression in a rat model of inflammatory pain. Pain 2017; 158: 705–716. [DOI] [PubMed] [Google Scholar]
- 25.Lv SY, Cui B, Yang Y, Du H, Zhang X, Zhou Y, Ye W, Nie X, Li Y, Wang Q, Chen WD, Wang YD. Spexin/NPQ induces FBJ osteosarcoma oncogene (fos) and produces antinociceptive effect against inflammatory pain in the mouse model. Am J Pathol 2019; 189: 886–899. [DOI] [PubMed] [Google Scholar]
- 26.Li HX, Xie SJ, Qi YL, Li HZ, Zhang R, Lian YY. TNF- increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp Ther Med 2018; 16: 4737–4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseuguem PP, Ngangoum DAM, Pouadjeu JM, Piegang BN, Sando Z, Kolber BJ, Tidgewell KJ, Nguelefack TB. Aqueous and methanol extracts of Paullinia pinnata L. (Sapindaceae) improve inflammation, pain and histological features in CFA-induced mono-arthritis: evidence from in vivo and in vitro studies. J Ethnopharmacol 2019; 236: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanelatto FB, Dias EV, Teixeira JM, Sartori CR, Parada CA, Tambeli CH. Anti-inflammatory effects of propranolol in the temporomandibular joint of female rats and its contribution to antinociceptive action. Eur J Pain 2018; 22: 572–582. [DOI] [PubMed] [Google Scholar]
- 29.Ibi M. Inflammation and temporomandibular joint derangement. Biol Pharm Bull 2019; 42: 538–542. [DOI] [PubMed] [Google Scholar]
- 30.Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum 2010; 62: 647–657. [DOI] [PubMed] [Google Scholar]
- 31.Choi H, Kim DJ, Nam S, Lim S, Hwang JS, Park KS, Hong HS, Won Y, Shin MK, Chung E, Son Y. Substance P restores normal skin architecture and reduces epidermal infiltration of sensory nerve fiber in TNCB-induced atopic dermatitis-like lesions in NC/Nga mice. J Dermatol Sci 2018; 89: 248–257. [DOI] [PubMed] [Google Scholar]
- 32.Utsumi D, Matsumoto K, Tsukahara T, Amagase K, Tominaga M, Kato S. Transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1 contribute to the progression of colonic inflammation in dextran sulfate sodium-induced colitis in mice: links to calcitonin gene-related peptide and substance P. J Pharmacol Sci 2018; 136: 121–132. [DOI] [PubMed] [Google Scholar]
- 33.Grassel S, Muschter D. Peripheral nerve fibers and their neurotransmitters in osteoarthritis pathology. Int J Mol Sci 2017; 18: E931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci 2002; 966: 343–354. [DOI] [PubMed] [Google Scholar]
- 35.Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, Vazquez E, von Banchet GS. Joint pain. Exp Brain Res 2009; 196: 153–162. [DOI] [PubMed] [Google Scholar]
- 36.Xie Q, Li X, Xu X. The difficult relationship between occlusal interferences and temporomandibular disorder—insights from animal and human experimental studies. J Oral Rehabil 2013; 40: 279–295. [DOI] [PubMed] [Google Scholar]
- 37.Davidson JA, Metzinger SE, Tufaro AP, Dellon AL. Clinical implications of the innervation of the temporomandibular joint. J Craniofac Surg 2003; 14: 235–239. [DOI] [PubMed] [Google Scholar]
- 38.Cao Y, Xie QF, Li K, Light AR, Fu KY. Experimental occlusal interference induces long-term masticatory muscle hyperalgesia in rats. Pain 2009; 144: 287–293. [DOI] [PubMed] [Google Scholar]
- 39.Butler RK, Ehling S, Barbar M, Thomas J, Hughes MA, Smith CE, Pogorelov VM, Aryal DK, Wetsel WC, Lascelles B. Distinct neuronal populations in the basolateral and central amygdala are activated with acute pain, conditioned fear, and fear-conditioned analgesia. Neurosci Lett 2017; 661: 11–17. [DOI] [PubMed] [Google Scholar]
- 40.Huang X, Yang J, Chang JK, Dun NJ. Amylin suppresses acetic acid-induced visceral pain and spinal c-fos expression in the mouse. Neuroscience 2010; 165: 1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hossaini M, Duraku LS, Kohli SK, Jongen JL, Holstege JC. Spinal distribution of c-Fos activated neurons expressing enkephalin in acute and chronic pain models. Brain Res 2014; 1543: 83–92. [DOI] [PubMed] [Google Scholar]