Short abstract
Background
Electroacupuncture has been elicited to effectively alleviate the pain sensation. Preemptive analgesic effect of pre-electroacupuncture has also been suggested in recent studies, while the underlying analgesic mechanism of pre-electroacupuncture requires further investigation. This study aimed to explore the preemptive analgesia of pre-electroacupuncture in formalin-induced acute inflammatory pain model.
Methods
Forty rats were randomly divided into control, model, pre-electroacupuncture, and post-electroacupuncture group. Inflammatory pain model was induced via injecting 50 µl 5% formalin into the plantar surface of right hind paw, while the equal volume of saline injection in the control group. Rats in the pre-electroacupuncture group were treated with electroacupuncture at ipsilateral Zusanli (ST36) and Weizhong (BL40) acupoints (2 Hz, 1 mA) for 30 min before formalin injection, while received the same electroacupuncture treatment immediately after formalin injection in the post-electroacupuncture group. Flinching number and licking time were recorded during 60 min after formalin injection. Immunofluorescence and Western blot were used to detect the expression of ionized calcium binding adapter molecule 1 (Iba1) and c-fos in spinal cord. Moreover, enzyme-linked immunosorbent assay was applied to measure the secretion of IL-6, IFN-γ, IL-4, substance P, and calcitonin gene-related peptide in spinal cord.
Results
Paw flinching and licking were obviously induced by formalin injection. Iba1, c-fos, proinflammatory cytokines (IL-6 and IFN-γ), and pain neurotransmitters (substance P and calcitonin gene-related peptide) were dramatically increased in the L4-5 spinal cord after formalin injection, while anti-inflammatory cytokine IL-4 was decreased. Pre-electroacupuncture and post-electroacupuncture administration significantly attenuated formalin-induced nociceptive effects, spinal microglia and neurons activation, proinflammatory cytokines and pain neurotransmitters upregulation, and upregulated the anti-inflammatory cytokine. Furthermore, these effects of pre-electroacupuncture were more significant than that of post-electroacupuncture.
Conclusions
This study illustrates the potential therapeutic effect of pre-electroacupuncture against acute inflammatory pain and reveals the mechanism underlying pre-electroacupuncture mediated analgesia, thus providing a novel preemptive analgesic treatment.
Keywords: Preemptive analgesia, pre-electroacupuncture, formalin-induced acute inflammatory pain, Iba1, c-fos, inflammatory cytokines, neurotransmitters
Introduction
Preemptive analgesia is an intervention that is conducted before initiating painful stimuli, which may alleviate or inhibit subsequent pain.1 Due to the “preventative” effect on nociceptive amplification, preemptive analgesia possesses the potential to be more effective than the similar analgesic therapy initiated after injury, as it alleviates following postoperative pain and forestalls the development of persistent pain by suppressing the altered central sensory processing.2,3 Notably, emerging studies confirmed that preemptive analgesia treatment prior to surgery can decrease postoperative analgesics requirements and improve the postoperative pain assessment and satisfaction scores.4,5 As is known, the present preemptive analgesic approaches are mainly consisted of pharmacological strategy, such as non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 inhibitors, local anesthetics (e.g., epidural), opioids, antidepressants, and anticonvulsants.6 Nevertheless, patients with analgesic needs may prefer non-pharmacological options because preemptive analgesia also comes with considerable risks for adverse effects. So, it is urgent for us to explore a valuable non-pharmacological preemptive analgesic approach. Electroacupuncture (EA), a combination of traditional acupuncture and modern medicine that apply various levels of stimulating currents to acupoints through acupuncture needles, represents a potentially useful adjunct to available pain relief strategies.7–9 Although the exact mechanisms in EA analgesia are unclear, research works have been well illustrated that the analgesic effect of EA is mainly associated with its function in regulating endogenous opioid peptides, cytokines, and other central neurotransmitter release.10–13
Interestingly, there is an evidence that pre-EA exerts a satisfactory analgesic effect against postincision pain in rats.14–16 Although there was a lack of clarification, these results showed us a novel strategy for non-pharmacological preemptive analgesic option. Thus, one purpose of our study is to confirm the preemptive analgesic effect of EA.
It is well established that the crosstalk between microglia and sensory neurons plays an indispensable role in the maintenance of persistent pain. During this pathological state, the microglial proinflammatory cytokines and the neurotransmitters released from nociceptive neurons not only drive the pain sensitization but also serve as mediators in the communication between microglia and sensory neurons.17–19 Furthermore, recent developments in the research of EA have heightened the role of microglia in EA analgesia, which inhibited the activation of microglia and improved the neuroinflammation.20
This study aimed to confirm the analgesic effect of pre-EA on inflammatory pain induced by formalin. In addition, this study also aimed to observe the inhibitory effect of pre-EA on the activation of microglia and nociceptive neurons in spinal cord in order to evaluate the preemptive analgesic ability of EA and explore its possible mechanisms.
Materials and methods
Animals
Forty male Sprague Dawley rats weighed 220 to 250 g were obtained from Experimental Animal Center of Southwest Medical University (Luzhou, China). Four rats were housed per cage at a temperature of 22°C to 24°C and with a 12-h reversed light-dark cycle. All animals were provided free access to food and water. All animal experimental procedures and protocols were approved by Animal Care and Ethics Committee of the Affiliated Traditional Chinese Medicine Hospital of Southwest Medical University, and the study was performed in accordance with the guidelines published in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85–23, revised 1996). The numbers of animals used and their suffering were minimized by all efforts.
Formalin test
Rats were moved to the test room at least 30 min before starting the experiment. The animals were randomly divided into four groups: control group, model group, pre-EA group, and post-EA group. In this study, rats were first bound for 30 min. In model group, the rats were subcutaneously injected with 50 µl of 5% formalin into the plantar surface of the right hind paw, while with the same volume of saline solution (0.9%) in control group. In pre-EA group, EA treatment was applied for 30 min before formalin injection, and in post-EA group, EA was conducted for 30 min immediately after formalin injection. Flinching number and licking time of pain-related behaviors were recorded continuously every 5 min immediately after formalin injection up to 60 min post-injection. At 2 h after formalin injection, animals were sacrificed for the followed experiments: immunofluorescence, Western blot, and enzyme-linked immunosorbent assay (ELISA).
EA treatment
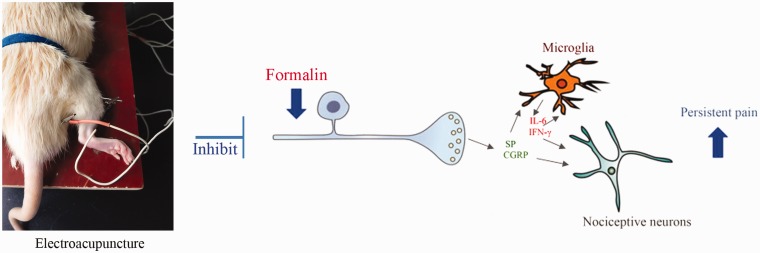
For EA treatment, the rats were bound without anesthesia. A pair of stainless steel acupuncture needles (diameter 0.35 mm, length 40 mm, Huatuo; Suzhou Medical Appliance Manufactory, Jiangsu, China) was inserted 2 mm deep into the rats’ ipsilateral Zusanli (ST36) and Weizhong (BL40) acupoints. Zusanli acupoint (ST36) is located 5 mm beneath the capitulum fibulae and lateral posterior to the knee-joint,7 and Weizhong acupoint (BL40) is located on the medial aspect of knee, at the midpoint of the joint between os femoris and tibia21 (Figure 5). EA stimulation was delivered using an electrical stimulation device (6805-D; Kangmai Medical Technology, Co., Ltd., Guangzhou, China) at 2 Hz and 1 mA, and a slight shrinkage in the muscle was observed. In pre-EA group, EA was performed for 30 min before formalin injection, while conducted after formalin injection for 30 min in post-EA group.
Figure 5.
EA inhibited the development of persistent pain in formalin-injected rats. Pre-EA treatment inhibited formalin induced the release of SP and CGRP from primary afferent neurons, thus suppressed the activation of spinal microglia, reduced the secretion of IL-6 and IFN-γ, and further inhibited the activation of nociceptive neurons, accordingly, inhibited the development of persistent pain. EA: electroacupuncture; SP: substance P; CGRP: calcitonin gene-regulated peptide.
Sample preparation and immunofluorescence
Rats were deeply anesthetized with 10% chloral hydrate (3 ml/kg) and were perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The L4-5 spinal cord segments were dissected out and post-fixed in the same fixative for 4 h at 4°C. Tissue was then maintained in 30% sucrose in 0.1 M phosphate-buffered saline (PBS) at 4°C overnight. Dissected tissue was embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan) frozen on dry ice. The transverse spinal cord was cut at a thickness of 20 μm in a cryostat (Microm HM 525). For immunofluorescence analysis, the sections were fixed for 10 min with the same fixative, and then were washed in 0.01 M PBS three times (5 min each). After blocking in 10% normal goat serum in 0.01 M PBS with 0.3% Triton X-100 for 1 h at room temperature, the sections were incubated overnight at 4°C with rabbit anti-ionized calcium binding adapter molecule 1 (Iba1) polyclonal antibody (1 : 500; Abcam) and rabbit anti-c-fos polyclonal antibody (1 : 250, Proteintech) dissolved in 10% normal goat serum. After rinsing in 0.01 M PBS three times 5 min each, the sections were incubated with fluorescein isothiocyanate-conjugated secondary antibody (1 : 600, Abcam) dissolved in 10% normal goat serum for 1 h at room temperature in the dark. The sections were rinsed again and were subsequently stained with diamidino-2-phenylindole dihydrochloride (1:1,000; Solarbio Science and Technology Ltd., Beijing, China). Finally, the sections were rinsed with PBS, and the coverslips were mounted with antifade solution (Solarbio Science and Technology Ltd., Beijing, China). Fluorescence images of Iba1 and c-fos were determined with a digital upright microscope (Olympus BX53, Anbiping Meditech Ltd., Guangzhou, China).
Western blot analysis
To examine protein expression of Iba1 and c-fos, the rats were deeply anesthetized as previously described at 2 h after formalin injection. The L4-5 segments were quickly excised and stored at −80°C for further analysis. The tissue was subjected to homogenize in the radioimmunoprecipitation assay (RIPA, Beyotime, Shanghai, China). The target proteins were harvested from the obtained supernatant after centrifugation (12,000 r/min for 30 min at 4°C). The total protein concentration was determined by using the bicinchoninic acid assay method (Beyotime, Beijing, China). In sum, 20 µg of protein was loaded in each lane and was separated by using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Beyotime, Shanghai, China), followed by transferring it onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). After blocking with 5% skim milk for 2 h at room temperature, the membranes were incubated with rabbit anti-c-fos (1:250, Proteintech), rabbit-anti Iba1 (1 : 500, Abcam), and anti-β-actin (1 : 2000, Cell Signaling, MA, USA) for loading control overnight at 4°C. After three washes with Tris-buffered saline with Tween 20 (TBST), these blots were subsequently incubated with horseradish peroxidase anti-rabbit secondary antibody (1 : 2000, ZSGB-Bio, Origene, Beijing, China) for 2 h at room temperature. Images were developed by enhanced chemiluminescence plus reagent, and Gel-Pol analyzer was used to analyze the intensity of bands.
ELISA for substance P, calcitonin gene-related peptide, IFN-γ, IL-6, and IL-4
After the EA experiments were conducted, the supernatants of L4-5 were collected. An ELISA was conducted by using these supernatants. The levels of substance P (SP), calcitonin gene-related peptide (CGRP), IFN-γ, IL-6, and IL-4 in the supernatants were detected using an ELISA kit (Shanghai bridge club, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
The obtained data in this study were expressed as the mean ± standard deviation (SD). SPSS 22.0 software was used for the statistical analysis. A statistical evaluation of the data was analyzed using a one-way analysis of variance (P < 0.05), followed by post hoc comparisons using the least significant difference or Dunnett’s post hoc test. P < 0.05 was considered statistically significant.
Results
EA attenuated formalin-induced pain-related behavior in rats
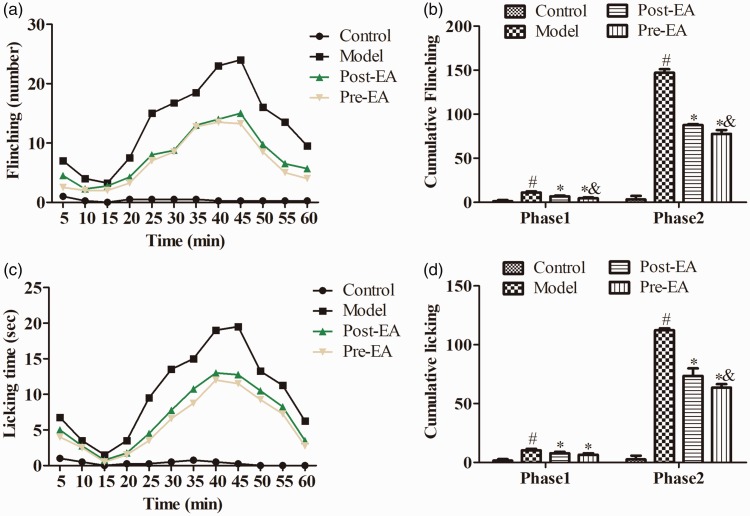
Formalin injection into the rat’s right hind paw induced a significant pain-related behavior characterized by flinching and licking the injected paw, as shown in Figure 1. To further validate the role of EA, EA was conducted at ST36 and BL40 for 30 min before and after the formalin injection. Both pre-EA and post-EA significantly reduced the paw flinching number and lifting time in the two phases following the injection, compared with the Model group (P < 0.05). Moreover, the administration of pre-EA dramatically reduced the flinching number in the two phases and licking time in phase 2 in comparison to post-EA, respectively (P < 0.05), while there was no significant difference in phase 1 of licking time between pre-EA and post-EA group (P > 0.05).
Figure 1.
Effects of EA on formalin-induced pain-related behavior (n = 16). Pain-related behaviors paw flinching number (a) and licking time (c), cumulative flinching (b) and cumulative licking (d) were recorded for 60 min immediately after formalin injection. Paw flinching and licking were observed immediately after formalin injection, and that behaviors were characterized by two distinct phases, respectively. EA dramatically inhibited the increased cumulative flinching and cumulative licking. Data were expressed as the Mean ± SD. #P<0.05, compared with control group; *P<0.05 compared with model group; &P<0.05 compared with post-EA group. EA: electroacupuncture.
EA inhibited spinal microglia and neurons activation in the L4-5 spinal cord of formalin-treated rats
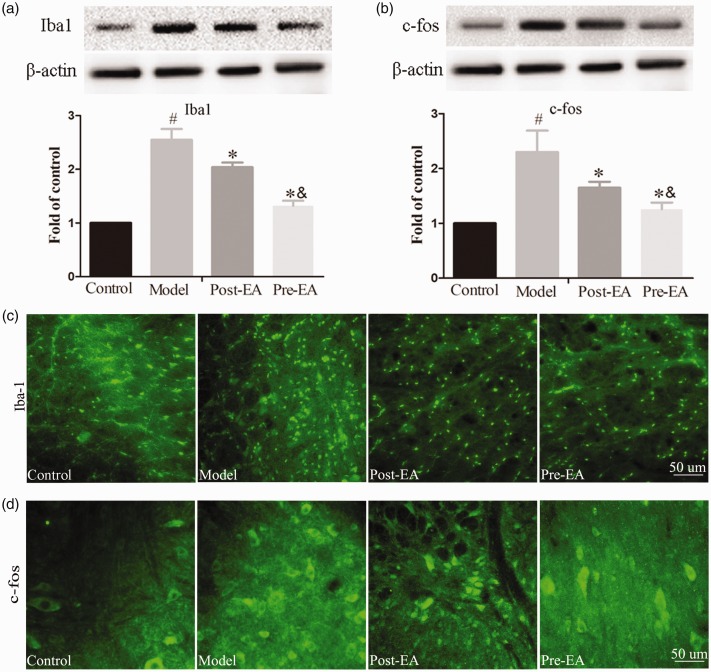
To determine whether glial-neuron activation was associated with formalin-induced acute inflammatory pain and the effect of EA treatment on glial-neuron activation, we measured the protein expression of Iba1 and c-fos at the L4-5 segments by immunofluorescence and Western blot. As is shown in Figure 2(c) and (d), microglia and neurons activation, represented by the Iba1 and c-fos fluorescence density, was increased in formalin-injected rats compared to pre-EA, post-EA, and control rats. Pre-EA or post-EA treatment for 30 min before or after formalin injection resulted in a remarkable decrease in the fluorescence density of Iba1 and c-fos compared to model rats. Western blot analysis validated that both treatment with EA significantly decreased microglia and neurons activation as reflected by the Iba1 and c-fos protein expression in the L4-5 spinal cord sections (P < 0.05) (Figure 2(a) and (b)). Furthermore, there was a significant change between pre-EA and post-EA group in Iba1 and c-fos protein expression, respectively (P < 0.05).
Figure 2.
Effects of EA on the increased expression of Iba1 and c-fos in the L4–L5 spinal cord induced by formalin injection (n = 12). (a) Representative bands and quantification of the Western blot analysis revealed that EA treatment dramatically reduced the increased Iba1 protein level. (b) Representative bands and quantification of Western blot analysis revealed that EA significantly reduced the increased c-fos protein level. (c) Representative immunostaining pictures revealed the change in Iba1 expression in the L4–L5 spinal cord in control, model, pre-EA, and post-EA group. (d) Representative immunostaining images revealed the change in c-fos expression in the L4–L5 spinal cord in control, model, pre-EA, and post-EA group. #P<0.05, compared with control group; *P<0.05, compared with model group, &P<0.05, compared with post-EA group; scale bars = 50 μm. EA: electroacupuncture; Iba1: ionized calcium binding adapter molecule 1.
EA altered formalin-induced production of cytokines in the L4-5 spinal cord
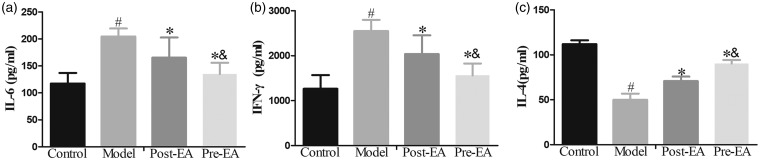
To further detect the effect of EA treatment on the spinal inflammatory response after formalin injection, ELISA was performed to measure the levels of inflammatory cytokines. Pre-EA or post-EA was conducted for 30 min before or after formalin injection, and the L4-5 spinal cord segments were separated at the 2 h after formalin injection or saline. ELISA analysis demonstrated that the levels of IL-6 and IFN-γ in formalin-injected rats were significantly increased compared with pre-EA, post-EA, and control rats, while the level of IL-4 was remarkably decreased (P < 0.05) (Figure 3). Pre-EA before formalin injection for 30 min significantly inhibited the increased levels of IL-6 and IFN-γ in the spinal cord and upregulated the level of IL-4 in comparison to post-EA (P < 0.05).
Figure 3.
Effects of EA on the levels of IL-6, IFN-γ, and IL-4 in the L4–L5 spinal cord of formalin-treated rats as determined by ELISA (n = 16). Compared with control group, the levels of IL-6 and IFN-γ were significantly increased in model group, while the level of IL-4 was remarkably reduced. Pre-EA and post-EA effectively blocked the increased levels of IL-6 and IFN-γ induced by formalin injection, upregulated the level of IL-4. Data were expressed as Mean ± SD. #P<0.05 compared with control group; *P<0.05 compared with model group; &P<0.05 compared with post-EA group. EA: electroacupuncture.
EA altered formalin-induced production of neurotransmitters in the L4-5 spinal cord
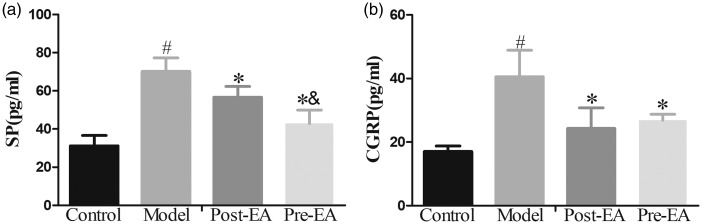
To further detect the effect of EA treatment on the spinal pain neurotransmitters after formalin injection, ELISA was also performed to measure the levels of SP and CGRP. ELISA analysis showed that the levels of SP and CGRP in formalin-injected rats were dramatically increased compared to that in pre-EA, post-EA, and control rats (P < 0.05) (Figure 4). Mover, pre-EA treatment significantly inhibited the increased level of SP in L4-5 spinal cord compared with post-EA, respectively (P < 0.05), while there was no significant difference in CGRP level between the two groups (P > 0.05).
Figure 4.
Effects of EA on the levels of SP and CGRP in the L4–L5 spinal cord of formalin-treated rats as determined by ELISA. Compared with control group, the levels of SP and CGRP were dramatically increased in model group. Pre-EA and post-EA effectively inhibited the increased levels of SP and CGRP induced by formalin injection, and pre-EA treatment significantly reduced the level of SP as compared to post-EA. Data were expressed as Mean ± SD. #P<0.05 compared with control group; *P<0.05 compared with model group; &P<0.05 compared with post-EA group. EA: electroacupuncture; SP: substance P; CGRP: calcitonin gene-regulated peptide.
Discussion
This study was designed to determine the preemptive analgesic effects of pre-EA on acute inflammatory pain. Our data demonstrated that formalin intraplantar injection-induced inflammatory pain can be attenuated by pre-EA at ipsilateral Zusanli (ST36) and Weizhong (BL40) acupoints. The related mechanisms of pre-EA may attribute to inhibit the activation of spinal microglia and sensory neurons. Accordingly, proinflammatory cytokines and nociceptive neurotransmitters in spinal cord, released from activated microglia and sensory neurons, were dramatically reduced by pre-EA. Besides, the analgesic effects of pre-EA on inflammatory pain are better than those of post-EA.
EA inhibits pain by activating numerous bioactive chemicals via peripheral, spinal, and supraspinal mechanisms. These include opioids, which inhibit the sensitization of peripheral nociceptors and decrease proinflammatory cytokines peripherally and in the spinal cord and activating supraspinal serotonergic and noradrenergic neurons that project to the spinal cord.9,22 Pre-EA is the treatment before initiating painful stimuli, while post-EA is initiated after injury. As the “preventative” effect on nociceptive amplification and the “gate control theory” of pain, pre-EA could effectively forestall the transmission of nociceptive information via blocking the altered peripheral and central sensory processing,23,24 thus may alleviate or inhibit subsequent pain.
In this study, right hind paw plantar injection of formalin was used to mimic the acute inflammatory pain. As is known, there is a biphasic pain response involved in the inflammatory injury.25 This phenomenon also occurred in our formalin model rats, and the underlying mechanisms of two phases are likely to be different: tissue injury contributes to the first phase (0–5 min) of pain response, representing nociceptive pain, while the second phase (10–45 min) attributes to the peripheral inflammation and central sensitization.26 Furthermore, paw flinching and paw licking were monitored and considered to be the parameters of pain intensity, as similar as previous studies.27 This study found that EA therapy significantly alleviated formalin-induced pain. Interestingly, our data also showed that 2-Hz pre-EA had better analgesic effects than post-EA, these findings indicated that EA might be considered to be a promising preemptive analgesic strategy. Consistent with our results, recent studies had demonstrated the expected outcome of EA and its preemptive analgesia.14,16 However, one of the previous study insisted that 100-Hz EA, instead of 2-Hz EA, was preemptive against incision pain in rats, whereas 2-Hz EA was more effective for postincision analgesia.16 A possible explanation for this contradiction might be the difference of EA acupoints and stimulating spontaneous time between two studies. Accordingly, ipsilateral Zusanli (ST36) and Weizhong (BL40) were stimulated for 30 min with 2-Hz/1 mA EA in our experiment, and based on the “JingMai” theory of traditional Chinese medicine, ST36 and BL40 are the most valuable acupoints for treating the lower limbs pain. Furthermore, the accumulative effect of EA on analgesia reveals that the stimulating time may act as a key factor for EA preemptive analgesia.28 Meanwhile, future studies are needed to design active control group for EA (e.g., acupuncture needle insertions without electrical stimulation or non-acupoint EA stimulation).
It is well established that microglia derive from primary macrophages.29 In almost all conditions, microglia are specifically distributed in the spinal cord and brain, maintaining the local environment.30 Once received the pathophysiological stimulus, microglia are activated, alter morphologically, increase the cell number, and change the expression of genes, including proinflammatory cytokines, such as IL-6 and IL-1β in the spinal cord.31 As mentioned in the previous literature, the proinflammatory cytokines, which works as the mediators in the progress of “crosstalk” with nociceptive neurons, finally promote pain processing and maintenance.32 This study demonstrated that the expression of microglial marker Iba1 was dramatically increased in L4-5 of spinal cord at 2 h after formalin injection, suggesting that microglia have already been activated by nociceptive stimuli in the early period of inflammation injury. As hypothesized, in the model group, ELISA analysis demonstrated that IL-6 and IFN-γ expression levels of L4-5 area of spinal cord were increased, while anti-inflammatory cytokine IL-4 decreased. Notably, pre-EA treatment significantly suppressed the activation of microglia and inhibited the expression levels of inflammatory cytokines, as compared with the post-EA group. These results indicated that microglia might be the target for EA preemptive analgesia, thus inhibited the progress of inflammation in central nervous system. Prior studies have noted the important role of microglia in EA analgesia both in chronic pain and acute pain model, via suppressing its activation to reverse the central sensitization of pain.7,33 However, there is no attention to the change in microglia and neurons in spinal cord by EA stimulation at different times in this study, which may provide the correlation between microglia and neuron activation.
Meanwhile, we also detected that the expression of c-fos was augmented in L4-5 after formalin injection; historically, c-fos has been used as the representative marker for neuronal activation following noxious stimulation.34,35 Coinciding with microglial activation, we found that neurons were polarized in this situation. As mentioned before, the crosstalk between microglia and sensory neurons in spinal cord is crucial for pain amplification. Microglia could communicate with neurons via releasing or receiving extracellular molecules, which initiate the intracellular activation signaling.36 For example, the upregulated IFN-γ and IL-6 in present research were confirmed that contributed to neurons polarization.17,37,38 SP and CGRP, the nociceptive neurotransmitters which were synthesized by small diameter sensory nerve fibers almost are C-fibers, also increased in L4-5 of spinal cord by formalin injection. Previous studies showed that the SP and CGRP were involved in pain transmission and contributed to the nociceptive synaptic strengthen.39 Moreover, a strong relationship between neurotransmitters and microglial activation has been reported in the literature, which indicated that SP and CGRP may act as the promotors of microglia activation.40,41 Following treatment with pre-EA, the expression levels of SP and CGRP as well as c-fos were significantly decreased, suggesting that pre-EA was able to inhibit the activation of nociceptive neurons in rat models of inflammatory pain. Therefore, as a promising preemptive analgesic strategy, EA could reduce the pain signal transmission from primary afferent neurons to spinal secondary sensory neurons in the early stage of inflammatory pain.
In conclusion, this study demonstrated that treatment with pre-EA significantly alleviated the formalin-induced acute inflammatory pain. Meanwhile, pre-EA dramatically decreased the activation of microglia and neurons by decreasing the expression of IFN-γ, IL-6, SP, and CGRP, thus interrupted the crosstalk between microglia and sensory neurons in spinal cord and, furthermore, inhibited spinal neuroinflammation and central nociceptive sensitization. Taken together, these finds provide a novel preemptive analgesic strategy for the clinical treatment of pain and give a clarification of the potential mechanisms involved in pre-EA analgesia.
Author contributions
Q Liu and YZ conceived and designed the experiments; YZ, YL, and Q Li performed the experiments; JB and Q Li analyzed the data; Q Liu and YL wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Long JB, Bevil K, Giles DL. Preemptive analgesia in minimally invasive gynecologic surgery. J Minim Invasive Gynecol 2018; 26: 198–218. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Chong MS. Preemptive analgesia–treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77: 362–379. [DOI] [PubMed] [Google Scholar]
- 3.Szedlak B, Mitre C, Fulesdi B. [Preemptive and preventive analgesia - an important element in perioperative pain management]. Orv Hetil 2018; 159: 655–660. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg AC, Schimpf MO, White AB, Mathews C, Ellington DR, Jeppson P, Crisp C, Aschkenazi SO, Mamik MM, Balk EM, Murphy M. Preemptive analgesia for postoperative hysterectomy pain control: systematic review and clinical practice guidelines. Am J Obstet Gynecol 2017; 217: 303.e6–313.e6. [DOI] [PubMed] [Google Scholar]
- 5.Aglio LS, Abd-El-Barr MM, Orhurhu V, Kim GY, Zhou J, Gugino LD, Crossley LJ, Gosnell JL, Chi JH, Groff MW. Preemptive analgesia for postoperative pain relief in thoracolumbosacral spine operations: a double-blind, placebo-controlled randomized trial. J Neurosurg Spine 2018; 29: 647–653. [DOI] [PubMed] [Google Scholar]
- 6.Sittl R, Irnich D, Lang PM. [Update on preemptive analgesia: options and limits of preoperative pain therapy]. Anaesthesist 2013; 62: 789–796. [DOI] [PubMed] [Google Scholar]
- 7.Tu WZ, Li SS, Jiang X, Qian XR, Yang GH, Gu PP, Lu B, Jiang SH. Effect of electro-acupuncture on the BDNF-TrkB pathway in the spinal cord of CCI rats. Int J Mol Med 2018; 41: 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JY, Rapson LM. Acupuncture in the management of pain of musculoskeletal and neurologic origin. Phys Med Rehabil Clin N Am 1999; 10: 531–545, vii–viii. [PubMed] [Google Scholar]
- 9.Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014; 120: 482–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Huang ZQ, Hu ZP, Jiang SZ, Li HT, Han JS, Wan Y. Electroacupuncture effects in a rat model of complete Freund's adjuvant-induced inflammatory pain: antinociceptive effects enhanced and tolerance development accelerated. Neurochem Res 2008; 33: 2107–2111. [DOI] [PubMed] [Google Scholar]
- 11.Cheng LL, Ding MX, Xiong C, Zhou MY, Qiu ZY, Wang Q. Effects of electroacupuncture of different frequencies on the release profile of endogenous opioid peptides in the central nerve system of goats. Evid Based Complement Alternat Med 2012; 2012: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Wang K, Deng J, Sun M, Jia J, Wang X. Electroacupuncture produces the sustained motor improvement in 6-hydroxydopamine-lesioned mice. PLoS One 2016; 11: e0149111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Li A, Xin J, Lao L, Ren K, Berman BM, Tan M, Zhang RX. Involvement of spinal serotonin receptors in electroacupuncture anti-hyperalgesia in an inflammatory pain rat model. Neurochem Res 2011; 36: 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves de Freitas AT, Lemonica L, De Faveri J, Pereira S, Bedoya Henao MD. Preemptive analgesia with acupuncture monitored by c-Fos expression in rats. J Acupunct Meridian Stud 2016; 9: 16–21. [DOI] [PubMed] [Google Scholar]
- 15.Coura LE, Manoel CH, Poffo R, Bedin A, Westphal GA. Randomised, controlled study of preoperative electroacupuncture for postoperative pain control after cardiac surgery. Acupunct Med 2011; 29: 16–20. [DOI] [PubMed] [Google Scholar]
- 16.Silva ML, Silva JR, Prado WA. 100-Hz Electroacupuncture but not 2-Hz electroacupuncture is preemptive against postincision pain in rats. J Acupunct Meridian Stud 2016; 9: 200–206. [DOI] [PubMed] [Google Scholar]
- 17.Lee KM, Jeon SM, Cho HJ. Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain 2010; 14: 682.e1–12. [DOI] [PubMed] [Google Scholar]
- 18.Nieto FR, Clark AK, Grist J, Chapman V, Malcangio M. Calcitonin gene-related peptide-expressing sensory neurons and spinal microglial reactivity contribute to pain states in collagen-induced arthritis. Arthritis Rheumatol 2015; 67: 1668–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma W, Eisenach JC. Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P, calcitonin gene-related peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation. Neuroscience 2003; 121: 681–690. [DOI] [PubMed] [Google Scholar]
- 20.Ji LL, Guo MW, Ren XJ, Ge DY, Li GM, Tu Y. Effects of electroacupuncture intervention on expression of cyclooxygenase 2 and microglia in spinal cord in rat model of neuropathic pain. Chin J Integr Med 2017; 23: 786–792. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Wang Y, Mo Q, Liu Z. A comparative study of electroacupuncture at Zhongliao (BL33) and other acupoints for overactive bladder symptoms. Front Med 2017; 11: 129–136. [DOI] [PubMed] [Google Scholar]
- 22.Fais RS, Reis GM, Silveira JW, Dias QM, Rossaneis AC, Prado WA. Amitriptyline prolongs the antihyperalgesic effect of 2- or 100-Hz electro-acupuncture in a rat model of post-incision pain. Eur J Pain 2012; 16: 666–675. [DOI] [PubMed] [Google Scholar]
- 23.Yang EJ, Koo ST, Kim YS, Lee JE, Hwang HS, Lee MS, Choi SM. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol Int 2011; 31: 725–730. [DOI] [PubMed] [Google Scholar]
- 24.Zhang SP, Zhang JS, Yung KK, Zhang HQ. Non-opioid-dependent anti-inflammatory effects of low frequency electroacupuncture. Brain Res Bull 2004; 62: 327–334. [DOI] [PubMed] [Google Scholar]
- 25.Yi M, Zhang H, Lao L, Xing GG, Wan Y. Anterior cingulate cortex is crucial for contra- but not ipsi-lateral electro-acupuncture in the formalin-induced inflammatory pain model of rats. Mol Pain 2011; 7: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain 1992; 51: 5–17. [DOI] [PubMed] [Google Scholar]
- 27.Han P, Liu S, Zhang M, Zhao J, Wang Y, Wu G, Mi W. Inhibition of spinal interlukin-33/ST2 signaling and downstream ERK and JNK pathways in electroacupuncture analgesia in formalin mice. PLoS One 2015; 10: e0129576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JY, Chen SP, Li YH, Meng FY, Gao YH, Liu JL. [Observation on the accumulative analgesic effect of electroacupuncture and the expression of protein kinase A in hypothalamus and hippocampus in chronic pain or/and ovariectomized rats]. Zhen Ci Yan Jiu 2008; 33: 80–87. [PubMed] [Google Scholar]
- 29.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010; 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K. Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc Jpn Acad, Ser B, Phys Biol Sci 2017; 93: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao H, Alam A, Chen Q, A Eusman M, Pal A, Eguchi S, Wu L, Ma D. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth 2017; 118: 504–516. [DOI] [PubMed] [Google Scholar]
- 32.Peng J, Gu N, Zhou L, B Eyo U, Murugan M, Gan W-B, Wu L-J. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun 2016; 7: 12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang WC, Hsu YC, Wang CC, Hu CY, Chio CC, Kuo JR. Early electroacupuncture treatment ameliorates neuroinflammation in rats with traumatic brain injury. BMC Complement Altern Med 2016; 16: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol 1990; 296: 517–530. [DOI] [PubMed] [Google Scholar]
- 35.Terashima T, Xu Q, Yamaguchi S, Yaksh TL. Intrathecal P/Q- and R-type calcium channel blockade of spinal substance P release and c-Fos expression. Neuropharmacology 2013; 75: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi J, Chen C, Meng QX, Wu Y, Wu H, Zhao TB. Crosstalk between activated microglia and neurons in the spinal dorsal horn contributes to stress-induced hyperalgesia. Sci Rep 2016; 6: 39442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marz P, Herget T, Lang E, Otten U, Rose-John S. Activation of gp130 by IL-6/soluble IL-6 receptor induces neuronal differentiation. Eur J Neurosci 1997; 9: 2765–2773. [DOI] [PubMed] [Google Scholar]
- 38.Vikman KS, Duggan AW, Siddall PJ. Interferon-gamma induced disruption of GABAergic inhibition in the spinal dorsal horn in vivo. Pain 2007; 133: 18–28. [DOI] [PubMed] [Google Scholar]
- 39.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol 2009; 194: 451–491. [DOI] [PubMed] [Google Scholar]
- 40.Zhu J, Qu C, Lu X, Zhang S. Activation of microglia by histamine and substance P. Cell Physiol Biochem 2014; 34: 768–780. [DOI] [PubMed] [Google Scholar]
- 41.Nees TA, Tappe-Theodor A, Sliwinski C, Motsch M, Rupp R, Kuner R, Weidner N, Blesch A. Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 2016; 157: 687–697. [DOI] [PubMed] [Google Scholar]