Abstract
Rheumatoid arthritis (RA) is an autoimmune disorder with multifactorial etiology; both genetic and environmental factors are known to be involved in pathogenesis. Treatment with disease-modifying antirheumatic drugs (DMARDs) plays an essential role in controlling disease progression and symptoms. DMARDs have immunomodulatory properties and suppress immune response by interfering in various pro-inflammatory pathways. Recent evidence has shown that the gut microbiota directly and indirectly modulates the host immune system. RA has been associated with dysbiosis of the gut microbiota. Patients with RA treated with DMARDs show partial restoration of eubiotic gut microbiome. Hence, it is essential to understand the impact of DMARDs on the microbial composition and its consequent influences on the host immune system to identify novel therapies for RA. In this review, we discuss the importance of antirheumatic-drug-induced host microbiota modulations and possible probiotics that can generate eubiosis.
Keywords: disease-modifying antirheumatic drugs, gut microbiota, immune modulation, microbial modulation, probiotics, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic chronic inflammatory disease that affects approximately 0.5–1.0% of the population.1 In most patients, it leads to joint destruction and functional disability due to the disease targeting the self-antigens present in the synovium, cartilage, and bone.2,3 Substantial insights into RA pathophysiology suggest that various inflammatory pathways lead to an altered immune system and onset of disease.4–6 Presence of autoantibodies prior to the onset of RA suggest that an autoreactive immune response occurs much before the clinical symptoms appear. The origin of a dysregulated immune response is suggested to occur due to environmental influences in the genetically predisposed individuals. Several gene variants have been identified by the genome-wide association studies in RA, though the most significant association has been observed with certain genes present in the major histocompatibility complex class II (MHC class II) region.7–9 However, several environmental and other factors such as geography, socioeconomic status, birth weight, diet, alcohol, smoking, and host microbiome also contribute to the risk of developing RA.10–13 Among these, smoking is known to strongly increase the risk of developing RA.13 While major research has focused on genetic factors, there is a growing realization that the host microbiota, and especially the gut microbiota, play a key role in the development and progression of RA. In concert with the gut-associated lymphoid tissue, the gut microbiome is involved in maintaining immune homeostasis and acts as an indicator of the health status of the host.14–16 Perturbation of this interaction can affect mucosal as well as systemic immunity, and promote various inflammatory and autoimmune diseases.17–19 Consequently, attempts have been made to define the beneficial microbes and their metabolites for their use in treating various diseases.20,21
The microbiome is a collection of genomes within an ecological community of microorganisms.22 However, only about 1% of microbes are culturable, making it hard to elucidate their role in health and disease. Recent advances in deoxyribonucleic acid (DNA) sequencing technologies have enabled researchers to study the unculturable microbial communities. International efforts such as Human Microbiome Project and the MetaHIT project have helped catalog microbial genes by high-throughput next-generation sequencing (NGS).23,24 Generally, microbes can be sequenced from samples using two methods, 16S recombinant DNA (rDNA) sequencing and whole-genome sequencing. While 16S rRNA provides a lower taxonomic level characterization, whole-genome sequencing provides detailed information about all the microbial communities and their functional potential in a sample. However, whole-genome sequencing requires high computational power and is relatively costly. NGS, along with contemporary bioinformatics, has improved our understanding of the human microbiome and its relation to human diseases.20,25,26 Certain factors such as lack of an updated database, reliability in analyzing lower taxonomical level, and the cost of sequencing limit application of NGS-based metagenomic studies in the medical field.25 Despite all the limitations, understanding core microbiome through a multiomics approach to identify novel pathways for treatment of diseases is essential.25
The human intestinal tract harbors trillions of bacteria. These microbes coevolve with the host and are involved in numerous functions, including metabolizing dietary nutrients and production of various vitamins.27 Host microbial composition is regulated by diet, as well as the environment a host is exposed to.28 Microbiota possess an extensive metabolic potential to metabolize xenobiotic compounds like drugs and can have significant implications for drug stability and activity.29–31 For example, sulfasalazine (SSZ), a drug used to treat RA, relies on the anaerobic bacteria in the colon to attain its active form.32 On the other hand, treatment with drugs can also have significant effects on the host microbial composition.33,34 Thus, the modulation of microbiota caused by drug treatment influences the interindividual variations in therapeutic outcome and host immune response.35
Today, most of the drugs available for the treatment of RA, including disease-modifying antirheumatic drugs (DMARDs), act by targeting cytokines, nonspecific immune suppression or T-cell and B-cell activation.36–38 Thus, almost all patients are prescribed with one or more DMARDs upon the diagnosis of RA. There are two main types of DMARDs: traditional DMARDs and biologic DMARDs (bDMARDs). Traditional DMARDs are potent drugs and act via suppressing immune response by blocking protein synthesis and interfering in critical reactions involved in the inflammatory cascades.38 These DMARDs reduce the damage to bone and cartilage and ultimately slow the progression of disease.39,40 Methotrexate is generally the first line of DMARD treatment of RA.41 Biologics, on the other hand, are target specific and selective in their mechanism of action.42 Biologics interfere with various cytokine production and functions, and inhibit T-cell and B-cell activation.38 Besides standard treatments, alternative medicine, including herbal medicines and probiotics, are also used by patients.43
In this review, we discuss the relationship between the microbiome and antirheumatic drugs. In addition, we examine the microbial modulation of RA by gut commensals that behave like probiotics.
Microbiome and rheumatoid arthritis
Microbial colonization occurs before birth and continues to change and diversify till it stabilizes around 3 years of age.44 The gut microbes can bind nod-like receptors and toll-like receptors (TLRs) to activate the immune system, as well as produce metabolites called short-chain fatty acids (SCFAs) that can directly interact with the host.45–47 A well-balanced microbiota maintains immune responses via interaction with intestinal epithelial cells, which helps to maintain a tolerant state within the gastrointestinal tract. To date, research has established that the gut microbiome has a profound association with the host immune system and disease.48 The first report that established a link between microbiota and pathology of arthritis was published in the late 1970s.49 The study showed that germ-free-condition-raised rats developed severe arthritis with 100% incidence, whereas conventionally raised rats developed less-severe arthritis with an incidence of only 20%.49 A role of gut microbiota in the causation of RA was further supported by a study comparing intestinal microbiota in humanized mice expressing RA-susceptible DRB1*0401 and RA-resistant DRB1*0402 mice which suggested that dysbiosis is associated with RA-susceptible genetic factors.50 However, contribution of the gut microbiota in RA remained relatively unclear until recently. Few studies have been conducted in the recent decade highlighting low diversity and dysbiotic gut microbiota in subjects with RA as compared with healthy subjects.51,52
A recent study compared the microbial composition between healthy subjects and new-onset untreated RA (NORA) patients and showed an increased abundance of Prevotella copri with reduced levels of Bacteroides in RA patients suggesting P. copri may be pathogenic.53 Pathogenicity of P. Copri was explained by the immune response generated by DR-presentation of a 27-kD protein from P. copri which could stimulate T-helper-cell 1 (Th1) responses in NORA patients.54 Further, a subset of RA patients also produced and immunoglobulin A (IgA) and immunoglobulin G (IgG) antibodies to P. copri. Another study showed that a novel strain of the prevotella genus, Prevotella histicola, isolated from a human upper gut, suppressed arthritis in humanized HLA-DQ8 mice.55 This suggests that the various species belonging to the prevotella genus possess different functional potential and impact the clinical outcome differentially.20 Two other studies compared the gut microbiome of RA patients with ongoing treatment with healthy controls.51,56 These studies highlighted that the gut dysbiosis in RA patients correlates with the depletion of Gram-negative bacteria and enrichment of Gram-positive bacteria.51,56 Both studies showed an abundance of collinsella in RA patients as compared with healthy subjects and a partially normalized microbial composition in patients treated with DMARDs. Chen and coworkers used first-degree relatives as comparators for RA patients and showed an abundance of Eggerthella lenta and collinsella in patients as compared with relatives. The study suggested that correlation of RA with these commensals was due to the disease status and not confounded by diet or genetic factors.51 A role of collinsella in the pathogenesis of RA was further confirmed in an animal model of arthritis using humanized mice.51 Microbial dysbiosis was associated with the change in inflammatory markers and disease activity.51 Overall, the available literature data suggest that the gut microbiome dysbiosis is associated with RA and treatment with DMARDs could lead to restoration of eubiosis.
Traditional DMARDs
The benefits of traditional DMARDs, including methotrexate (MTX), leflunomide, hydroxychloroquine, and SSZ,38 have been demonstrated at all stages of the disease, thus, they are an essential part of an overall treatment plan.57 DMARDs can attenuate inflammation, control disease activity, reduce joint destruction and improve clinical as well as quality-of-life parameters.58,59 DMARDs are used as first line of treatment as per The American College of Rheumatology and European League against Rheumatism recommendations.60 While DMARDs interfere or inhibit inflammatory cascade reaction via various pharmacological actions, some DMARDs like SSZ also possess antibacterial activity.61 Thus, their consumption may directly or indirectly induce an alteration in the microbiome of the patient. The two studies involving RA patients with ongoing treatments suggest that alteration in the microbiome could be beneficial for patients with RA.51,56 Table 1 summarizes the mechanism of action DMARDs and their effect on the host microbiome.
Table 1.
List of traditional DMARDs, their modes of action and effect on microbiota.
| Traditional DMARDs | Mode of action | Study animal | Induced microbial modulation | References |
|---|---|---|---|---|
| Methotrexate | Interferes with the synthesis of pyrimidine and purines, leads to the inhibition of lymphocyte proliferation | Balb/c mice | Decrease in Bacteroides fragilis post-treatment | Zhou et al.62 |
| Human | Reduced abundance of Enterobacteriaceae | Picchianti-Diamanti et al.63 | ||
| Human | Partially restored the gut microbiota in patients | Chen et al.;51 Zhang et al.56 | ||
| Sulfasalazine | Interferes in the conversion of arachidonic acid to prostaglandins, affects leukocyte function and production of cytokines | Human | Significant increase in bacillus and decrease in total aerobic bacteria, Escherichia coli and Bacteroides | Kanerud et al.64 |
| Human | Reduction in the numbers of total nonsporing anaerobes, Enterobacteria and opalescent-negative clostridia | West et al.65 | ||
| Leflunomide | Inhibits de novo synthesis of pyrimidine thereby regulating lymphocyte proliferation | Human | Data not available | Breedveld et al.66 |
| Hydroxychloroquine | Interferes with antigen processing in macrophages and its presentation to MHC class II proteins | Human | Increase in microbial species richness and diversity | Chen et al.51 |
| Human | Hydroxychloroquine plus doxycycline treatment led to the reduction in abundance of phylum Bacteroidetes and Firmicutes | Angelakis et al.67 | ||
| Azathioprine | Interferes in proliferation of leukocytes by inhibiting purine synthesis pathway | Human | Data not available | Trotter et al.68 |
| Minocycline | Inhibits T-cell activation and chemotaxis Downregulates pro-inflammatory cytokines Inhibits matrix metalloproteinases |
Human | Data not available | Smith et al.69 |
DMARD, disease-modifying antirheumatic drug; MHC, major histocompatibility complex.
Methotrexate
Among DMARDs, MTX is a widely used drug and has emerged as the preferred orally active agent for initial therapy of RA.70 It is derived from the parent compound aminopterin. Initially, aminopterin was used to treat childhood leukemia, but its ability to inhibit proliferation of connective tissue led to its use in RA.71 MTX acts via multiple pathways to inhibit the immune reaction to self-antigen. It inhibits T-cell proliferation by preventing de novo pyrimidine and purine synthesis. Additionally, it increases T-cell apoptosis and reduces cellular adhesion molecules which are essential for signal transduction and the activation of immune response.72,73 MTX has been shown to decrease the production of interleukins (IL-1, IL-4, IL-6, IL-13), tumor-necrosis-factor alpha (TNF-α), interferon gamma (IFN-γ), and granulocyte–monocyte–colony-stimulating factor (GM-CSF).74,75 Besides these physiological effects, MTX treatment also modulates the host microbiota.52
A study by Zhou and colleagues demonstrated the impact of MTX therapy on the gut microbiota and mucosal health of Balb/c mice.62 Interestingly, after MTX treatment, the gut microbiota in the mice was altered in a time-dependent manner with a significant decrease in Bacteroides fragilis after the treatment. Additionally, the bacterial family Ruminococcaceae showed a decreased abundance, whereas the Lachnospiraceae family members were dramatically increased.62 Another important observation from the study was the reduced species of phylum Bacteroidetes after 7 days of treatment with MTX as compared with before treatment. Previously, a significantly increased abundance of Bacteroidetes was noted in untreated juvenile idiopathic arthritis.76 Thus, treatment with MTX may reduce Bacteroidetes, resulting in eubiosis as observed in other studies. A similar study by Andrea Picchianti-Diamanti and coworkers demonstrated the effect of MTX on the gut microbiota of RA patients and clinical outcomes.63 It was observed that the taxa affiliated to Enterobacteriales were less abundant in RA patients receiving MTX therapy than RA patients without any treatment. This study suggested that the modulation of microbiota by MTX might be beneficial for patients as members of Enterobateriales have been associated with increased intestinal permeability and inflammation.77
In order to further discern the effects of MTX, Zhang and colleagues conducted sequencing of a cohort of RA patients and healthy participants comprising 212 fecal samples, 105 dental and 98 saliva samples.56 The patients who showed improvement after MTX treatment had an abundance of microbial linkage groups (MLGs) similar to healthy control samples. Prevotella maculosa showed an increased abundance in the healthy control group, as well as in patients who showed improvement after treatment. Moreover, after MTX therapy, MLGs that were differentially enriched in patients’ dental samples were negatively correlated with the levels of anticyclic citrullinated peptide antibody, rheumatoid factor, and C-reactive protein (CRP), indicating MTX-mediated improvement in the dental microbiome of patients. In the salivary microbiome, MLGs such as veillonella were abundant in patients but showed reduction after MTX therapy, suggesting that MTX therapy could alter the oral microbiome along with gut microbiome of RA patients.56 This suggests that MTX treatment has the potential to shift a ‘diseased’ microbiome toward ‘healthy’ microbiome.
Similar results were observed in another study, where patients treated with MTX harbored increased species richness and diversity as compared with the patients receiving other treatments, indicating potential restoration of normal microbiota with targeted treatment.51
Along with the microbial diversity, MTX altered the functional potential of the microbiome which included change in redox environment, transport, and metabolism of sulfur and other metals. Analysis of metagenome functions in the RA patients harboring high prevalence of P. Copri revealed a significant reduction in purine metabolic pathways. This can be explained by MTX’s ability to inhibit dihydrofolate reductase, an enzyme which takes part in purine biosynthesis.52 These observations suggest that MTX treatment could alter microbiome structure and function in RA patients to normalize and restore the gut microbiome.
Sulfasalazine
5-aminosalicylic acid (5-ASA) is used in the form of the prodrug SSZ as an anti-inflammatory drug given orally to reduce inflammation and pain for the treatment of RA and inflammatory bowel disease (IBD). It is also prescribed to reduce inflammation, diarrhea, rectal bleeding, and pain associated with ulcerative colitis.78 SSZ scavenges reactive oxygen species (ROS), leading to conversion of arachidonic acid to prostaglandins.79 SSZ affects leukocyte function and production of cytokines. In vitro studies have shown that SSZ inhibits cell proliferation.80 SSZ contains an antibiotic moiety, sulfonamide, and is likely to have a direct effect on the gut microbiome.81 On the other hand, the host’s gut microbiota can impact activation of SSZ and its response as this prodrug requires bacterial enzymatic cleavage for acetylation to the active metabolite, ASA.82 The concept that intestinal bacteria are required for acetylation of SSZ was demonstrated in the late 1980s.83 As per our knowledge, there are no reports demonstrating the effect of SSZ on the gut microbiome of RA patients using the NGS approach. However, one report showed that SSZ treatment altered the fecal microflora, leading to a decrease of Escherichia coli, Bacteroides and total aerobic bacteria in RA patients with an increase in Bacillus. However, no significant changes in oral microbiota were observed.64
Another study used a culture-dependent approach to study the antimicrobial effect of SSZ on the gut microflora of patients with Crohn’s disease and colitis.65 Patients receiving SSZ treatment had decreased numbers of enterobacteria opalescent-negative clostridia and total nonspore forming anaerobes. This study suggested that the SSZ treatment of colitis could be responsible for the reduction in bacterial numbers.65 Future studies using a metagenome approach may help to understand the crosstalk between SSZ and gut microbiota and its relevance to disease activity.
Hydroxychloroquine
Hydroxychloroquine (HCQ) is an antimalarial drug also used to treat RA because of its anti-inflammatory activity.84 It reduces arthritis pain, joint swelling and may prevent the risk of long-term disability. It is known to interfere with the processing of auto-antigens by increasing lysosomal pH and reducing antigen processing and peptide loading in MHC class II proteins, resulting in decreased proliferation of CD4+ T cells and reduction in the release of cytokines.85 In addition, it can also interfere with TLR-dependent signaling. An in vitro study confirmed that this drug could inhibit the production of ILs such as IL-6, IL-17, and IL-22.86
In a study by Chen and coworkers, patients receiving HCQ showed an increase in microbial species richness and diversity in comparison with the untreated patients.51 This suggests that treatment with HCQ may partially reinstate bacterial diversity in RA patients.51 In an another study, HCQ treatment in combination with doxycycline led to a significant reduction in the microbes belonging to phyla Bacteroidetes and Firmicutes in the microbiota of endocarditis patients.67 Further, linear regression analysis revealed that the reduction in those phyla was significantly associated with the duration of treatment.67 Patients with juvenile idiopathic arthritis were reported to harbor significantly increased abundance of the bacteria belonging to phylum Bacteroidetes.76 Thus, reduction of Bacteroidetes induced by HCQ treatment represents the restoration of eubiosis via its gut modulatory mechanisms.67
Biologic DMARDs
Generally, when patients fail to respond to traditional DMARD therapy, the next line of treatment is another class of DMARDs called biologic DMARDs (bDMARDs). bDMARDs target specific components of the immune response such as TNF-α and other ILs such as IL-1 and IL-6 and are highly effective in slowing the disease progression, reducing symptoms and overall improving the quality of life.87 Biologics are frequently used in combination with MTX or other synthetic DMARDs. The bDMARDs are categorized into groups based on the targets they bind (Table 2). bDMARDs binding TNF-α and blocking its downstream effect are classified as TNF blockers. DMARDs such as etanercept (ETN), infliximab, adalimumab and golimumab fall into this category.88 bDMARDs that target T-cell and antigen-presenting cell (APC) stimulatory receptor, CTLA4, are categorized as T-cell costimulation modulator. Other bDMARDs target CD20 marker present on B cells, and cytokines like IL-1, IL-1R antagonist, and IL-6, IL-6R antagonist.60 Among the TNF blockers, ETN is a widely used option for treating RA patients, as it inhibits TNF-α and associated downstream pathways. TNF-α is a potent pro-inflammatory cytokine involved in the activation of the inflammatory nuclear factor kappa light-chain enhancer of activated B cells
Table 2.
List of biologic DMARDs and their mechanisms of action.
| Type | Biologic DMARDs | Mode of action | References |
|---|---|---|---|
| TNF blockers | Adalimumab Etanercept Golimumab Certolizumab Infliximab | Inhibits TNF-α and inflammatory pathways and reduced IL-1 and IL-6 | Picchianti-Diamanti et al.;63 Busquets et al.;93 Mazumdar and Greenwald;94 Kavanaugh et al.;95 Perdriger96 |
| T-cell costimulation modulator (CTLA-4) | Abatacept | Inhibits the interaction of T cells and APCs by binding to CD80/CD86 | Blair and Deeks97 |
| IL-1-receptor antagonist | Anakinra | Receptor antagonist for IL-1RI, blocks IL-1 reducing the migration of T cells into the joint | Dinarello et al.98 |
| IL-6-receptor antagonist | Tocilizumab | Interferes with the binding of IL-6 to its receptor and prevents further IL-6-induced inflammatory cascade | Sebba99 |
| Anti-CD20 | Rituximab | Binds CD20 on B cells and depletes B cells by complement-dependent cytotoxicity | Boumans and Tak100 |
APC, antigen-presenting cell; CTLA-4, cytotoxic lymphocyte-associated protein 4; IL, interleukin; TNF, tumor necrosis factor.
(NFkB) pathway. It also causes an increase in the Janus kinase (JNK) pathways which causes cell differentiation and proliferation. Although it is unknown whether or not TNF-α genes are associated with RA susceptibility, it plays a crucial role in causation of inflammation and joint destruction, which is supported by the high levels of TNF-α in patients with RA.89 TNF-α is also a paracrine inducer of other cytokines involved in inflammation including ILs, IL-1, IL-6, IL-8, and granulocyte–monocyte−colony-stimulating factor (GM-CSF).90 Significant reduction in the production of these cytokines was observed when antibodies against TNF-α were used in the cultures of synovial cells from RA patients indicating that TNF-α can contribute to pathogenesis via production of these inflammatory cytokines.91,92 Much like traditional DMARDs, patients treated with bDMARDs modulate the microbial communities.63
Anti-TNF-α bDMARDs
Adalimumab
Adalimumab is an anti-TNF-α monoclonal antibody drug that neutralizes TNF-α to reduce disease severity in patients. It is administered subcutaneously and is prescribed alone or in combination with traditional DMARDs. Studies have reported that patients with RA who received adalimumab treatment in combination with MTX show greater improvements in clinical and work productivity outcomes.101 Patients treated with adalimumab show increased numbers of T-regulatory cells with lower Th17.102 The effect of adalimumab on the gut microbial diversity of patients with RA has not been thoroughly studied; however, analysis by denaturing gradient gel electrophoresis has shown that treatment with adalimumab positively modulated the gut microbiota composition with partial restoration in Crohn’s disease patients.93 A similar scenario can be envisaged in patients with RA where treatment with adalimumab may induce changes in gut microbial diversity to restoring eubiosis.
Etanercept
ETN is a recombinant human fusion protein where the TNF receptor linked to the fragment-crystallizable portion of human IgG1. It suppresses the host immune system by inhibiting TNF-α and related downstream pathways of inflammation.103,104 Treatment with ETN has shown an effect on the host intestinal microbiota. In 2018, Picchianti-Diamanti and coworkers compared the intestinal microbial profile of untreated RA patients with those treated with ETN alone or in combination with MTX or MTX alone and healthy individuals.63 The study showed a significant decrease in faecalibacterium in RA patients as compared with healthy individuals, similar to a previous study.51 Interestingly, there were no significant differences among the patient groups treated with ETN, MTX, or ETN with MTX, except the MTX treated RA patients showed a decrease in enterobacterials. However, a comparison of ETN-treated patients with untreated RA patients showed some significant differences. In the ETN-treated RA group, a significant increase in the abundance of Cyanobacteria as compared with untreated patients was observed. There was also an increased abundance of the species Nostocophycideae which belong to the Cyanobacteria. Interestingly, the study did not find Nostocophycideae and Nostocales in the microbiome of untreated patient’s microbiota. To date, there are no reports available to help understand the possible functional beneficial and deleterious impacts posed by these taxa. Certain species of Cyanobacteria are known to produce secondary metabolites and bioactive compounds which are potent toxins with cytotoxic properties and have been implicated in gastroenteritis.105 However, certain secondary metabolites produced by Cyanobacteria possess anti-inflammatory and immunosuppressive activities including inhibition of cell division and protease inhibition, which may benefit RA patients.63
The same study also reported a significantly decreased abundance in species belonging to the Deltaproteobacteria and Clostridiaceae in the ETN-treated group as compared with the intestinal microbiota of RA patients without treatment. Reduction in these taxa could be potentially beneficial to the RA subjects since these taxa were previously reported to be enriched in patients with ulcerative colitis, RA and IBD-associated arthropathy.63 The study also showed a correlation between the abundance of Pasteurellales and disease activity, though no difference was observed among the treatment groups. However, since the study was cross-sectional, it is difficult to determine if the changes observed were causative or the effects of disease and if microbial changes can be used as an index for predicting anti-TNF-α antibody response.
Alternative medicines
Herbal medicines have been used as alternative medicines for the treatment of various diseases since ancient times. To get relief, people with arthritis are increasingly seeking a natural approach using herbal remedies. Likewise, researchers are also showing interest in bioactive compounds derived from plant with medicinal properties for RA treatment.48
Paederia scandens
Paederia scandens is a common medicinal plant, prevalent in China and south Asian countries. It is generally recognized as safe and used in food and as medicine to treat arthritis and various gastrointestinal diseases.106 Studies have found iridoid glycosides, flavonoids, and volatile oil to be the bioactive compounds in P. scandens. Among these, iridoid glycosides were found to inhibit the expression of TNF-α, IL-1β, and transforming-growth-factor beta and exert a protective effect against uric acid nephropathy and gouty arthritis in rats.107,108
Recently, a study by Xiao and colleagues explored the therapeutic effect of P. scandens using the type II collagen-induced arthritis (CIA) mouse model. The authors also focused on the modulation of the gut microbial community followed by P. scandens extract (PSE) treatment.106 The observations indicated that PSE treatment suppressed CIA significantly in a dose-dependent manner. Interestingly, arthritic mice receiving PSE showed higher microbial diversity than the control arthritic mice receiving water. This implies that treatment with P. scandens helps in restoring ‘nondisease’ or normal gut microbial ecosystem which is altered in CIA. This trend of restoring microbial diversity was also observed at the phylum level. Mice treated with PSE harbored an increased abundance of Bacteriodetes with a decrease in the abundance of Deferribacteres, in comparison with mice receiving water. Microbial community of arthritic mice receiving water had increased relative abundance of genera mucispirillum, desulfovibrio, and helicobacter and reduced abundance of S24-7 and rikenella, compared with the nondisease group mice. However, the opposite was true for the gut microbial community of a group of arthritic mice treated with P. scandens. This group showed a higher abundance of S24-7 and rikenella, but a decrease in the abundance of mucispirillum, desulfovibrio, and helicobacter in comparison with the microbial community of a group of arthritic mice receiving water only.
The microbial community changes induced by P. scandens could regulate inflammation since the genera with increased abundance in arthritic mice (receiving water) are related to disease conditions or inflammation. For example, mucispirillum, helicobacter and desulfovibrio have been correlated to intestinal inflammation and autoimmune diseases.109,110 Overall, these results suggest that one of the pathways by which P. scandens could suppress arthritis in mice via modulating the intestinal microbiota.
Tripterygium wilfordii
Tripterygium wilfordii is another medicinal plant that grows in China and historically, has been used for treating RA. T. wilfordii extract was as effective as DMARD treatment in reducing the numbers of joints swellings and levels of CRP and erythrocyte sedimentation rate in RA patients.111 T. wilfordii acts upon nitric-oxide-synthase genes and is regarded as an inflammatory modulator.112 However, its consumption may have potential side effects such as adverse reproductive outcomes, kidney and liver damage, cardiac damage, skin and hair disorders, weight change and even death, thus, is not recommended in countries like UK and USA.113–115
In Zhang and coworkers’ study, they observed that microbiome of RA patients treated with glycosides of T. wilfordii harbored a reduced number of MLGs such as Holdemania filiformis and Bacteroides sp. which were observed to be present with abundance in the guts of RA patients.56 Furthermore, MLG Prevotella intermedia which were abundant in control samples were also found enriched in the microbiome of RA patients treated with MTX and T. wilfordii compared with patients treated with T. wilfordii alone or MTX alone.56 Thus, the observations suggest that the glycosides from T. wilfordii possess properties to modulate the host microbiome.
Probiotics
Probiotics are living microorganisms that upon consumption in adequate amounts can improve the health of an individual.116 Metabolic products such as SCFAs and vitamins can be produced by probiotic members and are energy sources for enterocytes.20 Unsurprisingly, these products can modulate the gut microbiome and intestinal immunity to maintain gut homeostasis. Presently, probiotic bacteria like Lactobacillus rhamnosus,117,118 Lactobacillus casei,119 Bacillus coagulans,120 Lactobacillus reuteri, Lactobacillus acidophilus, and Bifidobacterium bifidum121 have been studied for their ability to treat RA in randomized controlled trials. These probiotics have been shown to be safe and effective for patients suffering from RA.121 In patients with RA treated with a mixture of lactobacillus probiotics, L. rhamnosus GR-1 and L. reuteri RC-14, supplementation led to reduced serum levels of cytokines such as IL-1α, IL-6, IL-10, IL-12p70 and TNF-α following treatment.117 Similarly, another report in RA patients treated with probiotic L. casei showed lower IL-12 and TNF-α levels with reduced swollen joints after treatment.121 Analysis of the fecal microflora of healthy human subjects consuming a probiotic stain of L. rhamnosus observed a transient alteration with little fluctuations in lactobacillus and bifidobacterium numbers.122 During the probiotic consumption period, lactobacillus and enterococcus were detected in higher number in all tested patients. However, after secession of consumption, most subjects returned to a lower number of the probiotic strain of L. rhamnosus.122 A recent study in mice using the CIA model showed that oral treatment with L. casei reduced arthritis incidence mediated by a decrease in humoral immune response.123
In another study, administration of probiotic strain L. rhamnosus recovered the Bacteroidetes and Verrucomicrobia abundance which was lost in rats fed with high-fat diet.124 Moreover, administration of the lactobacillus probiotic strain led to a decrease in abundance of Firmicutes, which was increased in the rats on high-fat diet.124 At a lower taxonomic level, the numbers of escherichia and shigella were suppressed, whereas the numbers of Lactobacillus spp. were increased. Such modulations might be beneficial for host health, as escherichia and shigella are considered harmful and lactobacillus taxa are considered probiotic.124 Similarly, in another study, an increase in lactobacillus and bifidobacterium colonies and a decrease in the numbers of Escherichia coli colonies were observed in mice treated with lactic-acid bacteria.125 From this data, it can be predicted that consumption of probiotics affiliated to lactobacillus might induce gut modulation in RA patients that could help reduce gut dysbiosis and disease symptoms.
In a randomized double-blind, placebo-controlled trial on RA patients, intervention with bifidobacterium probiotic supplementation resulted in reduced disease severity of joints. Members of lactobacillus and bifidobacterium are well known to produce SCFAs such as lactate and or acetate.126 These SCFAs serve as a substrate for enterocytes and other gut microbial members. Thus, probiotic supplementation can lead to microbial modulation in the consumers. In the past decade, several studies have reported an association between periodontal disease, Porphyromonas gingivalis, and RA.53 Furthermore, antibodies to P. gingivalis were more common in RA subjects than controls, suggesting a pathogenic role in RA.127 Probiotic therapy of Bifidobacterium adolescentis might be helpful in this case, as this bacterium competes with P. gingivalis by reducing vitamin K concentration.128
Recently, the reduction in the abundance of Faecalibacterium prausnitzii was observed in patients with RA in comparison with healthy controls.51,20 F. prausnitzii is present in abundance in a healthy gut and is one of the main butyrate producers.129,130 Probiotic therapy with F. prausnitzii could be helpful in suppressing inflammation, as it produces high amounts of butyrate and leads to the production of IL-10, an anti-inflammatory cytokine.131 In addition, F. prausnitzii is known for its immune-modulatory activities and acts by suppressing the NFkB pathway and maintaining epithelial integrity.131,132 Butyrate, can modulate gut bacterial composition, as it acts as a substrate for other microbial members present in the gut.20 Thus, from these data, one can predict that the consumption of certain probiotics may lead to gut microbiota modulation in RA patients that might be helpful in restoring gut eubiosis and reducing disease symptoms. But reports on its effect on the gut microbial community are scarce.
Another study using a rat model of arthritis showed a change in intestinal microbiome and reduced TNF-α with a decrease in disease after treatment with probiotic B. coagulans.133 These studies demonstrate that probiotic treatment can lead to immune regulation, and modulate disease severity by changing intestinal composition and host immune response. Future research using longitudinal studies in RA patients at various time points post-treatment with probiotics might help elucidate probiotics’ impact on the gut microbiome.
P. histicola as a human gut-derived probiotic for RA
Recently, a novel strain of P. histicola (MCI 001) was isolated from human gut.55,134 Use of P. histicola in a humanized mouse model of CIA showed that it can suppress arthritis in mice expressing RA-susceptible HLA-DQ8.55 A comparison of arthritic mice treated with P. histicola by oral gavage in a prophylactic as well as therapeutic protocol showed a reduced incidence of disease with delayed onset. In comparison, P. melaninogenica treatment enhanced disease severity and caused earlier onset of arthritis in DQ8 mice. P. histicola-treated DQ8 mice showed a change in the gut immune system as observed by decrease in pro-inflammatory Th17 cytokines and increase in IL-10.55 This change was also reflected in the systemic immune response and was mediated by the differentiation of T cells into T-regulatory cells in the lamina propria of the P. histicola-treated mice as compared with media-treated control group. In addition, there was an increase in the intestinal regulatory dendritic cells and myeloid suppressors. In addition, treatment with P. histicola reduced cellular and humoral immunity as observed by lower autoantibodies and decrease in antigen-specific T-cell proliferation after treatment even though response via TLR binding was not reduced.55 The decrease in pro-inflammatory cytokines and increase in regulatory cells in the gut led to an increase in tight junction proteins thus maintaining the gut epithelial integrity. Oral gavage of P. histicola in mice did not cause any intestinal pathology even though it shifted the gut microbiome with increased abundance of lactobacillus and sutterella.135,136 A study comparing fecal microbiota of patients with RA and osteoarthritis and healthy controls has shown low levels of prevotella in patients with RA.137 The studies with P. histicola suggest that it can be used as a probiotic for treating RA. Since P. histicola is endogenous to human gut, it should have lower side effects. Another advantage of treating with known probiotics is that in case of side effects, patients can be treated with a targeted antibiotic.135
Summary
DMARDs are widely used to treat RA. Besides having immunomodulatory properties, DMARDs can also modulate the host microbiota.56 Several studies have pointed to the gut microbial dysbiosis in patients with RA.109,138 Interestingly, most of the studies indicated that subjects’ dysbiotic microbiomes were partially restored to normality or altered to increase abundance of beneficial microbial members after treatment with MTX and HCQ, which was related to disease activity.56 There are limited reports on the impact of drugs like chloroquine, and SSZ on the gut microbiome. Since these drugs have antibiotic properties, one can predict that they have a direct modulating effect on the gut microbiota. Based on the available data in patients with RA and animal models of arthritis, a critical role of the altered gut microbiota is indicated in disease severity. Since partial microbial restoration after treaments is associated with efficacy of treatment, drug–microbiome network could provide an effective strategy to future therapeutics for RA. However, one has to consider the heterogeneity of disease as well as of the variability of treatments. Considering that intestinal microbiome is influenced by genetics and environmental factors, one can envisage that for probiotics, one size fits all may not apply. There is a growing interest among patients to use alternate or complimentary treatments such as herbal medication or probiotics.139,140 Presently, information on drug–microbiome interactions and probiotics for RA is limited, more work needs to be done before a probiotic, can be used for immune homeostasis. This might also help in individualizing medicine where a probiotic commensal/metabolite with low abundance in a patient can be supplemented for generating immune homeostasis (Figure 1).
Figure 1.
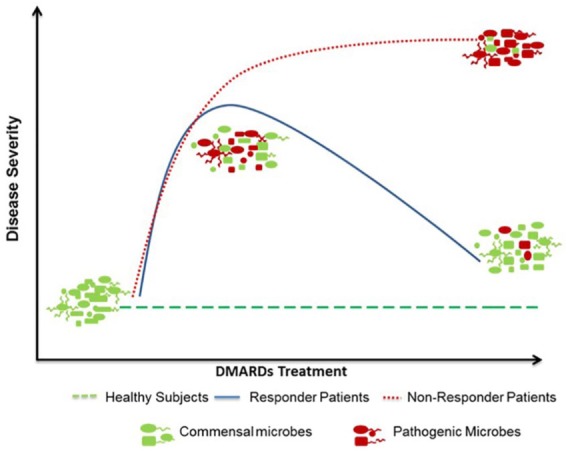
Disease-modifying drugs partially normalize the gut microbiomes of responders.
The pivotal contribution of gut microbiome in rheumatoid arthritis has been evidenced. Dynamic changes in gut microbiota during a lifetime determine host immunity. Expansion of certain clades of opportunistic commensals likely drives alterations in host’s microbial diversity, metabolic profile, and immune activation before and postdisease onset. Certain drugs like sulfasalazine require gut microbes for activity. Thus, distinct microbial profiles may determine the treatment with disease-modifying antirheumatic drugs (DMARDs). Responders to DMARDs show a partial normalization of the gut microbiota suggesting a crucial role of microbiota in treatment efficacy.
Footnotes
Authors’ Note: Rahul Bodkhe and Baskar Balakrishnan are now affiliated with National Center for Microbial Resource, National Center for Cell Science, Pune, India.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Veena Taneja is supported by the US Department of Defense (W81XWH-15-1-0213) and Department of Development, Mayo Clinic, Rochester, USA.
Rahul Bodkhe is supported by Fulbright-Nehru Doctoral Research Fellowship.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Veena Taneja  https://orcid.org/0000-0003-2401-694X
https://orcid.org/0000-0003-2401-694X
Contributor Information
Rahul Bodkhe, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Baskar Balakrishnan, Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Veena Taneja, Department of Immunology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA.
References
- 1. Xu B, Lin J. Characteristics and risk factors of rheumatoid arthritis in the United States: an NHANES analysis. PeerJ 2017; 5: e4035–e4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halpern MT, Cifaldi MA, Kvien TK. Impact of adalimumab on work participation in rheumatoid arthritis: comparison of an open-label extension study and a registry-based control group. Ann Rheum Dis 2009; 68: 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tran CN, Lundy SK, Fox DA. Synovial biology and T cells in rheumatoid arthritis. Pathophysiology 2005; 12: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burger D, Dayer J-M. The role of human T-lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res 2002; 4(Suppl. 3): S169–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klareskog L, Forsum U, Scheynius A, et al. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A 1982; 79: 3632–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yu MB, Langridge WHR. The function of myeloid dendritic cells in rheumatoid arthritis. Rheumatol Int 2017; 37: 1043–1051. [DOI] [PubMed] [Google Scholar]
- 7. Raychaudhuri S, Sandor C, Stahl EA, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 2012; 44: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stahl EA, Raychaudhuri S, Remmers EF, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 2010; 42: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luckey D, Behrens M, Smart M, et al. DRB1*0402 may influence arthritis by promoting naive CD4+ T-cell differentiation in to regulatory T cells. Eur J Immunol 2014; 44: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costenbader KH, Chang S-C, Laden F, et al. Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med 2008; 168: 1664–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bengtsson C, Nordmark B, Klareskog L, et al. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis 2005; 64: 1588–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips DIW, Barker DJP, Fall CHD, et al. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab 1998; 83: 757–760. [DOI] [PubMed] [Google Scholar]
- 13. Stolt P, Bengtsson C, Nordmark B, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis 2003; 62: 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122: 107–118. [DOI] [PubMed] [Google Scholar]
- 15. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004; 118: 229–241. [DOI] [PubMed] [Google Scholar]
- 16. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hill DA, Hoffmann C, Abt MC, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 2010; 3: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32: 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marietta E, Horwath I, Taneja V. Microbiome, immunomodulation, and the neuronal system. Neurotherapeutics 2018; 15: 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balakrishnan B, Taneja V. Microbial modulation of the gut microbiome for treating autoimmune diseases. Expert Rev Gastroenterol Hepatol 2018; 12: 985–996. [DOI] [PubMed] [Google Scholar]
- 21. Marietta E, Horwath I, Balakrishnan B, et al. Role of the intestinal microbiome in autoimmune diseases and its use in treatments. Cell Immunol 2019; 339: 50–58. [DOI] [PubMed] [Google Scholar]
- 22. Boon E, Meehan CJ, Whidden C, et al. Interactions in the microbiome: communities of organisms and communities of genes. FEMS Microbiol Rev 2014; 38: 90–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature 2012; 489: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D’Argenio V. Human microbiome acquisition and bioinformatic challenges in metagenomic studies. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang D, Leung RK, Guan W, et al. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog 2018; 10: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magnusdottir S, Ravcheev D, De Crecy-Lagard V, et al. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet 2015; 6: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothschild D, Weissbrod O, Barkan E, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018; 555: 210–215. [DOI] [PubMed] [Google Scholar]
- 29. Vande Voorde J, Sabuncuoglu S, Noppen S, et al. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J Biol Chem 2014; 289: 13054–13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehouritis P, Cummins J, Stanton M, et al. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep 2015; 5: 14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017; 357: 1156–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sousa T, Yadav V, Zann V, et al. On the colonic bacterial metabolism of azo-bonded prodrugs of 5-aminosalicylic acid. J Pharm Sci 2014; 103: 3171–3175. [DOI] [PubMed] [Google Scholar]
- 33. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018; 555: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sousa T, Paterson R, Moore V, et al. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm 2008; 363: 1–25. [DOI] [PubMed] [Google Scholar]
- 35. Wilson ID, Nicholson JK. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res 2017; 179: 204–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Isaacs JD. Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology (Oxford) 2008; 47: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 37. Kumar P, Banik S. Pharmacotherapy options in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord 2013; 6: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Benjamin O, Lappin SL. Disease modifying anti-rheumatic drugs (DMARD). Treasure Island, FL: StatPearls Publishing, 2019. [PubMed] [Google Scholar]
- 39. Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007; 66: 1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Keystone E, Emery P, Peterfy CG, et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann Rheum Dis 2009; 68: 216–221. [DOI] [PubMed] [Google Scholar]
- 41. Brasington R. Disease-modifying antirheumatic drugs. J Hand Surg 2009; 34: 347–348. [DOI] [PubMed] [Google Scholar]
- 42. Raychaudhuri SP, Raychaudhuri SK. Biologics: target-specific treatment of systemic and cutaneous autoimmune diseases. Indian J Dermatol 2009; 54: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phang JK, Kwan YH, Goh H, et al. Complementary and alternative medicine for rheumatic diseases: a systematic review of randomized controlled trials. Complement Ther Med 2018; 37: 143–157. [DOI] [PubMed] [Google Scholar]
- 44. Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 2017; 66: 515–522. [DOI] [PubMed] [Google Scholar]
- 45. Hasegawa M, Yang K, Hashimoto M, et al. Differential release and distribution of Nod1 and Nod2 immunostimulatory molecules among bacterial species and environments. J Biol Chem 2006; 281: 29054–29063. [DOI] [PubMed] [Google Scholar]
- 46. Inohara Chamaillard, McDonald C, et al. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 2005; 74: 355–383. [DOI] [PubMed] [Google Scholar]
- 47. Reichardt N, Duncan SH, Young P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 2014; 8: 1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choudhary M, Kumar V, Malhotra H, et al. Medicinal plants with potential anti-arthritic activity. J Intercult Ethnopharmacol 2015; 4: 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kohashi O, Kuwata J, Umehara K, et al. Susceptibility to adjuvant-induced arthritis among germfree, specific-pathogen-free, and conventional rats. Infect Immun 1979; 26: 791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gomez A, Luckey D, Yeoman CJ, et al. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One 2012; 7: e36095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen J, Wright K, Davis JM, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 2016; 8: 43–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scher JU, Abramson SB. Periodontal disease, Porphyromonas gingivalis, and rheumatoid arthritis: what triggers autoimmunity and clinical disease? Arthritis Res Ther 2013; 15: 122–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pianta A, Arvikar S, Strle K, et al. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol 2017; 69: 964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marietta EV, Murray JA, Luckey DH, et al. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol 2016; 68: 2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang X, Zhang D, Jia H, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med 2015; 21: 895–905. [DOI] [PubMed] [Google Scholar]
- 57. Strand V. Longer term benefits of treating rheumatoid arthritis: assessment of radiographic damage and physical function in clinical trials. Clin Exp Rheumatol 2004; 22: S57–S64. [PubMed] [Google Scholar]
- 58. Pisetsky DS, Ward MM. Advances in the treatment of inflammatory arthritis. Best Pract Res Clin Rheumatol 2012; 26: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Russell AS. Quality-of-life assessment in rheumatoid arthritis. Pharmacoeconomics 2008; 26: 831–846. [DOI] [PubMed] [Google Scholar]
- 60. Buckley F, Finckh AJ, Huizinga TW, et al. Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm 2015; 21: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ogrendik M. Antibiotics for the treatment of rheumatoid arthritis. Int J Gen Med 2013; 7: 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou B, Xia X, Wang P, et al. Induction and amelioration of methotrexate-induced gastrointestinal toxicity are related to immune response and gut microbiota. EBioMedicine 2018; 33: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Picchianti-Diamanti A, Panebianco C, Salemi S, et al. Analysis of gut microbiota in rheumatoid arthritis patients: disease-related dysbiosis and modifications induced by etanercept. Int J Mol Sci 2018; 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kanerud L, Scheynius A, Nord CE, et al. Effect of sulphasalazine on gastrointestinal microflora and on mucosal heat shock protein expression in patients with rheumatoid arthritis. Br J Rheumatol 1994; 33: 1039–1048. [DOI] [PubMed] [Google Scholar]
- 65. West B, Lendrum R, Hill MJ, et al. Effects of sulphasalazine (Salazopyrin) on faecal flora in patients with inflammatory bowel disease. Gut 1974; 15: 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Breedveld FC, Dayer JM. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis 2000; 59: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Angelakis E, Million M, Kankoe S, et al. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob Agents Chemother 2014; 58: 3342–3347. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68. Trotter JL, Rodey GE, Gebel HM. Azathioprine decreases suppressor T cells in patients with multiple sclerosis. N Engl J Med 1982; 306: 365–366. [DOI] [PubMed] [Google Scholar]
- 69. Smith CJ, Sayles H, Mikuls TR, et al. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res Ther 2011; 13: R168–R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vena GA, Cassano N, Iannone F. Update on subcutaneous methotrexate for inflammatory arthritis and psoriasis. Ther Clin Risk Manag 2018; 14: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 2013; 124: 16–25. [PMC free article] [PubMed] [Google Scholar]
- 72. Wessels JAM, Huizinga TWJ, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology 2007; 47: 249–255. [DOI] [PubMed] [Google Scholar]
- 73. Quéméneur L, Gerland L-M, Flacher M, et al. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol 2003; 170: 4986–4995. [DOI] [PubMed] [Google Scholar]
- 74. Aggarwal A, Misra R. Methotrexate inhibits interleukin-6 production in patients with juvenile rheumatoid arthritis. Rheumatol Int 2003; 23: 134–137. [DOI] [PubMed] [Google Scholar]
- 75. Gerards AH, de Lathouder S, De Groot ER, et al. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology 2003; 42: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 76. Tejesvi MV, Arvonen M, Kangas SM, et al. Faecal microbiome in new-onset juvenile idiopathic arthritis. Eur J Clin Microbiol Infect Dis 2016; 35: 363–370. [DOI] [PubMed] [Google Scholar]
- 77. Ramos-Romero S, Hereu M, Atienza L, et al. Mechanistically different effects of fat and sugar on insulin resistance, hypertension, and gut microbiota in rats. Am J Physiol Endocrinol Metabol 2018; 314: E552–E563. [DOI] [PubMed] [Google Scholar]
- 78. Xu C-T, Meng S-Y, Pan B-R. Drug therapy for ulcerative colitis. World J Gastroenterol 2004; 10: 2311–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Punchard NA, Greenfield SM, Thompson RP. Mechanism of action of 5-arninosalicylic acid. Mediators Inflamm 1992; 1: 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. MacDermott RP. Progress in understanding the mechanisms of action of 5-aminosalicylic acid. Am J Gastroenterol 2000; 95: 3343–3345. [DOI] [PubMed] [Google Scholar]
- 81. Horta-Baas G, Romero-Figueroa MDS, Montiel-Jarquín AJ, et al. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. J Immunol Res. Epub ahead of print 30 August 2017. DOI: 10.1155/2017/4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scher JU, Abramson SB. The microbiome and rheumatoid arthritis. Nat Rev Rheumatol 2011; 7: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dull BJ, Salata K, Goldman P. Role of the intestinal flora in the acetylation of sulfasalazine metabolites. Biochem Pharmacol 1987; 36: 3772–3774. [DOI] [PubMed] [Google Scholar]
- 84. Sebastiani S, Fresina M, Cellini M, et al. Hydroxychloroquine for treatment of rheumatoid arthritis: multifocal electroretinogram and laser flare-cell photometry study. Clin Ophthalmol 2017; 11: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling. Nat Clin Pract Rheumatol 2006; 2: 458–459. [DOI] [PubMed] [Google Scholar]
- 86. Da Silva JC, Mariz HA, Da Rocha LF, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics 2013; 68: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011; 33: 679–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Fenix-Caballero S, Alegre-del Rey EJ, Castano-Lara R, et al. Direct and indirect comparison of the efficacy and safety of adalimumab, etanercept, infliximab and golimumab in psoriatic arthritis. J Clin Pharm Ther 2013; 38: 286–293. [DOI] [PubMed] [Google Scholar]
- 89. Farrugia M, Baron B. The role of TNF-α in rheumatoid arthritis: a focus on regulatory T cells. J Clin Transl Res 2016; 2(3): 84–90. [PMC free article] [PubMed] [Google Scholar]
- 90. Cromwell O, Hamid Q, Corrigan CJ, et al. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology 1992; 77: 330–337. [PMC free article] [PubMed] [Google Scholar]
- 91. Butler DM, Maini RN, Feldmann M, et al. Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures. Comparison of monoclonal anti TNF-alpha antibody with the interleukin-1 receptor antagonist. Eur Cytokine Netw 6: 225–230. [PubMed] [Google Scholar]
- 92. Vasanthi P, Nalini G, Rajasekhar G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: a review. APLAR J Rheum 2007; 10: 270–274. [Google Scholar]
- 93. Busquets D, Mas-de-Xaxars T, López-Siles M, et al. Anti-tumour necrosis factor treatment with adalimumab induces changes in the microbiota of Crohn’s disease. J Crohns Colitis 2015; 9: 899–906. [DOI] [PubMed] [Google Scholar]
- 94. Mazumdar S, Greenwald D. Golimumab. MAbs 2009; 1: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kavanaugh A, Smolen JS, Emery P, et al. Effect of certolizumab pegol with methotrexate on home and work place productivity and social activities in patients with active rheumatoid arthritis. Arthritis Rheum 2009; 61: 1592–1600. [DOI] [PubMed] [Google Scholar]
- 96. Perdriger A. Infliximab in the treatment of rheumatoid arthritis. Biologics 2009; 3: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Blair HA, Deeks ED. Abatacept: a review in rheumatoid arthritis. Drugs 2017; 77: 1221–1233. [DOI] [PubMed] [Google Scholar]
- 98. Dinarello CA, Simon A, Van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 2012; 11: 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst 2008; 65: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 100. Boumans MJ, Tak PP. Rituximab treatment in rheumatoid arthritis: how does it work? Arthritis Res Ther 2009; 11: 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Emery P, Smolen JS, Ganguli A, et al. Effect of adalimumab on the work-related outcomes scores in patients with early rheumatoid arthritis receiving methotrexate. Rheumatology 2016; 55: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 102. Nguyen DX, Ehrenstein MR. Anti-TNF drives regulatory T cell expansion by paradoxically promoting membrane TNF-TNF-RII binding in rheumatoid arthritis. J Exp Med 2016; 213: 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Haraoui B, Bykerk V. Etanercept in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 2007; 3: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Alldred A. Etanercept in rheumatoid arthritis. Expert Opin Pharmacother 2001; 2: 1137–1148. [DOI] [PubMed] [Google Scholar]
- 105. Rao PVL, Gupta N, Bhaskar ASB, et al. Toxins and bioactive compounds from cyanobacteria and their implications on human health. J Environ Biol 2002; 23: 215–224. [PubMed] [Google Scholar]
- 106. Xiao M, Fu X, Ni Y, et al. Protective effects of Paederia scandens extract on rheumatoid arthritis mouse model by modulating gut microbiota. J Ethnopharmacol 2018; 226: 97–104. [DOI] [PubMed] [Google Scholar]
- 107. Yang T, Kong B, Gu J-W, et al. Anticonvulsant and sedative effects of paederosidic acid isolated from Paederia scandens (Lour.) Merrill. in mice and rats. Pharmacol Biochem Behav 2013; 111: 97–101. [DOI] [PubMed] [Google Scholar]
- 108. Zhu W, Pang M, Dong L, et al. Anti-inflammatory and immunomodulatory effects of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on uric acid nephropathy rats. Life Sci 2012; 91: 369–376. [DOI] [PubMed] [Google Scholar]
- 109. Berry D, Kuzyk O, Rauch I, et al. Intestinal microbiota signatures associated with inflammation history in mice experiencing recurring colitis. Front Microbiol 2015; 6: 1408–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol 2014; 20: 6374–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang J, Chen N, Fang L, et al. A systematic review about the efficacy and safety of Tripterygium wilfordii Hook. f. Preparations used for the management of rheumatoid arthritis. J Evid Based Complementary Altern Med 2018; 2018: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Yuan J, Ye H, Li S, et al. Comparison of methotrexate with Tripterygium wilfordii Hook F in the management of rheumatoid arthritis. J Bioanal Biomed 2015; 07: 1–2. [Google Scholar]
- 113. Cameron M, Gagnier JJ, Chrubasik S. Herbal therapy for treating rheumatoid arthritis. Cochrane Database Syst Rev 2011: CD002948. [DOI] [PubMed] [Google Scholar]
- 114. Zhang C, Sun PP, Guo HT, et al. Safety profiles of Tripterygium wilfordii Hook F: a systematic review and meta-analysis. Front Pharmacol 2016; 7: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Canter PH, Lee HS, Ernst E. A systematic review of randomised clinical trials of Tripterygium wilfordii for rheumatoid arthritis. Phytomedicine 2006; 13: 371–377. [DOI] [PubMed] [Google Scholar]
- 116. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 2013; 6: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pineda ML, Thompson SF, Summers K, et al. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit 2011; 17: CR347–CR3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hatakka K, Martio J, Korpela M, et al. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis–a pilot study. Scand J Rheumatol 2003; 32: 211–215. [DOI] [PubMed] [Google Scholar]
- 119. Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, et al. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014; 30: 430–435. [DOI] [PubMed] [Google Scholar]
- 120. Mandel DR, Eichas K, Holmes J. Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med 2010; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zamani B, Golkar HR, Farshbaf S, et al. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int J Rheum Dis 2016; 19: 869–879. [DOI] [PubMed] [Google Scholar]
- 122. Tannock GW, Munro K, Harmsen HJ, et al. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol 2000; 66: 2578–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci 1998; 63: 635–644. [DOI] [PubMed] [Google Scholar]
- 124. Chen D, Yang Z, Chen X, et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complement Altern Med 2014; 14: 386–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xie N, Cui Y, Yin Y-N, et al. Effects of two lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement Altern Med 2011; 11: 53–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Aoki R, Kamikado K, Suda W, et al. A proliferative probiotic bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep 2017; 7: 43522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mikuls TR, Payne JB, Reinhardt RA, et al. Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol 2009; 9: 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hojo K, Nagaoka S, Murata S, et al. Reduction of vitamin K concentration by salivary bifidobacterium strains and their possible nutritional competition with Porphyromonas gingivalis. J Appl Microbiol 2007; 103: 1969–1974. [DOI] [PubMed] [Google Scholar]
- 129. Barcenilla A, Pryde SE, Martin JC, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol 2000; 66: 1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khan MT, Duncan SH, Stams AJ, et al. The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic-anoxic interphases. ISME J 2012; 6: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Rossi O, Van Berkel LA, Chain F, et al. Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses. Sci Rep 2016; 6: 18507–18507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yan F, Polk DB. Disruption of NF-kappaB signalling by ancient microbial molecules: novel therapies of the future? Gut 2010; 59: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Khadijeh Abhari SSS, Saeid Hosseinzadeh, Saeid Nazifi, et al. The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr Res 2016; 60: 30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Balakrishnan B, Luckey D, Marietta E, et al. Development of a real-time PCR method for quantification of Prevotella histicola from the gut. Anaerobe 2017; 48: 37–41. [DOI] [PubMed] [Google Scholar]
- 135. Marietta EV, Murray JA, Luckey DH, et al. Suppression of inflammatory arthritis by human gut-derived Prevotella histicola in humanized mice. Arthritis Rheumatol 2016; 68: 2878–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mangalam A, Shahi SK, Luckey D, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep 2017; 20: 1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Vaahtovuo J, Munukka E, Korkeamäki M, et al. Fecal microbiota in early rheumatoid arthritis. J Rheumatol 2008; 35: 1500–1505. [PubMed] [Google Scholar]
- 138. Wu X, He B, Liu J, et al. Molecular insight into gut microbiota and rheumatoid arthritis. Int J Mol Sci 2016; 17: 431–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Khanna S, Jaiswal KS, Gupta B. Managing rheumatoid arthritis with dietary interventions. Front Nutr 2017; 4: 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Reid R, Steel A, Wardle J, et al. Complementary medicine use by the Australian population: a critical mixed studies systematic review of utilisation, perceptions and factors associated with use. BMC Complement Altern Med 2016; 16: 176–176. [DOI] [PMC free article] [PubMed] [Google Scholar]


