Abstract
Background: Nuclear imaging biomarkers illustrate unique aspects of lung physiology and are useful for assessing therapeutic effects in cystic fibrosis (CF) lung disease. We have developed a multiprobe method to simultaneously measure mucociliary clearance (MCC) and paracellular absorption (ABS). MCC is a direct measure of mucus clearance. ABS has been related to airway surface liquid (ASL) absorption through previous in vitro studies.
Methods: We describe baseline factors affecting MCC and ABS using data from a retrospective baseline group (n = 22) and the response of the measures to inhaled 7% hypertonic saline (HS) and dry powder mannitol using data from a prospective response group (n = 7). A retrospective healthy control group (n = 15) is also described. The baseline and control groups performed single measurements of MCC/ABS. The response group performed baseline measurements of MCC/ABS and measurements after each intervention.
Results: ABS was correlated (Spearman's ρ = 0.51, p = 0.06) to sweat chloride, a systemic measure of cystic fibrosis transmembrane conductance regulator (CFTR) function, whereas MCC was not. Baseline MCC was depressed after Pseudomonas aeruginosa infection as we have previously described. MCC provided a more sensitive indication of therapeutic effect and indicated improved clearance with mannitol compared with HS.
Conclusion: MCC provides a useful and well-established means of testing therapies directed at improving mucus clearance in the lung. ABS may provide a means of detecting local changes in ASL absorption and CFTR function in the lung. Both are useful tools for studying the key aspects of CF lung pathophysiology (ASL hyperabsorption and MCC depression) that link the basic genetic defects of CF to disease manifestations in the lung.
Keywords: airway surface liquid, CFTR, DTPA, mucociliary clearance
Introduction
The basic defect of cystic fibrosis (CF) lung disease is the absence or dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel on airway surfaces. Chloride and bicarbonate secretion from CFTR are needed to maintain normal airway surface liquid (ASL) hydration and pH, respectively. CF lung disease is associated with airway mucus dehydration and accumulation, chronic infection and inflammation, and respiratory failure. Failed mucociliary clearance (MCC) is often described as a link between the basic ion and liquid transport defects of CF and chronic infection in the airways.(1)
Here, we consider two nuclear imaging biomarkers of CF lung disease: MCC, a measure of particle clearance associated with MCC rate, and paracellular absorption (ABS), a measure of the ABS of a small-molecule probe associated with ASL absorption and lung permeability. The MCC measurement involves the inhaled delivery of radiolabeled particle probes (technetium 99m-sulfur colloid; Tc-SC) in a nebulized liquid aerosol, using delivery techniques that preferentially deposit the aerosol in the airways. Serial gamma camera images are then collected that depict the clearance of the nonabsorbable particles in mucus, allowing an MCC rate to be measured. It has been used to demonstrate the MCC changes associated with inhaled hypertonic saline (HS)(2) and mannitol,(3) and the CFTR potentiator ivacaftor.(4) MCC may also be altered by CF-related changes in mucus viscosity. ABS was developed as a potential means of detecting changes in ASL absorption. It is measured by adding a small-molecule radiolabeled probe (indium 111-DTPA; In-DTPA) to the same aerosol delivered to measure MCC.(5–7) In-DTPA clears by both MCC and absorption through the epithelium, whereas Tc-SC is cleared by MCC only. ABS is the absorptive component of In-DTPA clearance and is calculated by subtracting the Tc-SC clearance rate from the total clearance rate of In-DTPA. DTPA absorption is increased in human bronchial epithelial cell cultures from CF lungs, proportional to liquid absorption, and decreases when an osmotic gradient is established favoring ASL hydration.(8)
Our previous clinical studies have demonstrated increased ABS in the lungs of adults and children with CF(5,7) and that MCC and ABS respond to oral inhalation of HS.(7) We have established the repeatability of both measurements, described an association between a previous history of Pseudomonas aeruginosa (PA) infection and depressed MCC in CF,(6) and used the measurements to evaluate a nasal aerosol system for pulmonary delivery of HS.(9)
Here, we describe variation in baseline MCC and ABS based on CFTR function through a consideration of sweat chloride measurements, subject genotype, and subject use of ivacaftor. We also consider the effect of PA infection on MCC/ABS relative to CFTR function. Finally we compare therapeutic response demonstrated with MCC and ABS after the inhalation of HS and dry powder mannitol.
Materials and Methods
Subject groups
We analyzed three subject groups. Our baseline group includes 27 CF subjects, ages 9–30 years, who performed baseline MCC and ABS scans as a part of previous studies performed at our center from 2010 to 2014. We have previously reported data on how PA infection affects MCC and ABS using a data set that included multiple measurements from this subject group.(6) Here, we use a more limited version of the data set that includes only the first measurement from each subject to consider effects related to CFTR function. The effect of PA infection on MCC is analyzed in this subgroup and reported here so its relative effect on MCC and ABS can be compared. Our retrospective control group includes 15 healthy controls who performed MCC and ABS scans during previous studies at our center. And our prospective response group includes 12 CF subjects who performed 3 measurements of MCC/ABS, including a baseline measurement and 2 postintervention measurements.
Since our previous studies had demonstrated a confounding effect of high levels of central airway aerosol deposition that affected both ABS and MCC,(6) we removed subjects from the baseline, control, and response groups who had any single study day measurement with >50% central deposition. This included 5/27 subjects in the baseline group, 0/15 subjects in the control group, and 3/12 subjects in the response group. One subject who did not complete all response study days was also removed as was another subject with a genotype questionable for CF, yielding the following group sizes: baseline (n = 22), control (n = 15), and response (n = 7).
In some of these analyses, we compare subjects having “functional CFTR,” defined here as F508del/G551D subjects utilizing ivacaftor or subjects with genotypes associated with residual CFTR function, to subjects having “no CFTR,” defined here as F508del/F508del subjects and F508del heterozygous subjects with a second mutation associated with minimal CFTR function. No subjects were utilizing lumacaftor or tezacaftor.
Clinicaltrials.gov registrations: NCT01887197, NCT01223183, NCT01486199, and NCT00541190. All studies were approved by the University of Pittsburgh Institutional Review Board.
MCC/ABS measurements and interventions
Subjects in the baseline and control groups inhaled a liquid aerosol containing the particle and small-molecule probes [8 mCi (296 MBq) of Tc-SC and 1.5 mCi (55.5 MBq) of In-DTPA] in 3 mL of isotonic saline for 2 minutes (13 years or younger) or 4 minutes (14 years or older). Delivery techniques favored aerosol deposition in the airways.(10) Sequential images were then collected for 80 minutes with the subjects lying recumbent. Isotonic saline was inhaled from t = 10–20 minutes while recumbent. This was done to provide a stimulus for liquid absorption in the airways. These methods have been previously described.(5–7)
Subjects in the response group inhaled two puffs of albuterol before inhaling the Tc-SC/In-DTPA mixture described earlier for 5 minutes. Sequential images were then collected for 82 minutes with the subjects lying recumbent, except for t = 10–22 minutes when they were seated for intervention delivery. During that period, they performed no intervention (baseline), inhaled 4 mL of 7% HS from a Pari Plus nebulizer with a Pulmo-Aide compressor, or inhaled 10 × 40 mg doses of dry powder mannitol (Bronchitol; Pharmaxis Ltd., Frenchs Forest, Australia). The intervention order was randomized for three study days. Follow-up images were also collected at t = 2 and 3 hours (15 minutes each).
Statistics
Nonpaired group comparisons used the nonparametric Wilcoxon rank-sum test. Paired comparisons used the Wilcoxon matched-pairs signed-rank test. Correlations between continuous variables were evaluated by the Spearman's rank correlation coefficient. Multivariate regression was used to consider potential confounding effects. All calculations were performed using Stata 14 (StataCorp LLC, College Station, TX). Unless otherwise indicated, data reported are mean ± standard deviation.
Results
Subject characteristics along with measurements of MCC and ABS are included in Table 1 for the baseline group and Table 2 for the response group. The control group included 15 healthy subjects (11 male) with an average age of 28 ± 13 years and an average one-second forced expiratory volume (FEV1) of 99% ± 11% of predicted.
Table 1.
Subject Characteristics, Pulmonary Function (% of Predicted), Cystic Fibrosis Transmembrane Conductance Regulator Genotype, Mucociliary Clearance (% Cleared/80 Minutes), Paracellular Absorption (% Absorbed/80 Minutes), and Central Aerosol Deposition Percentage for the Cystic Fibrosis Baseline Group
| Subject | Age | Gender | FEV1 | FEF2575 | Genotype | MCC | ABS | Cen% | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Male | 88 | 65 | F508del | F508del | 24.9 | 44.8 | 42 |
| 2 | 9 | Female | 106 | 82 | F508del | F508del | 33 | 19.5 | 37 |
| 3 | 9 | Female | 41 | 25 | F508del | F508del | 10.5 | 30.0 | 36 |
| 4 | 12 | Female | 103 | 134 | F508del | F508del | 27.9 | 55.0 | 37 |
| 5 | 11 | Male | 78 | 45 | F508del | F508del | 13.6 | 26.8 | 48 |
| 6 | 10 | Male | 94 | 91 | F508del | F508del | 38.8 | 30.3 | 42 |
| 7 | 9 | Female | 100 | 111 | F508del | F508del | 40.2 | 30.4 | 48 |
| 8 | 12 | Male | 80 | 56 | F508del | R1162X | 0 | 33.3 | 49 |
| 9 | 11 | Male | 93 | 80 | F508del | F508del | 15.9 | 35.7 | 38 |
| 10 | 19 | Female | 91 | 54 | F508del | F508del | 18.4 | 29.3 | 41 |
| 11 | 21 | Female | 76 | 56 | F508del | F508del | 41.8 | 16.3 | 39 |
| 12 | 19 | Female | 51 | 26 | F508del | F508del | 21.1 | 26.7 | 34 |
| 13 | 20 | Female | 70 | 70 | F508del | F508del | 8.8 | 27.6 | 39 |
| 14 | 18 | Male | 98 | 62 | F508del | F508del | 14.2 | 46.1 | 42 |
| 15 | 21 | Male | 65 | 34 | F508del | F508del | 9.2 | 47.3 | 38 |
| 16 | 25 | Male | 90 | 68 | 2711delt | 5T** | 23.2 | 16.6 | 50 |
| 17 | 29 | Female | 49 | 25 | F508del | F508del | 30.5 | 30.6 | 43 |
| 18 | 23 | Male | 79 | 50 | F508del | G551D* | 34.3 | 15.7 | 46 |
| 19 | 18 | Male | 64 | 26 | F508del | G551D* | 27.2 | 11.2 | 45 |
| 20 | 30 | Male | 81 | 56 | F508del | 2789 + 5G>A** | 14.4 | 40.2 | 40 |
| 21 | 20 | Male | 60 | 28 | F508del | G551D* | 31.2 | 33.4 | 43 |
| 22 | 24 | Male | 101 | 91 | F508del | S977F** | 26.1 | 34.1 | 42 |
| Average | 17 | 80 | 61 | 23.0 | 31.0 | 42 | |||
| SD | 7 | 19 | 29 | 11.3 | 11.2 | 4 | |||
Studies performed from 2010 to 2014, before approval of ivacaftor/lumacaftor (Orkambi®). Pulmonary function was measured on MCC/ABS test day.
G551D subjects utilizing ivacaftor at the time of MCC/ABS measurement.
Genotype associated with residual CFTR function.
ABS, paracellular absorption; CFTR, cystic fibrosis transmembrane conductance regulator; FEF, forced expiratory flow rate; FEV1, one-second forced expiratory volume; MCC, mucociliary clearance; SD, standard deviation.
Table 2.
Subject Characteristics, Pulmonary Function (% of Predicted), Cystic Fibrosis Transmembrane Conductance Regulator Genotype, Baseline Mucociliary Clearance (% Cleared/80 Minutes), Baseline Paracellular Absorption (% Absorbed/80 Minutes), and Baseline Central Aerosol Deposition Percentage for the Cystic Fibrosis Response Group
| Subject | Age | Gender | FEV1 | FEF2575 | Genotype | MCC | ABS | Cen% | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 38 | Male | 55 | 36 | F508del | F508del | 23.7 | 38.0 | 39 |
| 2 | 23 | Male | 68 | 50 | F508del | G542X | 11.1 | 47.9 | 41 |
| 3 | 44 | Male | 52 | 26 | F508del | G542X | 18.1 | 48.1 | 37 |
| 4 | 22 | Female | 84 | 94 | F508del | N1303K | 11.2 | 51.3 | 40 |
| 5 | 33 | Male | 99 | 94 | F508del | 1717-1G>A | 5.4 | 56.8 | 36 |
| 6 | 37 | Female | 89 | 77 | F508del | F508del | 9.0 | 49.0 | 45 |
| 7 | 32 | Male | 82 | 66 | F508del | F508del | 26.6 | 47.7 | 47 |
| Average | 33 | 76 | 63 | 15.0 | 48.4 | 41 | |||
| SD | 8 | 18 | 27 | 7.9 | 5.6 | 4 | |||
No subjects were utilizing ivacaftor or ivacaftor/lumacaftor (Orkambi). Pulmonary function was measured on MCC/ABS test day.
Baseline group results
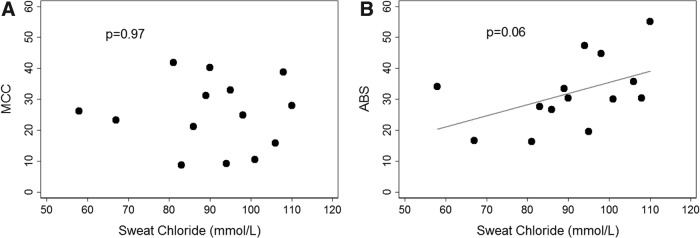
Sweat chloride correlates inversely with CFTR function.(11) In diagnostic use, values ≥60 mmol/L are indicative of CF. Figure 1 compares ABS and MCC with sweat chloride in the 14 subjects from the baseline group and who had sweat data available from their time of CF diagnosis. Subjects using ivacaftor were not included since sweat chloride values are known to change with treatment and their post-treatment sweat chloride values were not available.(11) There was no relationship between MCC and sweat chloride. The correlation between ABS and sweat chloride approaches significance, ρ = 0.51, p = 0.06, likely indicating that absorption of the small-molecule probe in the lung increases with decreasing CFTR function.
FIG. 1.
Comparing measurements of (A) MCC and (B) ABS versus subject sweat chloride values as assessed at the time of CF diagnosis in the baseline group. (Values are not included for subjects receiving ivacaftor.) MCC and ABS are % cleared/80 minutes. MCC: Spearman's ρ = 0.01, p = 0.97; ABS: ρ = 0.51, p = 0.06. ABS, paracellular absorption; CF, cystic fibrosis; MCC, mucociliary clearance.
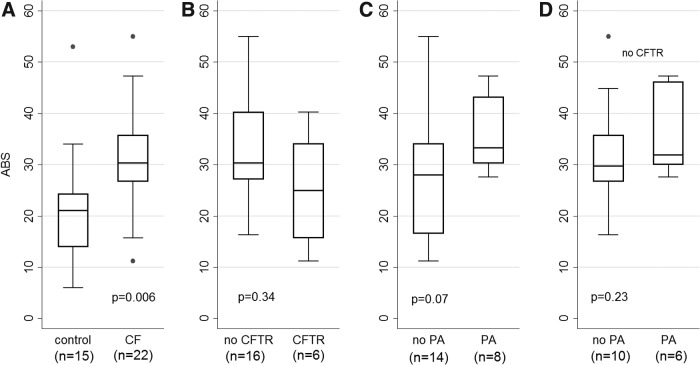
Figure 2A compares MCC between the baseline (CF) and control groups. No significant difference is demonstrated although some portion of the CF group does appear to have depressed clearance based on the range of values measured. Figure 3A compares ABS between the baseline (CF) and control groups. ABS is significantly increased with CF (p = 0.006) as we have previously described.(7) Figure 2B compares MCC based on CFTR function where “CFTR” indicates subjects using ivacaftor or subjects with genotypes associated with retained CFTR function and “no CFTR” indicates F508del/F508del subjects and F508del heterozygous subjects with a second mutation associated with minimal CFTR function. See Tables 1 and 2 for full details on included subjects by group. Figure 3B presents the same comparison for ABS. Neither comparison demonstrates a significant difference based on the CFTR functional grouping.
FIG. 2.
Comparing measurements of MCC in (A) CF baseline group versus controls, (B) subjects with and without retained CFTR function, (C) subjects who had PA+ throat or sputum cultures at last surveillance collection before the MCC/ABS scan, (D) subjects who had PA+ throat or sputum cultures at last surveillance collection before the MCC/ABS scan within the “no CFTR” group. “No CFTR” includes F508del homozygous subjects and heterozygous subjects with a second mutation associated with minimal CFTR function. “CFTR” includes F508del/G551D subjects taking ivacaftor and subjects with genotypes associated with residual CFTR function (Table 1). MCC and ABS are % cleared/80 minutes. CFTR, cystic fibrosis transmembrane conductance regulator; PA, Pseudomonas aeruginosa.
FIG. 3.
Comparing measurements of ABS in (A) CF baseline group versus controls, (B) subjects with and without retained CFTR function, (C) subjects who had PA+ throat or sputum cultures at last surveillance collection before the MCC/ABS scan, and (D) subjects who had PA+ throat or sputum cultures at last surveillance collection before the MCC/ABS scan within the “no CFTR” group. “No CFTR” includes F508del homozygous subjects and heterozygous subjects with a second mutation associated with minimal CFTR function. “CFTR” includes F508del/G551D subjects taking ivacaftor and subjects with genotypes associated with residual CFTR function (Table 1). MCC and ABS are % cleared/80 minutes.
Data from the baseline group were previously utilized to demonstrate an association between PA infection history and MCC depression.(6) Eight of 22 subjects in the baseline group had PA positive throat or sputum cultures at their last clinical visit ahead of the MCC scan. (The average time between the culture and scan was 36 ± 20 days.) As shown in Figure 2C those subjects had significantly lower MCC than the rest of the baseline group (p = 0.01). There was a trend toward increased ABS in this group as well (p = 0.07) as shown in Figure 3C. Sweat chloride values did not vary significantly between the PA positive and PA negative groups although the numbers available for comparison were small: PA positive average = 92 ± 8 mmol/L, n = 4; PA negative average = 90 ± 17 mmol/L, n = 10 (p = 0.88). Multivariate regression of MCC versus sweat chloride, age, and PA status approached significance only for PA status (p = 0.07). A similar regression ABS was approached significance only for sweat chloride (p = 0.09). Six of the eight PA positive subjects were in the “no CFTR” group described earlier. Those 6 subjects had significantly lower MCC than the 10 PA negative subjects in the “no CFTR” group as shown in Figure 2D (p = 0.01). These results imply that the association between MCC depression and PA infection is independent of CFTR function. ABS did not vary significantly between the PA positive and PA negative subjects within the “no CFTR” group (Fig. 3D). To consider the possibility of false negative cultures, we compared MCC in PA positive subjects (n = 8) with subjects with two confirmed PA negative cultures ahead of the MCC/ABS measurement (n = 6). Statistical significance was maintained with p = 0.04.
Response group results
Figure 4 shows the average retention of Tc-SC over time for each intervention (clearance = 1-retention). Table 3 includes average measurements of MCC and ABS at 80 minutes in whole, central, and peripheral lung zones and at 120 and 180 minutes in whole lung zones on the baseline measurement day and after the inhalation of 7% HS and dry powder mannitol. Individual measurements of 80, 120, and 180 minutes MCC and ABS are included in Supplementary Tables S1, S2, S3. Changes in MCC and ABS on the intervention days versus baseline are shown in Table 4. MCC increased significantly more with mannitol than HS in all whole lung measurements. Whole lung ABS decreased more with mannitol than HS. This difference was statistically significant at 80 minutes (p = 0.04) and approached significance at 120 and 180 minutes (both p = 0.06). ABS responses were generally smaller than MCC responses. For example, 80-minute MCC increased by 14.5% ± 6.5% and ABS decreased by 7.1% ± 4.3% with mannitol. For comparison, the standard deviations from previous intrasubject repeatability studies were ABS = 5% and MCC = 8%.(6) High levels of central aerosol deposition can result in an upward bias in MCC measurements and very low ABS measurements (Please see the Independence of Absorption and Mucociliary Clearance section in Supplementary Data and Supplementary Figure S1). The percentage of centrally deposited aerosol was slightly but significantly increased on the HS day versus baseline: 2.6% ± 1.6% (p = 0.02). There was also a nonsignificant increase on the mannitol day versus baseline: 4.1% ± 6.3% (p = 0.40). There was no relationship between the change in MCC and the change in central deposition percentage with either intervention (p = 0.64 0.48 by Spearman) and, therefore, we do not believe these changes confounded the outcomes. Parameters describing the deposition of the radioisotope aerosol are included in Supplementary Table S4.
FIG. 4.
Retention curves showing the MCC of the response group versus time (n = 7). (Clearance = 1-retention). Therapies were delivered from t = 10–22 minutes. Error bars shown are standard error of the mean. Comparisons by Wilcoxon signed-rank test of cleared percentages for each intervention versus baseline (Table 3). HS, hypertonic saline.
Table 3.
Measurements of Mucociliary Clearance and Paracellular Absorption at Baseline and After Inhalation of 7% Hypertonic Saline or Mannitol
| Compartment | Time (minutes) | Baseline | 7% HS | Mannitol | p Base vs. HS | p Base vs. mannitol | p HS vs. mannitol |
|---|---|---|---|---|---|---|---|
| MCC | |||||||
| Whole lung | 80 | 15.0 ± 7.9 | 22.2 ± 9.5 | 29.6 ± 7.7 | 0.06 | 0.02 | 0.03 |
| Whole lung | 120 | 25.1 ± 6.0 | 34.0 ± 10.0 | 40.3 ± 8.1 | 0.09 | 0.02 | 0.24 |
| Whole lung | 180 | 30.4 ± 8.0 | 36.0 ± 10.0 | 44.1 ± 5.6 | 0.15 | 0.02 | 0.11 |
| Central lung | 80 | 18.1 ± 11.2 | 24.8 ± 9.0 | 31.6 ± 12.1 | 0.31 | 0.09 | 0.31 |
| Peripheral lung | 80 | 12.9 ± 11.2 | 20.3 ± 13.9 | 27.4 ± 12.8 | 0.06 | 0.09 | 0.09 |
| ABS | |||||||
| Whole lung | 80 | 48.4 ± 5.6 | 47.4 ± 6.8 | 41.2 ± 2.8 | 0.67 | 0.03 | 0.04 |
| Whole lung | 120 | 51.4 ± 8.0 | 49.3 ± 7.3 | 42.7 ± 3.9 | 0.50 | 0.03 | 0.06 |
| Whole lung | 180 | 52.0 ± 5.7 | 50.1 ± 7.3 | 42.7 ± 6.1 | 0.74 | 0.02 | 0.06 |
| Central lung | 80 | 48.0 ± 7.7 | 44.1 ± 10.5 | 39.3 ± 8.4 | 0.40 | 0.06 | 0.17 |
| Peripheral lung | 80 | 48.2 ± 6.4 | 49.5 ± 10.2 | 43.5 ± 5.6 | 1.00 | 0.24 | 0.24 |
Comparisons by Wilcoxon signed-rank test. All ± SD. MCC and ABS are % cleared/time period of scan.
HS, hypertonic saline.
Table 4.
Comparing the Changes in Mucociliary Clearance and Paracellular Absorption from the Baseline Measurement Associated with the Inhalation of 7% Hypertonic Saline and Dry Powder Mannitol
| ΔMCC | Time (minutes) | 7% HS | Mannitol | p |
|---|---|---|---|---|
| Whole lung | 80 | 7.2 ± 9.7 | 14.5 ± 6.5 | 0.03 |
| Central lung | 80 | 6.7 ± 16.1 | 13.5 ± 16.2 | 0.31 |
| Peri lung | 80 | 7.3 ± 12.9 | 14.4 ± 16.6 | 0.09 |
| Whole lung | 120 | 5.3 ± 12.1 | 17.3 ± 9.6 | 0.02 |
| Whole lung | 180 | 3.9 ± 13.0 | 13.6 ± 8.2 | 0.03 |
| ΔABS | Time (minutes) | 7% HS | Mannitol | p |
|---|---|---|---|---|
| Whole lung | 80 | −1.0 ± 9.3 | −7.1 ± 4.3 | 0.04 |
| Central lung | 80 | −3.9 ± 15.9 | −8.7 ± 11.0 | 0.18 |
| Peri lung | 80 | 1.2 ± 11.2 | −4.7 ± 9.8 | 0.24 |
| Whole lung | 120 | −2.1 ± 10.3 | −8.7 ± 7.4 | 0.06 |
| Whole lung | 180 | −1.8 ± 7.9 | −9.3 ± 3.8 | 0.06 |
ΔMCC and ΔABS are % cleared/time period of scan (n = 7). Comparisons by Wilcoxon signed-rank test.
Comparisons with previous studies of HS delivery
MCC after HS inhalation was significantly higher in a previous study by our group (42% ± 18%/80 minutes vs. 22.2% ± 9.5%/80 minutes in this study(7)). The previous study utilized a Sidestream jet nebulizer and a high gas flow rate [∼10 liters per minute (LPM)] from a large compressor (DeVilbiss 8650D) to deliver the aerosol while the subjects were recumbent in the camera. Here, subjects used Pari-Plus nebulizer with a DeVilbiss Pulmo-Aide compressor and were seated for delivery for a slightly longer period (12 vs. 10 minutes). In vitro studies with both systems, delivering 4 mL of 7% HS for a 12-minute period, and using a simulated breathing pattern and (dried) filter capture, demonstrated similar NaCl output rates (Sidestream: 26.9 ± 4.0 mg vs. Pari-Plus 25.2 ± 5.0 mg, p = 0.60 by t-test). Recumbent delivery has been shown to increase aerosol deposition in medium and small airways, which may have improved the MCC performance of the HS treatment. Lying recumbent is thought to decrease airway size and functional residual capacity, which would increase aerosol resident in the airways and deposition due to sedimentation.(12)
Discussion
Here, we have utilized two imaging-based biomarkers, MCC and ABS, which are intended to quantify two key aspects of CF lung pathophysiology: MCC and ASL absorption. These biomarkers have received previous use in screening therapies and helping to demonstrate mechanism of action in CF studies.(2–4,9)
We considered the effect of CFTR function on baseline MCC and ABS through comparisons with sweat chloride, a well-accepted indicator of systemic CFTR function, and subject groupings based on CFTR genotypes associated with residual function or the use of the CFTR potentiator ivacaftor. There was a trend toward correlation between ABS and sweat chloride (p = 0.06). There was no relationship between sweat chloride and MCC (p = 0.97). The relationship between ABS and sweat chloride likely indicates that depressed CFTR function results in higher rates of ASL absorption in the airways that in turn transports more DTPA through the epithelium through solvent drag. Previous in vitro studies with CF bronchial epithelial cell cultures have demonstrated a proportional relationship between DTPA and ASL volume absorption, despite the fact that ASL absorption occurs through both transcellular and paracellular paths, whereas DTPA absorption is only paracellular.(8) Factors beyond ASL absorption such as tight junction diameter could also affect ABS, however, and there is no direct proof that ABS reflects ASL absorption in vivo.
We and other groups have described an association between a history of PA infection and MCC depression in CF(6,13,14) and have speculated that the bacteria may directly depress clearance through previously described virulence factors.(15) Another possibility would be a PA-associated change in mucus production/expression in a way that impairs MCC. Our previous report on this topic used data from the baseline group, which is described here as well.(6) Here, we found that MCC within the baseline group was significantly lower in subjects who had a PA positive throat or sputum culture at their last clinical visit, which was on average more than a month before the MCC scan. There was no difference in sweat chloride between the PA positive and negative groups, and PA infection was still associated with depressed clearance when only subjects in the “no CFTR” group were included in the comparison, indicating that this result is likely independent of CFTR function. There was a trend toward increased ABS in PA positive subjects (p = 0.07), but this trend was not apparent when only subjects in the “no CFTR” group were considered (p = 0.23). Potentially PA infection may cause changes in epithelial permeability that are reflected in the ABS measurement.
To assess the utility of MCC and ABS as measures of therapeutic effect, we measured their response to the inhalation of nebulized 7% HS and dry powder mannitol. Mannitol provided larger increases in whole lung MCC from baseline at all time points considered. Mannitol use was also associated with larger decreases in whole lung ABS than HS. These differences were significant at t = 80 minutes and trended toward significance t = 2 and t = 3 hours. The therapeutic effect of an osmotic therapy would be largely determined by the osmotic gradient it establishes at the airway epithelium. The “osmotic load” in the airways provided by mannitol and HS is difficult to assess directly. Airway doses of HS and mannitol are affected by device loaded dose (400 mg for mannitol and 280 mg for HS), delivery efficiency, and aerosol deposition location. Salt would dissociate in an aqueous environment approximately doubling its osmolarity; however, it is also actively absorbed by the airway and thus may not endure for as long in the ASL as mannitol. Therefore, it is difficult to assess why mannitol provide an improved therapeutic effect. HS response was notably lower here versus our previous studies that utilized a different nebulizer and involved recumbent positioning of the patient during aerosol delivery (to accommodate continued imaging). Aerosol output from both devices was similar, which leads us to speculate that deposition of the aerosol may have been altered favorably by recumbent positioning in the previous study, resulting in increased rates of MCC after therapy.(12) In general, MCC demonstrated more significant response in these studies and would be a more useful biomarker for testing similar osmotic therapies.
Method-related limitations to the study include difficulties in targeting aerosol delivery to only the airways of the lungs. Here both probes are delivered in a liquid aerosol, inhaled using a breathing pattern and aerosol size that favors airway deposition but certainly does not exclude alveolar delivery. CF baseline MCC rates in the peripheral portion of the lung, which excludes the large central airways, were 12.9% ± 11.2%/80 minutes on average. This is much higher than would be anticipated if the Tc-SC probe was deposited in alveoli, where retention up to 24 hours would be anticipated. A more definitive assessment of alveolar dose could be provided by measuring retained Tc-Sc activity after 24 hours. We would anticipate that DTPA absorption may be increased in alveoli versus airways and that aerosol distribution may, therefore, substantially affect the ABS measurement.
Another potential method-related limitation is the duration of the MCC/ABS measurement (80 minutes in baseline group and 3 hours in the response group). Extended measurements might reveal more effects related to small airway clearance that were not detectable through our methods. Another potential method-related limitation is the linkage between the MCC and ABS measurements. This addressed in The Independence of Absorption and Mucociliary Clearance section in Supplementary Data. Clearance measurements in the response group may have been affected by cough during or after intervention delivery, based on the delivery of the radioisotope aerosol ahead of the interventions.
Several of our outcomes are limited by small subject numbers. The study was not prospectively designed to detect effects related to CFTR genotype or potentiator use. Subjects with genotypes associated with retained CFTR function represent a small portion of the total CF population as do subjects with genotypes suitable for treatment with ivacaftor alone. The study was performed ahead of large-scale use of corrector/potentiator combinations. As corrector and potentiator use expands, the CF population is likely to include more subjects with functional CFTR and our outcomes may provide some insight into factors to be assessed through future studies to define the physiological baseline and therapeutic needs of this population.
In summary, we have described how MCC can provide a sensitive indication of therapeutic response and how mucus clearance is affected by PA infection history. Our results indicate a relationship between baseline ABS and CFTR function. This biomarker may be of use for detecting the local effect of CFTR correctors/potentiators in the lung. However, ABS was less sensitive in detecting the therapeutic effect of the two osmotic therapies tested here.
Supplementary Material
Acknowledgments
Authors received funding from the NIH grants (R01HL108929 and P30DK72506). Mannitol for use in these studies was provided by Pharmaxis Ltd., Frenchs Forest, NSW, Australia, but did not provide funding. Dr. Corcoran is the sponsor on IND 117,639, which includes the procedures described herein.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
Supplementary Material
Reviewed by:
Steve Rowe
Scott Donaldson
References
- 1. Boucher RC: An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371 [DOI] [PubMed] [Google Scholar]
- 2. Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, and Boucher RC: Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250 [DOI] [PubMed] [Google Scholar]
- 3. Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, and Bye PT: The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: A pilot study. Eur Respir J. 1999;14:678–685 [DOI] [PubMed] [Google Scholar]
- 4. Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, Sagel SD, Khan U, Mayer-Hamblett N, Van Dalfsen JM, Joseloff E, and Ramsey BW; Network GIotCFFTD: Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med. 2014;190:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corcoran TE, Thomas KM, Myerburg MM, Muthukrishnan A, Weber L, Frizzell R, and Pilewski JM: Absorptive clearance of DTPA as an aerosol-based biomarker in the cystic fibrosis airway. Eur Respir J. 2010;35:781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Locke LW, Myerburg MM, Weiner DJ, Markovetz MR, Parker RS, Muthukrishnan A, Weber L, Czachowski MR, Lacy RT, Pilewski JM, and Corcoran TE: Pseudomonas infection and mucociliary and absorptive clearance in the cystic fibrosis lung. Eur Respir J. 2016;47:1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Locke LW, Myerburg MM, Markovetz MR, Parker RS, Weber L, Czachowski MR, Harding TJ, Brown SL, Nero JA, Pilewski JM, and Corcoran TE: Quantitative imaging of airway liquid absorption in cystic fibrosis. Eur Respir J. 2014;44:675–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corcoran TE, Thomas KM, Brown S, Myerburg MM, Locke LW, and Pilewski JM: Liquid hyper-absorption as a cause of increased DTPA clearance in the cystic fibrosis airway. EJNMMI Res. 2013;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corcoran TE, Godovchik JE, Donn KH, Busick DR, Goralski J, Locke LW, Markovetz MR, Myerburg MM, Muthukrishnan A, Weber L, Lacy RT, and Pilewski JM: Overnight delivery of hypertonic saline by nasal cannula aerosol for cystic fibrosis. Pediatr Pulmonol. 2017;52:1142–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, Wu J, Mogayzel PJ, Jr., Pilewski J, and Donaldson S: Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv. 2013;26:157–164 [DOI] [PubMed] [Google Scholar]
- 11. Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, Rowe SM, Clancy JP, Konstan MW, Hoch HE, Heltshe SL, Ramsey BW, Campbell PW, and Ashlock MA: Sweat chloride as a biomarker of CFTR activity: Proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sa RC, Zeman KL, Bennett WD, Prisk GK, and Darquenne C: Effect of posture on regional deposition of coarse particles in the healthy human lung. J Aerosol Med Pulm Drug Deliv. 2015;28:423–431 [DOI] [PubMed] [Google Scholar]
- 13. Laube BL, Sharpless G, Benson J, Carson KA, and Mogayzel PJ , Jr.: Mucus removal is impaired in children with cystic fibrosis who have been infected by Pseudomonas aeruginosa. J Pediatr. 2014;164:839–845 [DOI] [PubMed] [Google Scholar]
- 14. Donaldson SH, Laube BL, Corcoran TE, Bhambhvani P, Zeman K, Ceppe A, Zeitlin PL, Mogayzel PJ, Jr., Boyle M, Locke LW, Myerburg MM, Pilewski JM, Flanagan B, Rowe SM, and Bennett WD: Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight 2018;3:122695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunter RC, Klepac-Ceraj V, Lorenzi MM, Grotzinger H, Martin TR, and Newman DK: Phenazine content in the cystic fibrosis respiratory tract negatively correlates with lung function and microbial complexity. Am J Respir Cell Mol Biol. 2012;47:738–745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.