Abstract
Bartonella henselae is a zoonotic vector-borne pathogen affecting both humans and dogs. Little is known about the epidemiology of B. henselae in dogs, including risk factors associated with exposure. The objectives of this study were to map the current distribution of B. henselae in dogs in North Carolina (NC) and to identify ecological and socioeconomic factors influencing B. henselae seroreactivity.
Results from 4446 B. henselae serology samples from dogs in NC submitted by veterinarians for clinical diagnostic testing to the North Carolina State University College of Veterinary Medicine Vector Borne Disease Diagnostic Laboratory between January 1, 2004 and December 31, 2015 were retrospectively reviewed. These results were used to generate a map of B. henselae seroreactivity. To account for sparsely sampled areas, statistical smoothing using head banging and areal interpolation kriging was performed. Using previously described risk factors for exposure to canine tick-borne diseases, eight multivariable logistic regression models based on biologically plausible hypotheses were tested, and a final model was selected using an Akaike's Information Criterion weighted-average approach.
Seroreactivity among dogs tested for vector-borne disease was variable across the state: higher along the southern/eastern coastal plains and eastern Piedmont, and lower in the western mountains. Of 25 explanatory factors considered, the model combining demographic, socioeconomic, climatic, and land use variables fits best. Based on this model, female intact sex and increasing percentage of the county with low-intensity development and evergreen forest were associated with higher seroreactivity. Conversely, moderate development, increasing median household income, and higher temperature range and relative humidity were associated with lower seroreactivity. This model could be improved, however, by including local and host-scale factors that may play a significant role in dogs' exposure.
Keywords: canine, seroreactivity, tick, flea, zoonoses, vector-borne
Background
Members of the bacterial genus Bartonella are important emerging pathogens in dogs and humans worldwide (Harms and Dehio 2012, Breitschwerdt et al. 2017). There are >30 named species, more than half of which have been associated with human and animal diseases (Breitschwerdt et al. 2014, 2017). One of the most common zoonotic species of Bartonella in humans and dogs is Bartonella henselae, the causative agent of human cat scratch disease (CSD) (Breitschwerdt et al. 2014, Regier et al. 2016, Lashnits et al. 2018). In humans, B. henselae is transmitted by inoculation of infected flea feces through the patient's skin through cat scratch (Zangwill 2013, Regier et al. 2016). Although the cat flea, Ctenocephalides felis, serves as the primary arthropod vector for transmission of B. henselae among the domestic cat reservoir, the primary vector for transmission to dogs is unknown (Billeter et al. 2008, Angelakis et al. 2010, Mosbacher et al. 2011). Ticks (primarily Ixodes spp., but also Dermacentor spp., Amblyomma americanum, and Rhipicephalus sanguineus) and fleas (C. felis and Pulex spp.) have been proposed as vectors for B. henselae in dogs based on case reports, serosurveys, surveys of arthropod vectors, and laboratory transmission studies investigating Bartonella spp. transmission (Pappalardo et al. 1997, 2000, Breitschwerdt et al. 1998, Kordick et al. 1999, Chang et al. 2001, Honadel et al. 2001, Chang et al. 2002, Adelson et al. 2004, MacDonald et al. 2004, Morozova et al. 2004, Solano-Gallego et al. 2004, Henn et al. 2005, Holden et al. 2006, Foley et al. 2007, Wikswo et al. 2007, Billeter et al. 2008, 2012, Cotté et al. 2008, Reis et al. 2011, Yancey et al. 2014, Lashnits et al. 2018, Regier et al. 2017, Duplan et al. 2018). There is also evidence suggesting that occasionally human B. henselae infection may be due to tick transmission, including with reports of CSD, in patients with no reported cat contact, or with reported tick exposure or Lyme disease (Lucey et al. 1992, Zangwill et al. 1993, Arnez et al. 2003, Podsiadły et al. 2003, Breitschwerdt et al. 2007, Billeter et al. 2008, Angelakis et al. 2010, Rigaud et al. 2016, Donà et al. 2018).
Climatic conditions, geographical factors, and socioeconomic factors have been associated with the prevalence of tick-borne diseases in dogs, including Anaplasma spp. (McMahan et al. 2016), Borrelia burgdorferi (Watson et al. 2017), and Ehrlichia spp. (Liu et al. 2017). Although B. burgdorferi and Ehrlichia spp. have different tick vectors, modeling studies suggest higher exposure to either of these diseases in locations with lower population density and more forest (away from urban centers) (Liu et al. 2017, Watson et al. 2017). However, no such analysis for Bartonella exposure in dogs has been published.
The availability of a large amount of Bartonella serology data from a national diagnostic laboratory (North Carolina State University College of Veterinary Medicine Vector Borne Disease Diagnostic Laboratory [NCSU-VBDDL], North Carolina State University, NC) has previously allowed us to investigate trends across dog populations and over many years, to identify demographic and geographical risk factors associated with Bartonella spp. exposure in dogs (Solano-Gallego et al. 2004, Yancey et al. 2014, Lashnits et al. 2018). However, ecological and socioeconomic factors associated with B. henselae exposure in dogs have not previously been studied.
The goal of this study was, therefore, to provide further insight into ecological and socioeconomic factors associated with B. henselae exposure in dogs, using NCSU-VBDDL clinical diagnostic serology data from dogs residing in North Carolina (NC) and suspected of having one or more canine vector-borne diseases (CVBDs). NC is a logical choice to identify large-scale patterns of association between Bartonella exposure and ecological and socioeconomic factors, because it is a large and diverse state, with wide variation in these factors (see Supplementary Fig. S1), as well as having the largest number of sample submissions for Bartonella diagnostic testing each year.
The specific aims of this study were to map the current spatial distribution of B. henselae in dogs in NC and to characterize ecological and socioeconomic factors associated with B. henselae exposure based on NCSU-VBDDL serology data. We hypothesized that risk factors previously associated with vector-borne diseases of dogs, including climatic conditions, geographical factors, and societal factors, are associated with B. henselae exposure in dogs.
Materials and Methods
Study design, setting, and participants
We performed a retrospective cross-sectional observational analysis of dog blood samples submitted to the NCSU-VBDDL for B. henselae serology. The NCSU-VBDDL routinely tests sera for antibodies against B. henselae as an individual serological test or as a part of comprehensive panel that includes multiple Bartonella spp. as well as other CVBDs. Samples originate from veterinary hospitals and practices throughout North America.
In this study, we included samples from dogs submitted from either from the North Carolina State University Veterinary Hospital or other veterinary clinics located throughout NC between January 1, 2004, and December 31, 2015. If dogs had multiple tests submitted, only one test per year was included. If multiple samples were submitted within 1 year, samples were excluded after the first positive result. If no samples were positive, the chronologically first sample was chosen and the others excluded. Dogs enrolled with the NCSU Veterinary Hospital Blood Bank were identified by manual review of medical records, and excluded.
Data source for outcome variable
Samples were tested for B. henselae H-1 strain through immunofluorescent antibody (IFA) as previously described, using a cutoff of 1:64 to define a seroreactive titer (Hegarty et al. 2014).
Map creation
To characterize the spatial distribution of B. henselae in dogs tested for vector-borne disease in NC during the study period, a map of the average percentage of samples seroreactive over all sample years, for each county, was created using ArcGIS (ArcMap v. 10.4.1; Environmental Systems Research Institute [ESRI], Redlands, CA). Boundaries were created from publicly available data from the U.S. Census Bureau (United States Census Bureau 2017) and ESRI using the North American Datum 1983 geographic coordinate system with Geodetic Reference System 1980 spheroid.
Two smoothing techniques were applied to the empiric map. First, a weighted head-banging algorithm (NCI 2016) was used to reduce the influence of sparsely sampled counties (Wang et al. 2014, McMahan et al. 2016). Missing values were replaced with the average proportion of B. henselae seropositive dogs for adjacent counties with sampling. Parameters used were 6 nearest neighbors, 4 triples, 10 iterations, and 135 degrees angle. Second, to aid visualization, we smoothed the map into a continuously variable surface using areal interpolation kriging with baseline parameters in the geostatistical analyst extension of ArcGIS (Wang et al. 2014, McMahan et al. 2016). Maps for explanatory factors, averaged over all study years, were also created (Supplementary Fig. S1).
Data sources for explanatory variables
Patient information available from the NCSU-VBDDL included date of sample collection, signalment (age, breed, gender), and veterinary practice location. County of sample origin was assigned based on owner's zip code if available, or veterinary clinic location if not.
Previous studies have examined risk factors for exposure to CVBDs (Stich et al. 2014, Wang et al. 2014, McMahan et al. 2016). These factors were initially investigated for analysis, and included climate factors (annual temperature, precipitation, and humidity); socioeconomic factors (median household income, population density, and estimate of number of dogs per county); and geographic factors (elevation and land cover). In addition, the presence or absence of Ixodes spp. ticks on a county-wide scale across the United States. was recently reported (Eisen et al. 2016a), and this presence/absence data were used as an additional factor. Year of sample submission was initially explored, but ultimately not included as an explanatory factor since it does not provide any mechanistic information about the underlying drivers of exposure. A list of considered factors and the publicly available data sources are provided in Table 1, and the range of values for these variables within NC is given in Table 2 and Supplementary Figure S1.
Table 1.
Candidate Explanatory Variables. Candidate Factors with Abbreviations for Explanatory Variables Included in Models, Units, Spatial Resolution, and Data Sources
| Category | Factor | Abbreviations | Scale | Years | Source |
|---|---|---|---|---|---|
| Demographic | Sex | SEX | Individual | — | NCSU-VBDDL |
| Breed | BRD | ||||
| Climate | Mean, minimum, and maximum temperatures, temperature range (°F) | TR | County | Annual | PRISM Climate Group http://prism.oregonstate.edu |
| Mean precipitation (inches) | County | Annual | PRISM Climate Group | ||
| Mean dew point temperature (°F) | County | Annual | PRISM Climate Group | ||
| Relative humidity | RH | County | Annual | Calculated from dew point and mean temperature | |
| Geographic | Elevation (ft. above sea level) | ELEV | County | 1986 | North Carolina Geodetic Survey www.ncgs.state.nc.us |
| Land cover (12 classes) Grass and shrub Forest Development Wetland and water Crops Pasture |
GS FOR DEV WET CR PST |
30 meters | 2006, 2011 | National Land Cover Database www.mrlc.gov |
|
| Socioeconomic | Population density (persons/sq. mi) | PD | County | 2009, 2010, 2015 | U.S. Census Bureau, American Community Survey and 2010 Census www.socialexplorer.com |
| Median household income ($) | INC | County | 2009, 2010, 2015 | U.S. Census Bureau, American Community Survey and 2010 Census | |
| Number of dogs | County | 2009, 2010, 2015 | U.S. Census Bureau, American Community Survey and 2010 Census U.S. Pet Ownership and Demographics Sourcebook (AVMA, 2012) |
||
| Tick vector presence | Ixodes spp. tick presence reported | TICK | County | 1998, 2015 | Eisen et al. 2016a |
NCSU-VBDDL, North Carolina State University College of Veterinary Medicine Vector Borne Disease Diagnostic Laboratory.
Table 2.
Median and Range for County-Level Explanatory Variables
| Variable | Abbreviation | Median | Range |
|---|---|---|---|
| Climate | |||
| Maximum annual temperature (°F) | MaxTemp | 71.05 | 56.6–76.1 |
| Mean annual temperature (°F) | MeanTemp | 60.1 | 47.8–65 |
| Minimum annual temperature (°F) | MinTemp | 49 | 38–55.6 |
| Mean annual dew point (°F) | DP | 47 | 38.9–55.4 |
| Annual precipitation (inches) | Precip | 48.4 | 27.08–97.51 |
| Temperature range (°F) | TempRange | 22.2 | 15.1–28.6 |
| Relative humidity (%) | MeanRH | 63.40 | 51.40–75.71 |
| Socioeconomic | |||
| Population density (100 persons/sq. mi.) | PD | 1.094 | 0.0858–18.904 |
| Number of dogs (per county) | DogEst | 13,751.6 | 986.7–258,254.5 |
| Median household income ($1000/year) | Inc | 39.642 | 27.487–67.309 |
| Geographic | |||
| Elevation (100 ft. above sea level) | Elev | 4.35 | 0.01–35.82 |
| Land use (% of county) | |||
| Developed–high | DevHi | 0.10 | 0–4.34 |
| Developed–medium | DevMed | 0.40 | 0.01–8.94 |
| Developed–open+low | DevOpLow | 7.53 | 1.27–49.34 |
| Evergreen forest | EvFor | 9.63 | 0.50–34.57 |
| Deciduous forest | DecFor | 28.95 | 0–84.01 |
| Mixed forest | MixFor | 2.00 | 0.02–7.44 |
| Grass+shrub | GS | 8.00 | 0.44–21.07 |
| Pasture | Pst | 7.09 | 0–38.04 |
| Crops | Crop | 1.30 | 0–46.27 |
| Wetland+open water | Wet | 4.62 | 0.12–92.86 |
Median and range over all 100 North Carolina counties for all study years (2004–2015). Land use type represented by the percentage of each county with specified land use type.
Detailed data collection and management information are available in Supplementary Data. All data management and analyses were performed in R 3.3.1 (R Core Team 2016).
Descriptive statistics
To better characterize differences between seroreactive and nonseroreactive dogs, descriptive statistics were obtained for gender, breed, and county-level tick reporting. Differences between seroreactive and nonseroreactive dogs were calculated using chi-squared tests.
Model development
To evaluate ecological and socioeconomic factors associated with B. henselae exposure, we followed a model selection approach in which we combined explanatory variables representing biologically plausible hypotheses (Johnson and Omland 2004). There were 25 explanatory variables initially considered, and a subset of these variables was included in each hypothesis-based model (Fig. 1). We first evaluated all pairwise correlations among the explanatory variables, using Pearson's correlation coefficients, to minimize statistical issues associated with collinearity. Combinations of excessively correlated explanatory variables were avoided in the hypothesis-based models.
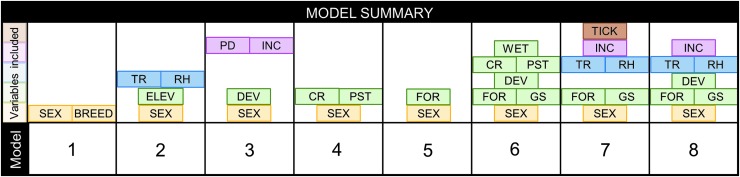
FIG. 1.
Hypothesis model structures. Model summary, showing variables included in each hypothesis model. Colored boxes show individual explanatory variables included in each hypothesis-based model, with color based on the variable category. Yellow, demographic variable; blue, climate variables; green, geographic variables; pink, socioeconomic variables; brown, tick vector presence. BRD, breed group; CR, crops; DEV, developed land (open/low or moderate); ELEV, elevation; FOR, forest (evergreen, deciduous, or mixed); GS, grass and shrub; INC, median household income; PD, population density; PST, pasture; RH, relative humidity; TR, temperature range; TICK, Ixodes spp. ticks established, reported, or not reported; WET, wetland and open water.
The combinations of explanatory variables for each hypothesis-driven model are shown in Fig. 1. Dog gender was included in all hypothesis-based models, based on previous studies showing significant differences in Bartonella spp. exposure in different genders (Henn et al. 2005, Lashnits et al. 2018). The basis for the specific combinations of variables in each hypothesized model is as follows:
-
1.
In model 1, we hypothesize that host factors, primarily dog demographics, are most important in explaining the variation in B. henselae exposure in dogs. Dog demographics include gender and breed, but not age, based on previous studies investigating demographics (Pappalardo et al. 1997, Honadel et al. 2001, Henn et al. 2005, Foley et al. 2007, Lashnits et al. 2018). Based on a recent and large-scale seroepidemiologic study of Bartonella spp. exposure in dogs (Lashnits et al. 2018), we hypothesize that the odds of exposure is highest in male intact mixed breed dogs.
-
2.
In model 2, we explore the hypothesis that climatic factors alone are most important in explaining the variation in B. henselae exposure in dogs. The hypothesis for this model is that exposure is highest in areas with high relative humidity and in less extreme climates (lower temperature range).
-
3.
In model 3, we consider the hypothesis that socioeconomic and development factors are most important in explaining the variation in B. henselae exposure in dogs. The hypothesis is that the highest seroreactivity is found where there is high median household income and high levels of development.
-
4.
In model 4, we assume access to active farmland is most important in explaining the variation in B. henselae exposure in dogs. This model is based on a case–control study performed in the southeast United States in the 1990s, indicating that Bartonella vinsonii subsp. berkhoffii exposure was higher in dogs in rural environments, particularly on farms (Pappalardo et al. 1997). For this model, land covers including crops and pasture are tested, with the hypothesis that exposure is highest in counties with a high percentage of farmland.
-
5.
In model 5, we assume that different types of forest cover are most important in explaining the variation in B. henselae exposure in dogs. In NC, the forest type follows an elevation gradient from the coastal eastern counties, which are predominantly evergreen forest, to the mountainous western counties, which are predominantly deciduous forest. A previous study of landscape risk factors for tick borne diseases of dogs in northern California found the highest seroprevalence for Bartonella vinsonii subsp. berkhoffii in evergreen forests (Foley et al. 2007). Based on this, the hypothesis is that exposure is highest in counties with large proportions of mixed or evergreen forest.
-
6.
In model 6, we take into account multiple different land uses to explain the variation in B. henselae exposure in dogs. Including all land use categories produces excessive collinearity, particularly with all levels of development and all forest types. Therefore, high-intensity development and mixed forest were not included. The hypothesis is that the highest seroreactivity will be seen in areas with large percentage of forest, grass/shrub, and development.
-
7.
In model 7, we include multiple categories of factors to explain the variation in B. henselae exposure in dogs. Particular types of land cover (forest and grass/shrub) as well as climate variables (temperature range and relative humidity) were included based on previous studies of factors important in predicting other CVBDs (Springer et al. 2015, Hahn et al. 2016, Alkishe et al. 2017, Eisen et al. 2018, Soucy et al. 2018). Whether Ixodes spp. ticks had been previously reported in each county was also included (Eisen et al. 2016a). The hypothesis is that exposure is highest in counties with established Ixodes spp. ticks, high percentage of land dedicated to forest or grass/shrub, and low temperature ranges with high relative humidity.
-
8.
In model 8, we also include multiple categories of factors that may be associated with positive B. henselae serology, but we leave out the direct assessment of reported presence of Ixodes spp. ticks and instead include a measure of development. The hypothesis is that exposure is highest in counties with high percentage of land dedicated to forest or grass/shrub, low temperature ranges with high relative humidity, and high levels of development and income.
We used logistic regression to quantify the log odds of B. henselae exposure. The dependent variable was positive (vs. negative) B. henselae IFA sample.
Model selection and assessment
For each of the eight models, model p value was calculated based on ANOVA test compared with a null model, and goodness of fit (GOF) was assessed using the Hosmer–Lemeshow GOF test with the “Resource Selection” package (Subhash et al. 2017) and McFadden's Pseudo-R2 using the “pscl” package (Jackman 2017). Akaike's Information Criterion (AIC) was calculated for each model, and the relative importance of each model was assessed by assigning AIC weights (Anderson 2008) using the “MuMIn” package (Barton 2018). A ninth model, the final weighted model, was selected based on averaging the models within ΔAIC of 9 (Anderson 2008), allowing for evaluation of the relative support in the data for each model, and therefore quantitatively measure support for each model (Johnson and Omland 2004). Odds ratios (ORs) and 95% confidence intervals for the ORs were estimated for the final weighted-average model. Unless otherwise stated, p ≤ 0.05 was considered statistically significant.
Results
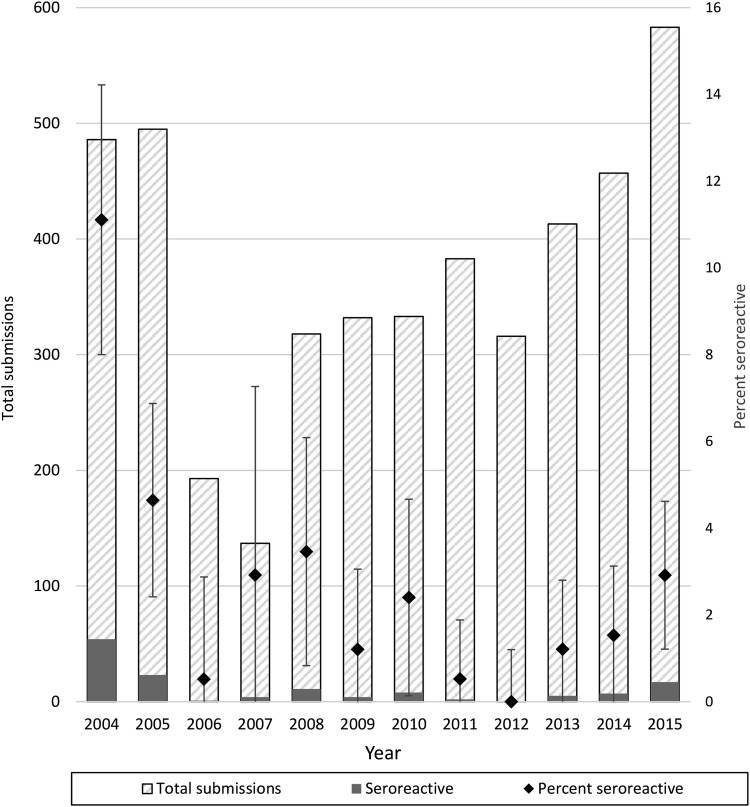
During the 12-year study period, there were 4446 blood samples tested for B. henselae, comprising 4343 unique dogs (demographic characteristics available in Supplementary Table S1). There were samples submitted from 88 counties (out of 100 counties in NC). Within a given year, the number of sampled counties ranged from 35 counties in 2007 to 59 counties in 2004. The counties from which the highest number of samples was submitted included Wake, Durham, and Mecklenburg; samples from these three counties made up 55% of the sample size. The largest number of samples (583, 13.1%) was submitted in 2015, the smallest number (137, 3.1%) in 2007 (Fig. 2).
FIG. 2.
Bartonella henselae seroreactivity by year during the study period. Total IFA submissions on left axis; positive samples in solid gray, negative samples in striped gray. Percentage of samples seroreactive per year on right axis; error bars represent 95% confidence intervals for the estimated proportions, and are cut off at 0%. IFA, immunofluorescent antibody.
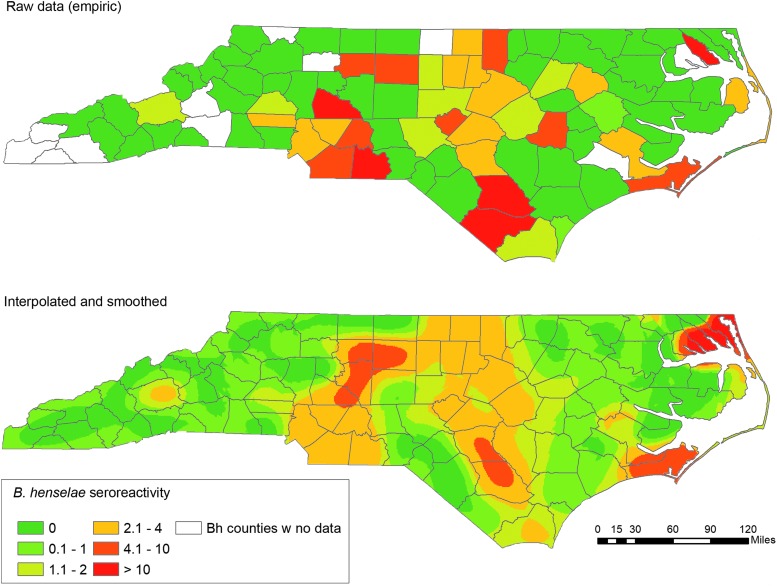
There were 136 dogs (3.1%) that had serological evidence of B. henselae exposure. Test results by county of origin are shown in Fig. 3 (top panel). The smoothed map showing estimated percentages of dogs with seroreactivity to B. henselae across NC over the entire study period, based on head-banging and areal interpolation kriging, is shown in Fig. 3 (bottom panel). There are areas of higher seroreactivity on the coast as well as through the middle of the state, with areas of lower seroreactivity in the western part of the state and through the middle of the coastal plains.
FIG. 3.
Map of B. henselae seroreactivity in dogs. Top panel shows the empiric map (raw data), with the percentage of seroreactive dogs in each county. Bottom panel shows the smoothed and interpolated map, with estimated percentage of seroreactive dogs.
Female intact dogs had higher seroreactivity (5.5%) compared with the other genders (male castrated 1.9%, p = 0.0002; male intact 2.0%, p = 0.148; female spayed 2.3%, p = 0.0324). There were no statistically significant differences in seroreactivity when compared between American Kennel Club (AKC) breed groups, or when comparing specific breeds that made up >5% of the samples. There was higher seroreactivity in counties with Ixodes spp. ticks reported (4.35%) than in counties with Ixodes spp. ticks established or not reported (2.69% and 2.53%, respectively; p = 0.0225). Seroreactivity was highest in 2004 (12.5%), and otherwise ranged from 0% in 2012 to 4.9% in 2005; overall annual seroreactivity is shown in Fig. 2.
The most informative model was model 8, which included as explanatory factors dog gender, median household income, relative humidity, temperature range, and percentage of land with evergreen forest, grass/shrub, open or low-intensity development, and moderate intensity development. This model had the lowest AIC (1152.7) and an AIC weight of 0.9 (Table 3), with a McFadden pseudo-R2 of 0.072 (where a value of ≥0.2 indicates an excellent fit) (McFadden 1979). The Hosmer–Lemeshow GOF test had a nonsignificant p value (p = 0.2998), indicating that this model appropriately fit the data. The next best model was model 7, with an AIC weight of 0.08. Models 1 and 3–6 had ΔAIC >9, and, therefore, did not contribute to the AIC weighted average. Results of the initial eight hypothesis-based models are shown in Table 4. The weighted-average model (Table 5) showed that female intact or unknown gender status and increasing percentage land cover with open or low-intensity development or evergreen forest were independently associated with increased log odds of B. henselae exposure. Conversely, increasing percentage of moderate intensity developed land, increasing median household income, increasing temperature range, and increasing relative humidity were independently associated with decreased log odds of B. henselae exposure.
Table 3.
Akaike's Information Criterion Weighted Model Average
| Model | Rank | DF | logLik | AICc | ΔAIC | Weight | Pseudo R2 |
|---|---|---|---|---|---|---|---|
| 8 | 1 | 12 | −564.29 | 1152.66 | 0 | 0.9 | 0.072 |
| 7 | 2 | 11 | −567.69 | 1157.43 | 4.78 | 0.08 | 0.067 |
| 2 | 3 | 9 | −571.5 | 1161.05 | 8.39 | 0.01 | 0.060 |
| 3 | 4 | 8 | −580.76 | 1177.54 | 24.89 | 0.00 | 0.045 |
| 6 | 5 | 13 | −576.67 | 1179.42 | 26.76 | 0.00 | 0.052 |
| 4 | 6 | 7 | −583.03 | 1180.08 | 27.42 | 0.00 | 0.041 |
| 5 | 7 | 8 | −582.92 | 1181.87 | 29.21 | 0.00 | 0.041 |
| 1 | 8 | 14 | −578.65 | 1185.4 | 32.74 | 0.00 | 0.048 |
Models listed in descending order of AIC.
AIC, Akaike's Information Criterion.
Table 4.
Hypothesis-Based Model Results
| Estimate | Standard error | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Model 8 | |||||
| (Intercept) | 16.3458 | 4.5227 | 0.0003* | ||
| Gender | |||||
| MI | 0.1690 | 0.3800 | 0.6565 | 1.18 | 0.54–2.42 |
| FS | 0.2524 | 0.2577 | 0.3273 | 1.29 | 0.78–2.16 |
| FI | 1.2709 | 0.3292 | 0.0001* | 3.56 | 1.84–6.75 |
| Unk | 1.0531 | 0.2742 | 0.0001* | 2.87 | 1.68–4.96 |
| DevOpLow | 0.0832 | 0.0318 | 0.0089* | 1.09 | 1.02–1.16 |
| DevMed | −0.3939 | 0.1616 | 0.0148* | 0.67 | 0.49–0.92 |
| Inc | −0.0391 | 0.0128 | 0.0022* | 0.96 | 0.94–0.99 |
| EvFor | 0.0969 | 0.0320 | 0.0025* | 1.10 | 1.03–1.17 |
| GS | −0.0751 | 0.0459 | 0.1018 | 0.93 | 0.85–1.01 |
| MeanRH | −0.1328 | 0.0439 | 0.0025* | 0.88 | 0.8–0.95 |
| TempRange | −0.5219 | 0.1019 | <0.0001* | 0.59 | 0.49–0.72 |
| Model 7 | |||||
| (Intercept) | 18.5113 | 4.4983 | <0.0001* | ||
| Gender | |||||
| MI | 0.1226 | 0.3794 | 0.7466 | 1.15 | 0.52–2.34 |
| FS | 0.2371 | 0.2569 | 0.3561 | 1.27 | 0.77–2.12 |
| FI | 1.1914 | 0.3278 | 0.0003* | 3.33 | 1.72–6.29 |
| Unk | 1.2795 | 0.2608 | <0.0001* | 3.46 | 2.07–5.89 |
| EvFor | 0.0942 | 0.0318 | 0.0030* | 1.11 | 1.04–1.19 |
| GS | −0.0923 | 0.0464 | 0.0465* | 0.91 | 0.82–0.99 |
| MeanRH | −0.1667 | 0.0422 | 0.0001* | 0.85 | 0.78–0.93 |
| TempRange | −0.5047 | 0.1021 | <0.0001* | 0.61 | 0.50–0.74 |
| Inc | −0.0362 | 0.0117 | 0.0019* | 0.97 | 0.94–0.99 |
| Tick status | |||||
| Not reported | −0.0409 | 0.3844 | 0.9153 | 0.96 | 0.43–1.97 |
| Reported | 0.1961 | 0.26344 | 0.4566 | 1.22 | 0.72–2.03 |
| Model 2 | |||||
| (Intercept) | 15.6572 | 4.1363 | 0.0002* | ||
| Gender | |||||
| MI | 0.1416 | 0.3795 | 0.7092 | 1.15 | 0.52–2.35 |
| FS | 0.2450 | 0.2568 | 0.3401 | 1.28 | 0.78–2.14 |
| FI | 1.2598 | 0.3250 | 0.0001* | 3.52 | 1.83–6.62 |
| Unk | 1.3705 | 0.2591 | <0.0001* | 3.94 | 2.39–6.62 |
| TempRange | −0.4099 | 0.0899 | <0.0001* | 0.66 | 0.56–0.79 |
| MeanRH | −0.1535 | 0.0403 | 0.0001* | 0.86 | 0.79–0.93 |
| Inc | −0.0229 | 0.0102 | 0.0246* | 0.98 | 0.96–1.00 |
| Elev | −0.0320 | 0.0261 | 0.2197 | 0.97 | 0.91–1.01 |
| Model 3 | |||||
| (Intercept) | −2.7989 | 0.7075 | 0.0001* | ||
| Gender | |||||
| MI | 0.0554 | 0.3767 | 0.8832 | 1.06 | 0.01–0.24 |
| FS | 0.2298 | 0.2560 | 0.3693 | 1.26 | 0.48–2.15 |
| FI | 1.1275 | 0.3198 | 0.0004* | 3.09 | 0.77–2.10 |
| Unk | 1.3848 | 0.2653 | <0.0001* | 3.99 | 1.62–5.73 |
| Inc | −0.0269 | 0.0153 | 0.0789 | 0.97 | 2.39–6.79 |
| DevMed | −0.5035 | 0.2472 | 0.0417* | 0.60 | 0.94–1.00 |
| PopDens | 0.2689 | 0.1304 | 0.0392* | 1.31 | 0.37–0.96 |
| Model 6 | |||||
| (Intercept) | −4.8029 | 7.0339 | 0.4947 | ||
| Gender | |||||
| MI | 0.1279 | 0.3775 | 0.7348 | 1.14 | 0.52–2.31 |
| FS | 0.2485 | 0.2562 | 0.3321 | 1.28 | 0.78–2.14 |
| FI | 1.2050 | 0.3235 | 0.0002* | 3.34 | 1.74–6.24 |
| Unk | 1.1748 | 0.2790 | <0.0001* | 3.24 | 1.89–5.65 |
| DevMed | −0.5380 | 0.2148 | 0.0123* | 0.58 | 0.38–0.89 |
| DevOpLow | 0.1081 | 0.0772 | 0.1614 | 1.11 | 0.96–1.29 |
| DecFor | −0.0128 | 0.0736 | 0.8622 | 0.99 | 0.85–1.14 |
| EvFor | 0.0501 | 0.0912 | 0.5832 | 1.05 | 0.88–1.25 |
| Crop | 0.0070 | 0.0738 | 0.9241 | 1.01 | 0.87–1.16 |
| Pst | 0.0250 | 0.0731 | 0.7327 | 1.03 | 0.89–1.18 |
| GS | −0.0661 | 0.0785 | 0.3999 | 0.94 | 0.80–1.09 |
| Wet | 0.0111 | 0.0702 | 0.8741 | 1.01 | 0.88–1.16 |
| Model 4 | |||||
| (Intercept) | −4.0361 | 0.2500 | <0.0001* | ||
| Gender | |||||
| MI | 0.0691 | 0.3759 | 0.8542 | 1.07 | 0.49–2.17 |
| FS | 0.2355 | 0.2558 | 0.3573 | 1.27 | 0.77–2.11 |
| FI | 1.1232 | 0.3190 | 0.0004* | 3.07 | 1.62–5.70 |
| Unk | 1.5154 | 0.2552 | <0.0001* | 4.55 | 2.78–7.60 |
| Crop | 0.0006 | 0.0107 | 0.9526 | 1.00 | 0.98–1.02 |
| Pst | 0.0065 | 0.0123 | 0.5983 | 1.01 | 0.98–1.03 |
| Model 5 | |||||
| (Intercept) | −4.0828 | 0.3569 | <0.0001* | ||
| Gender | |||||
| MI | 0.0625 | 0.3760 | 0.8680 | 1.06 | 0.49–2.16 |
| FS | 0.2364 | 0.2558 | 0.3555 | 1.27 | 0.77–2.11 |
| FI | 1.1139 | 0.3198 | 0.0005* | 3.05 | 1.60–5.66 |
| Unk | 1.5359 | 0.2588 | <0.0001* | 4.65 | 2.82–7.80 |
| EvFor | 0.0145 | 0.0215 | 0.5001 | 1.01 | 0.97–1.06 |
| DecFor | 0.0010 | 0.0080 | 0.9005 | 1.00 | 0.98–1.02 |
| MixFor | −0.0277 | 0.0591 | 0.6391 | 0.97 | 0.87–1.09 |
| Model 1 | |||||
| (Intercept) | −3.7057 | 0.2846 | <0.0001* | ||
| Gender | |||||
| MI | 0.0465 | 0.3769 | 0.9018 | 1.05 | 0.48–2.13 |
| FS | 0.2466 | 0.2563 | 0.3361 | 1.28 | 0.78–2.14 |
| FI | 1.1385 | 0.3197 | 0.0004* | 3.12 | 1.64–5.80 |
| Unk | 1.4410 | 0.2583 | <0.0001* | 4.22 | 2.56–7.09 |
| Breed group | |||||
| Herding | −0.3751 | 0.3351 | 0.2629 | 0.69 | 0.35–1.32 |
| Hound | −0.1409 | 0.3414 | 0.6799 | 0.87 | 0.44–1.68 |
| Non AKC | 0.1477 | 0.7598 | 0.8459 | 1.16 | 0.18–4.16 |
| Nonsporting | −0.6257 | 0.4397 | 0.1547 | 0.53 | 0.21–1.21 |
| Sporting | −0.0570 | 0.2686 | 0.8319 | 0.94 | 0.56–1.62 |
| Terrier | −0.9167 | 0.5021 | 0.0679 | 0.40 | 0.13–0.99 |
| Toy | −0.6664 | 0.4205 | 0.1130 | 0.51 | 0.21–1.13 |
| Working | −0.2937 | 0.3412 | 0.3895 | 0.75 | 0.37–1.44 |
| Unk | 0.2217 | 0.6507 | 0.7333 | 1.25 | 0.28–3.93 |
Logistic regression models based on biologically plausible hypotheses for factors driving differences in Bartonella henselae exposure in dogs, with B. henselae seroreactivity as dependent variable. Models are listed in ranked order based on AIC. Baseline sex male castrated; baseline breed mixed; baseline tick status established. Models listed in descending order of AIC. Statistical significance considered at p < 0.05 for individual factors (indicated by *).
AKC, American Kennel Club; CI, confidence interval; OR, odds ratio; Unk, gender/breed not recorded.
Table 5.
Akaike's Information Criterion Weighted-Average Model Results
| Variable | Estimate | Standard error | p | OR | 95% CI |
|---|---|---|---|---|---|
| Gender | |||||
| MI | 0.1673 | 0.3801 | 0.6600 | 1.18 | 0.56–2.49 |
| FS | 0.2517 | 0.2576 | 0.3287 | 1.29 | 0.78–2.13 |
| FI | 1.2679 | 0.3294 | 0.0001* | 3.55 | 1.86–6.78 |
| Unk | 1.0654 | 0.2786 | 0.0001* | 2.9 | 1.68–5.01 |
| EvFor | 0.0959 | 0.0340 | 0.0048* | 1.1 | 1.03–1.17 |
| DevOpLow | 0.0785 | 0.0363 | 0.0307* | 1.09 | 1.02–1.16 |
| DevMed | −0.3719 | 0.1813 | 0.0402* | 0.67 | 0.49–0.93 |
| Inc | −0.0386 | 0.0129 | 0.0028* | 0.96 | 0.94–0.99 |
| MeanRH | −0.1342 | 0.0442 | 0.0024* | 0.87 | 0.8–0.95 |
| TempRange | −0.5194 | 0.1027 | 0.0000* | 0.59 | 0.49–0.73 |
| Elev | −0.0005 | 0.0049 | 0.9260 | 0.97 | 0.92–1.02 |
| GS | −0.0751 | 0.0468 | 0.1087 | 0.93 | 0.85–1.01 |
| Tick status | |||||
| Not reported | −0.0017 | 0.0789 | 0.9828 | 0.96 | 0.45–2.04 |
| Reported | 0.0082 | 0.0666 | 0.9022 | 1.22 | 0.73–2.04 |
Estimates and standard error of slope, p value for each explanatory variable, and ORs with 95% CIs for explanatory variables included in AIC weighted-average logistic regression model.
Statistical significance considered at p < 0.05 for individual factors.
Sex baseline MC, tick status baseline established; Unk, gender not recorded; EvFor, percentage of county with evergreen forest classification; DevOpLow, percentage of county with open or low-intensity development classification; DevMed, percentage of county with medium development classification; Inc, median household income/1000; MeanRH, mean relative humidity; TempRange, difference between annual average highest temperature and annual average lowest temperature (°F); Elev, county average elevation/100; GS, percentage of county with grass or shrub classification.
Discussion
This study provides a statistical modeling approach to understanding B. henselae exposure in dogs suspected of vector-borne disease across NC. There was variable seroreactivity across the state, with areas of apparent higher exposure along the coastal counties in the east, in the southern coastal plains counties, and in the eastern Piedmont counties. There was lower seroreactivity in the western mountain counties. Of the initial hypotheses for associations between explanatory variables and seroreactivity, the data provided the most support for a combination of patient demographic factors, owner socioeconomic factors, and climate and land use factors. This model could be improved, however, by including local and host-scale factors that may play a significant role in dogs' exposure. Unmeasured factors that may influence exposure include, among others, local effects of a dog's particular living environment; host factors including acaricide usage, immunocompromise or other comorbidities, or genetic susceptibility; and direct evidence for proposed arthropod vector abundance and activity including possible seasonal trends.
The likelihood of a positive IFA test is dependent on three basic categories of factors: vector presence, vector contact, and detection of exposure. As direct evidence for any of these three variables is lacking, indirect associations with socioeconomic and ecological variables were assessed in this study. Because of this, any interpretation of these findings with regard to their implication for vector transmission must be done with caution.
That said, these findings suggest that the variation in seroreactivity may reflect variation in exposure not only to fleas, the widely accepted vector for B. henselae transmission in cats and humans, but also potentially to ticks. Since climate and habitat are well known to play a key role in the prevalence and activity of many species of ticks, the model provides support for transmission through ticks on a population scale but does not specify a particular species of tick vector (Springer et al. 2015, Eisen et al. 2016b, Ogden and Lindsay 2016, Minigan et al. 2017).
Flea abundance depends on temperature and humidity, but the suitable climatic range is wide [temperatures between 37°F and 95°F, with relative humidity >33% (Traversa 2013)] and climate extremes sufficient to limit flea development are rarely found in NC based on our data. In addition, exposure to fleas, particularly C. felis, may be independent of climatic and habitat factors due to their ability to complete their entire lifecycle indoors (Gracia et al. 2008, Rust 2017). However, in some cases, flea infestation may have a seasonal component, and C. felis thrive in warm humid environments (Cruz-Vazquez et al. 2001, Gracia et al. 2008, Traversa 2013). This model does not, therefore, preclude the involvement of fleas (or other arthropod vectors) in transmission, but rather suggests that there is an additional more climate- and habitat-dependent route of transmission than fleas alone.
It is possible that the variation in seroreactivity reflects variation in exposure to both fleas and ticks, or even a nonvector-borne pathway of transmission. Future epidemiologic studies surveying the ectoparasites present on dogs and cats and investigating risk factors for vector exposure would help define the role of these potential vectors, and address the variables of both vector presence and vector contact.
In addition to highlighting the role of climate and habitat in B. henselae exposure, this model showed that some of the variability in exposure was due to patient gender. We hypothesized that male intact dogs would have highest B. henselae seroreactivity, but in this sample in fact female intact dogs had highest B. henselae seroreactivity. The explanation for gender differences in Bartonella spp. exposure remains controversial. Whether there is a biological component to being a female or intact dog that increases exposure, such as the possibility of sexual transmission of B. henselae or immunological differences in intact dogs, or whether being an intact female is a marker of another confounding lifestyle factor that increases exposure, such as living outdoors or lack of acaricide use, is unknown.
In a report of patients presented to a Pennsylvania teaching hospital, patient age, owner household income, and being neutered were associated with an increased likelihood of heartworm preventative compliance, but it is difficult to generalize these localized small-scale survey-based findings to wider scale or to use of flea and tick preventatives (Gates and Nolan 2010). However, gender differences in prevalence of vector-borne disease have been previously found in the case of heartworm, with intact dogs more likely to have heartworm disease (Selby et al. 1980, Levy et al. 2007), so this result is in keeping with patterns of exposure for other CVBDs and infectious disease generally (Hoffman et al. 2013).
Finally, in this model as median household income increased, exposure to B. henselae decreased (in contrast to our hypothesis of a positive association between median household income and B. henselae seroreactivity). Thus, assuming that the knowledge of—and financial ability to test for—Bartonella spp. as pathogens in dogs is not associated with climatic or land-use variables, then the differences in seroreactivity across counties did not appear to be based solely on increased detection. This may be indicative of lifestyle factors in dogs residing in counties with lower average median household income, such as higher risk of contacting flea or tick vectors due to lower use of acaricides, or reduced access to veterinary care (Brown et al. 2012, LaVallee et al. 2017).
Counties with larger percentages of moderate development had lower B. henselae seroreactivity, and counties with larger percentages of low-level development or open developed space, or evergreen forest, had higher B. henselae seroreactivity. As defined by the NLCD, areas of moderate development mainly include buildings and impervious surfaces such as roads and sidewalks, in contrast to low-level development or open space, which most commonly includes large-lot single-family housing and vegetation such as parks or lawns (Homer et al. 2015).
Along with the possibility that lower income levels are associated with decreased detection, this pattern suggests a rural–urban gradient of exposure. For example, counties with the largest cities did not have the highest seroreactivity: Mecklenburg, containing the city of Charlotte, had an average seroreactivity of 3.2%, compared with the adjacent suburban to rural counties to the east, Union (4.2%) and Stanly (8.3%). However, this model also provided little support for an association between farms and B. henselae exposure in dogs on a statewide level, in contrast to a previous study showing increased exposure to B. vinsonii subsp. berkhoffii in dogs in rural environments or with access to farms (Pappalardo et al. 1997). In fact, no single variable explained the distribution of B. henselae exposure well, further highlighting the complexity of B. henselae disease ecology in dogs.
Limitations of this study include the limitations inherent in a retrospective serology study using a convenience sample. Although the motivation for submission of samples to the VBDDL is not specified on submission forms, typically most testing is performed diagnostically for sick dogs; therefore, our study sample does not represent a random sample from the general dog population in NC. The decision to submit a sample for testing may be biased by both owners and veterinarians, based on previous experience with or knowledge of Bartonella, as well as perception of vector-borne disease risk in certain locations or seasons. Whether testing was done to confirm a suspected clinical diagnosis, to rule out a possible underlying etiology for a clinical syndrome typically associated with Bartonella or another vector-borne disease, or to screen a healthy dog (e.g., military or other working dogs), is unknown. These samples, however, do not include experimental animals from research institutions or blood donor dogs screened at NCSU, but rather diagnostic submissions only.
Limited knowledge of, and access to, Bartonella serology testing by both dog owners and veterinarians may lead to dogs not being tested by serology for this emerging infectious disease. The population examined in our study may overestimate or underestimate the true prevalence of exposure in healthy or sick populations of dogs. Because sampling was not uniformly distributed throughout the state, and there was scant data from rural counties and counties in the far western part of the state, extrapolations to these under-represented regions should be done with caution. However, even when excluding counties with low number of samples, there were areas with apparently higher exposure, including Granville (3/39 seroreactive), Wayne (3/45 seroreactive), and Mecklenburg (30/557 seroreactive), compared with areas with low exposure (Wilson county, 0/44 and Hanover, 2/187); these findings were confirmed with the smoothed map.
Travel histories for the dogs were not available, and it is possible that dogs in this sample were exposed to B. henselae in other locations besides their home county. Despite these limitations, the NCSU-VBDDL database provides one of the best sources for existing Bartonella spp. serology data in dogs to date. This study included data from >80% of the counties in the state and >4300 dogs, which is a fairly large and comprehensive sample for a retrospective study of this nature.
Although serology is the current gold standard for determination of exposure to B. henselae for both diagnostic and serosurvey purposes, this modality does have limitations (Perez et al. 2011, Brenner et al. 2012, Hegarty et al. 2014, Maggi et al. 2014). Previous studies have shown poor associations between seroreactivity and bacteremia (Brenner et al. 2012), with antibody reactivity to Bartonella species antigens detected in ≤50% of dogs in which active infection can be documented (Perez et al. 2011). Therefore, IFA antibody testing lacks sensitivity, and may underestimate the true prevalence of B. henselae exposure in dogs.
Finally, limitations are inherent in the statistical model itself. This model does not account for factors that are not routinely measured with publicly available data. Importantly, this model analyzed factors at the county level on an annual timescale, and there may be important drivers of exposure at smaller scales or seasonally that we were not able to assess (Robertson and Feick 2018). Household-level effects may drastically change exposure risk for dogs within similar environments, particularly when considering variation in acaricide use. Because of this, care must be taken in interpreting the results from this model, particularly at smaller spatial scales. Further studies should focus on methods to assess previously undefined factors, such as household-level risks for vector exposure, and different spatial scales.
Conclusions
In this study, we report a statistical model for B. henselae seroreactivity in dogs in NC, providing a better understanding of its endemic range and highlighting the importance of considering ecological factors when evaluating B. henselae exposure. The model with the best fit included demographic, socioeconomic, climatic, and landscape factors. The maps created herein may help inform public health and veterinary professionals in NC about B. henselae in their areas, and may suggest areas where humans are at increased risk for B. henselae exposure. Humans and dogs share environments both indoors and outdoors, and are thus often exposed to similar vectors and vector-borne diseases. Indeed, if B. henselae in dogs shares similar ecology with that in people, it could be expected that seroreactivity in dogs may be correlated with exposure risk in humans. In the future, this model may be expanded to investigate transmission risk and explore alternative vectors for B. henselae in humans, used to evaluate possible consequences of ecological and socioeconomic changes to the range and prevalence of B. henselae in dogs, or expanded to wider geographic areas as serology data become available.
Supplementary Material
Acknowledgments
The authors thank the personnel of the NCSU-VBDDL and, in particular, Barbara Hegarty, who organized and managed the database of sample submissions at this laboratory until her retirement in 2017. Research reported in this publication was supported by the Comparative Medicine and Translational Research Program of the National Institutes of Health under award number T32OD011130.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- Adelson ME, Rao R-VS, Tilton RC, Cabets K, et al. Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in Northern New Jersey. J Clin Microbiol 2004; 42:2799–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkishe AA, Townsend Peterson A, Samy AM. Climate change influences on the potential geographic distribution of the disease vector tick Ixodes ricinus. PLoS One 2017; 12:e0189092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR. Model Based Inference in the Life Sciences: A Primer on Evidence. New York, NY: Springer Science+Business Media, LLC, 2008 [Google Scholar]
- Angelakis E, Billeter SA, Breitschwerdt EB, Chomel BB, et al. Potential for tick-borne bartonelloses. Emerg Infect Dis 2010; 16:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnez M, Luznik-Bufon T, Avsic-Zupanc T, Ruzic-Sabljic E, et al. Causes of febrile illnesses after a tick bite in Slovenian children. Pediatr Infect Dis J 2003; 22:1078–1083 [DOI] [PubMed] [Google Scholar]
- Barton K. MuMIn: Multi-Model Inference. R package version 1.43.6. 2018
- Billeter SA, Kasten RW, Killmaster LF, Breitschwerdt EB, et al. Experimental infection by capillary tube feeding of Rhipicephalus sanguineus with Bartonella vinsonii subspecies Berkhoffii. Comp Immunol Microbiol Infect Dis 2012; 35:9–15 [DOI] [PubMed] [Google Scholar]
- Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol 2008; 22:1–15 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB. Bartonellosis: One health perspectives for an emerging infectious disease. ILAR J 2014; 55:46–58 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB. Bartonellosis, one health and all creatures great and small. Vet Dermatol 2017; 28:96-e21 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Hegarty BC, Hancock SI. Sequential evaluation of dogs naturally infected with Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia equi, Ehrlichia ewingii, or Bartonella vinsonii. J Clin Microbiol 1998; 36:2645–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, et al. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 2007; 13:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner EC, Chomel BB, Singhasivanon OU, Namekata DY, et al. Bartonella infection in urban and rural dogs from the tropics: Brazil, Colombia, Sri Lanka and Vietnam. Epidemiol Infect 2012; 141:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown HE, Harrington LC, Kaufman PE, McKay T, et al. Key factors influencing canine heartworm, Dirofilaria immitis, in the United States. Parasit Vectors 2012; 5:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Chomel BB, Kasten RW, Romano V, et al. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol 2001; 39:1221–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-C, Hideki H, Pusterla N, Kasten RW, et al. Investigation of Bartonella infection in ixodid ticks from California. Comp Immunol Microbiol Infect Dis 2002; 25:229–236 [DOI] [PubMed] [Google Scholar]
- Cotté V, Bonnet S, Le Rhun D, Le Naour E, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis 2008; 14:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Vazquez C, Castro Gamez E, Parada Fernandez M, Ramos Parra M. Seasonal occurrence of Ctenocephalides felis and Ctenocephalides canis (Siphonaptera: Pulicidae) infesting dogs and cats in an urban area in Cuernavaca, Mexico. J Med Entomol 2001; 38:111–113 [DOI] [PubMed] [Google Scholar]
- Donà D, Nai Fovino L, Mozzo E, Cabrelle G, et al. Osteomyelitis in cat-scratch disease: A never-ending dilemma-a case report and literature review. Case Rep Pediatr 2018; 2018:1679306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplan F, Davies S, Filler S, Abdullah S, et al. Anaplasma phagocytophilum, Bartonella spp., Haemoplasma species and Hepatozoon spp. in ticks infesting cats: A large-scale survey. Parasit Vectors 2018; 11:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol 2016a; 53:349–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, Beard CB. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and lyme disease in North America. J Med Entomol 2016b; 53:250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Feirer S, Padgett KA, Hahn MB, et al. Modeling climate suitability of the western blacklegged tick in California. J Med Entomol 2018; 55:1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley JE, Brown RN, Gabriel MW, Henn J, et al. Spatial analysis of the exposure of dogs in rural north-coastal California to vectorborne pathogens. Vet Rec 2007; 161:653–657 [DOI] [PubMed] [Google Scholar]
- Gates MC, Nolan TJ. Factors influencing heartworm, flea, and tick preventative use in patients presenting to a veterinary teaching hospital. Prev Vet Med 2010; 93:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia MJ, Calvete C, Estrada R, Castillo JA, et al. Fleas parasitizing domestic dogs in Spain. Vet Parasitol 2008; 151:312–319 [DOI] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, Eisen RJ. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J Med Entomol 2016; 53:1176–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Dehio C. Intruders below the Radar: Molecular pathogenesis of Bartonella spp. Clin Microbiol Rev 2012; 25:42–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty BC, Bradley JM, Lappin MR, Balakrishnan N, et al. Analysis of seroreactivity against cell culture-derived Bartonella spp. Antigens in dogs. J Vet Intern Med 2014; 28:38–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn JB, Liu C-H, Kasten RW, VanHorn B, et al. Seroprevalence of antibodies against Bartonella species and evaluation of risk factors and clinical signs associated with seropositivity in dogs. Am J Vet Res 2005; 66:688–694 [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Creevy KE, Promislow DEL. Reproductive capability is associated with lifespan and cause of death in companion dogs. PLoS One 2013; 8:61082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis 2006; 6:99–102 [DOI] [PubMed] [Google Scholar]
- Homer CG, Dewitz JA, Yang L, Jin S, et al. Completion of the 2011 National Land Cover Database for the Conterminous United States—Representing a decade of land cover change information. Photogrammetr Eng Remote Sensing 2015; 81:345–354 [Google Scholar]
- Honadel TE, Chomel BB, Yamamoto K, Chang C, et al. Seroepidemiology of Bartonella vinsonii subsp Berkhoffii exposure among healthy dogs. J Am Vet Med Assoc 2001; 219:480–484 [DOI] [PubMed] [Google Scholar]
- Jackman S. Pscl: Classes and Methods for R Developed in the Political Science Computational Laboratory. R package version 1.5.2. 2017
- Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol 2004; 19:101–108 [DOI] [PubMed] [Google Scholar]
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound Kennel in North Carolina. J Clin Microbiol 1999; 37:2631–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashnits E, Correa M, Hegarty BC, Birkenheuer A, et al. Bartonella seroepidemiology in dogs from North America, 2008–2014. J Vet Intern Med 2018; 32:222–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVallee E, Mueller MK, McCobb E. A systematic review of the literature addressing veterinary care for underserved communities. J Appl Anim Welf Sci 2017; 20:381–394 [DOI] [PubMed] [Google Scholar]
- Lele SR, Keim JL, Solymos P. ResourceSelection: Resource Selection (Probability) Functions for Use-Availability Data. R package version 0.3-2. 2017
- Levy JK, Edinboro CH, Glotfelty C-S, Dingman PA, et al. Seroprevalence of Dirofilaria immitis, feline leukemia virus, and feline immunodeficiency virus infection among dogs and cats exported from the 2005 gulf coast hurricane disaster area. J Am Vet Med Assoc 2007; 231:218–225 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lund RB, Nordone SK, Yabsley MJ, et al. A Bayesian spatio-temporal model for forecasting the prevalence of antibodies to Ehrlichia species in domestic dogs within the contiguous United States. Parasit Vectors 2017; 10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey D, Dolan MJ, Moss CW, Garcia M, et al. Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: Implication for therapy and new epidemiological associations. Clin Infect Dis 1992; 14:683–688 [DOI] [PubMed] [Google Scholar]
- MacDonald KA, Chomel BB, Kittleson MD, Kasten RW, et al. A prospective study of canine infective endocarditis in Northern California (1999–2001): Emergence of bartonella as a prevalent etiologic agent. J Vet Intern Med 2004; 18:56–64 [DOI] [PubMed] [Google Scholar]
- Maggi RG, Birkenheuer AJ, Hegarty BC, Bradley JM, et al. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit Vectors 2014; 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. Quantitative methods for analyzing travel behaviour on individuals: Some recent developments. In: Hensher D, Stopher P, eds. Behavioural Travel Modelling. London: Croom Helm, 1979 [Google Scholar]
- McMahan CS, Wang D, Beall MJ, Bowman DD, et al. Factors associated with Anaplasma spp. seroprevalence among dogs in the United States. Parasit Vectors 2016; 9:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan JN, Hager HA, Peregrine AS, Newman JA. Current and potential future distribution of the American dog tick (Dermacentor Variabilis, Say) in North America. Ticks Tick Borne Dis 2017; 9:354–362 [DOI] [PubMed] [Google Scholar]
- Morozova OV, Cabello FC, Dobrotvorsky AK. Semi-nested PCR detection of Bartonella henselae in Ixodes persulcatus ticks from Western Siberia, Russia. Vector Borne Zoonotic Dis 2004; 4:306–309 [DOI] [PubMed] [Google Scholar]
- Mosbacher ME, Klotz S, Klotz J, Pinnas JL. Bartonella henselae and the potential for arthropod vector-borne transmission. Vector Borne Zoonotic Dis 2011; 11:471–477 [DOI] [PubMed] [Google Scholar]
- NCI. Hansen Simonson and Statistical Research and Applications Branch. Headbang Software, 2016
- Ogden NH, Lindsay LR. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol 2016; 32:646–656 [DOI] [PubMed] [Google Scholar]
- Pappalardo BL, Brown T, Gookin JL, Morrill CL, et al. Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med 2000; 14:37–42 [DOI] [PubMed] [Google Scholar]
- Pappalardo BL, Correa MT, York CC, Peat CY, et al. Epidemiologic evaluation of the risk factors associated with exposure and seroreactivity to Bartonella vinsonii in dogs. Am J Vet Res 1997; 58:467–471 [PubMed] [Google Scholar]
- Perez C, Maggi RG, Diniz PPVP, Breitschwerdt EB. Molecular and serological diagnosis of Bartonella infection in 61 dogs from the United States. J Vet Intern Med 2011; 25:805–810 [DOI] [PubMed] [Google Scholar]
- Podsiadły E, Chmielewski T, Tylewska-Wierzbanowska S. Bartonella henselae and Borrelia burgdorferi infections of the central nervous system. Ann N Y Acad Sci 2003; 990:404–406 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2016
- Regier Y, Ballhorn W, Kempf VAJ. Molecular detection of Bartonella henselae in 11 Ixodes ricinus ticks extracted from a single cat. Parasit Vectors 2017; 10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier Y, O'Rourke F, Kempf VAJ. Bartonella spp.—A chance to establish one health concepts in veterinary and human medicine. Parasit Vectors 2016; 9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis C, Cote M, Le Rhun D, Lecuelle B, et al. Vector competence of the tick Ixodes ricinus for transmission of Bartonella birtlesii. PLoS Negl Trop Dis 2011; 5:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud E, Jaulhac B, Garcia-Bonnet N, Hunfeld KP, et al. Seroprevalence of seven pathogens transmitted by the Ixodes ricinus tick in forestry workers in France. Clin Microbiol Infect 2016; 22:735.e1–735.e9 [DOI] [PubMed] [Google Scholar]
- Robertson C, Feick R. Inference and analysis across spatial supports in the big data era: Uncertain point observations and geographic contexts. Trans GIS 2018; 22:455–476 [Google Scholar]
- Rust M. The biology and ecology of cat fleas and advancements in their pest management: A review. Insects 2017; 8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby LA, Corwin RM, Hayes HM. Risk factors associated with canine heartworm infection. J Am Vet Med Assoc 1980; 176:33–35 [PubMed] [Google Scholar]
- Solano-Gallego L, Bradley JM, Hegarty BC, Sigmon B, et al. Bartonella henselae IgG antibodies are prevalent in dogs from Southeastern USA. Vet Res 2004; 34:585–595 [DOI] [PubMed] [Google Scholar]
- Soucy J-PR, Slatculescu AM, Nyiraneza C, Ogden NH, et al. High-resolution ecological niche modeling of Ixodes scapularis ticks based on passive surveillance data at the northern frontier of lyme disease emergence in North America. Vector Borne Zoonotic Dis 2018; 18:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer YP, Jarnevich CS, Monaghan AJ, Eisen RJ, et al. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the continental United States. Am J Trop Med Hyg 2015; 93:875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich RW, Blagburn BL, Bowman DD, Carpenter C, et al. Quantitative factors proposed to influence the prevalence of canine tick-borne disease agents in the United States. Parasit Vectors 2014; 7:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors 2013; 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. Cartographic Boundary Shapefiles—Counties. 2017
- U.S. Pet Ownership & Demographics Sourcebook. Schaumburg, Ill: American Veterinary Medical Association, 2012 [Google Scholar]
- Wang D, Bowman DD, Brown HE, Harrington LC, et al. Factors influencing U.S. canine heartworm (Dirofilaria immitis) prevalence. Parasit Vectors 2014; 7:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SC, Liu Y, Lund RB, Gettings JR, et al. A bayesian spatio-temporal model for forecasting the prevalence of antibodies to Borrelia burgdorferi, causative agent of lyme disease, in domestic dogs within the contiguous United States. PLoS One 2017; 12:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikswo ME, Hu R, Metzger ME, Eremeeva ME. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J Med Entomol 2007; 44:158–162 [DOI] [PubMed] [Google Scholar]
- Yancey CB, Hegarty BC, Qurollo BA, Levy MG, et al. Regional seroreactivity and vector-borne disease co-exposures in dogs in the United States from 2004–2010: Utility of canine surveillance. Vector Borne Zoonotic Dis 2014; 14:724–732 [DOI] [PubMed] [Google Scholar]
- Zangwill KM. Cat scratch disease and other Bartonella infections. In: Curtis N, Finn A, Pollard AJ, eds. Hot Topics in Infection and Immunity in Children IX. Advances in Experimental Medicine and Biology. New York, NY: Springer New York, 2013; 764:159–166 [DOI] [PubMed] [Google Scholar]
- Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, et al. Cat scratch disease in connecticut—Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 1993; 329:8–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.