Abstract
Prostate autonomic and sensory axons control glandular growth, fluid secretion, and smooth muscle contraction and are remodeled during cancer and inflammation. Morphogenetic signaling pathways reawakened during disease progression may drive this axon remodeling. These pathways are linked to proliferative activities in prostate cancer and benign prostate hyperplasia. However, little is known about which developmental signaling pathways guide axon investment into prostate. The first step in defining these pathways is pinpointing when axon subtypes first appear in prostate. We accomplished this by immunohistochemically mapping three axon subtypes (noradrenergic, cholinergic, and peptidergic) during fetal, neonatal, and adult stages of mouse prostate development. We devised a method for periprostatic axon density quantification and tested whether innervation is uniform across the proximo-distal axis of dorsal and ventral adult mouse prostate. Many axons directly interact with or innervate neuroendocrine cells in other organs, so we examined whether sensory or autonomic axons innervate neuroendocrine cells in prostate. We first detected noradrenergic, cholinergic, and peptidergic axons in prostate at embryonic day (E)14.5. Noradrenergic and cholinergic axon densities are uniform across the proximal-distal axis of adult mouse prostate while peptidergic axons are denser in the periurethral and proximal regions. Peptidergic and cholinergic axons are closely associated with prostate neuroendocrine cells whereas noradrenergic axons are not. These results provide a foundation for understanding mouse prostatic axon development and organization and provides strategies for quantifying axons during progression of prostate disease.
Keywords: mouse, axon development, prostate innervation, sympathetic, parasympathetic
Introduction
Peripheral neurons regulate cellular functions of breast (Harburg and Hinck 2011), skin (Belle et al. 2017), lung (Nassenstein et al. 2008), intestine (Hatch and Mukouyama 2015), bladder (Keast et al. 2015) and kidney (Kopp 2015). Although modulatory actions of peripheral neurons in prostate cancer, benign hyperplasia, and inflammation are increasingly supported by peer-reviewed publications, the function of peripheral neurons in the prostate is not fully understood (McVary et al. 1994). The structure and function of prostate is maintained by axons, as indicated by denervation studies, but the mechanisms are unclear (Wang et al. 1991; McVary et al. 1994). Noradrenergic axons in healthy mouse prostate contract vascular and non-vascular smooth muscle (Pennefather et al. 2000). Cholinergic axons change ion fluxes across the epithelium, promoting secretion in healthy prostate tissue (Ventura et al. 2002). In prostate cancer, prostatic noradrenergic and cholinergic axon outgrowth promotes cancer development, cancer dissemination and tumor invasion, and positively correlates with tumor severity in patients with prostate adenocarcinoma (S. Batra et al. 1990; Magnon et al. 2013; Zahalka et al. 2017). The role of sensory axons in the prostate are relatively uncharacterized. In other organs such as the bladder, heart, and stomach, sensory axons sense stretch as the organ fills (Umans and Liberles 2018). Prostatic sensory axon density and nerve growth factor (NGF) abundance in prostatic fluid are elevated in men with prostatitis (Pontari and Ruggieri 2004; Watanabe et al. 2011). How noradrenergic, cholinergic, and peptidergic axons contribute to prostatic disease is poorly understood, in part because axon development and innervation patterns in healthy developing and mature prostate are incompletely characterized.
Previous studies have examined autonomic and sensory prostate axon innervation. Early studies focused almost exclusively on sympathetic innervation, reporting noradrenergic innervation of the prostate stroma and capsule using immunohistochemistry and pharmacological stimulation studies (Owman and Sjöstrand 1965; Sjöstrand 1965; Baumgarten et al. 1968; Raz et al. 1973; Caine et al. 1975). Further, prostate glandular tissue was reported to be innervated by an unidentified type of sympathetic axon (Farrell and Lyman 1937). A rat study examined location, properties, distribution and spinal connections of autonomic postganglionic pathways innervating prostate using retrograde labeling, nerve lesions, and immunofluorescence (Kepper and Keast 1995). This study identified two different cholinergic axons innervating the prostate, parasympathetic and sympathetic (Kepper and Keast 1995). These neurons were classified as cholinergic because they lacked detectable tyrosine hydroxylase (TH) and all TH-negative neurons in the rat pelvic ganglion express the synthetic enzyme for acetylcholine, choline acetyltransferase. Another study examined, over a limited time period, the development of peripheral axons in the prostate and concluded there were very few autonomic axons in mouse reproductive tissue prior to birth, with the density and depth of penetration increasing throughout the postnatal period (Yan and Keast 2008). However, this study did not examine embryonic periods or quantify axon densities in different prostate regions. The current study uniquely builds on previous work by studying the innervation and development patterns of noradrenergic, cholinergic, and peptidergic axons from before birth to sexual maturity in mice. Further, this is the first study, to our knowledge to describe the location and density of sensory axons in mouse prostate. We have also directly identified cholinergic axons using antibodies raised against vesicular acetylcholine transporter (VAChT), whereas many previous studies used the indirect makers, VIP or nNOS that potentially label only a subclass of cholinergic axons.
Here, we immunohistochemically mapped noradrenergic (tyrosine hydroxylase, TH), cholinergic (VAChT) and peptidergic (calcitonin gene-related peptide; CGRP) axon subtypes in fetal, neonatal, and adult mouse prostate to determine when axons first appear. We created a method to quantify axon density within periductal stroma of the periurethral, proximal, and distal regions of dorsal and ventral adult mouse prostate ducts and tested the hypothesis that axon density differs across the proximal-distal duct axis. This will form the foundation for investigating the impact of prostate disease, drug exposure, or environmental contaminants on axon density. We also used multiplex immunostaining to determine which axon subtypes innervate neuroendocrine cells. We found that both autonomic and sensory axons develop at the same time.
MATERIALS AND METHODS
Mice
All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice were C57BL/6J and purchased from Jackson Laboratories (stock number 000664, Bar Harbor, ME). Mice were housed in Innovive® HDPE plastic microisolator cages in a room maintained on a 12-h light and dark cycle, ambient temperature of 20.5 ± 1°C and relative humidity of 30–70%. Mice were fed a 5015 Diet (PMI Nutrition International, Brentwood, MO) from conception through weaning (P21) and a 8604 Teklad Rodent Diet thereafter (Harlan Laboratories, Madison, WI). Feed and water were available ad libitum and cages contained corncob bedding. All mice were euthanized by CO2 asphyxiation. Mice were time-mated by pairing males and females overnight. The morning of definitive copulatory plug identification was considered E0.5. Urogenital sinus and prostate tissues were collected at E13.5, E14.5, E15.5, E16.5, E17.5, P9, and P50, fixed overnight at 4°C in 4% paraformaldehyde dissolved in neutral buffered saline (Thermo Scientific) and dehydrated through a series of graded ethanols. Tissues were cleared in xylenes, embedded in paraffin wax and sectioned at 10 μm thickness on a microtome (Surgipath Medical Industries).
Immunohistochemistry
Tissue sections were deparaffinized with xylene and rehydrated in a series of graded ethanols. Immunofluorescence staining was conducted as described previously (Abler et al. 2011) with the following modifications: microwave decloaking was performed in 10 mM sodium citrate (pH 6.0) and non-specific sites were blocked for 1 h in TBSTw containing 1% Blocking Reagent (Roche Diagnostics, Indianapolis, IN), 5% normal donkey sera, and 1% bovine serum albumin fraction 5 (RDBTw). Immunofluorescence was detected using the recommended settings for each secondary antibody fluorophore. Antibodies are listed in Supplementary Table 1. Tissue sections were imaged using an SP8 confocal microscope (Leica, Wetzlar, Germany) fitted with a 20X oil immersion objective (HC PL Apo CS2 NA = 0.75; Leica, Wetzlar, Germany). Ten z-images were captured at 1024×1024 resolution and a Z-interval of 1 μm using LASX 8 software at a speed of 700 fps (Leica, Wetzlar, Germany). The detector gain ranged between 10–30 using Hy-D lasers. All fluorescent images were stained using Alexa 488, Rhodamine red, and Alexa 647 nm fluorophores. These fluorophores were detected using the 488, 546, and 633 lasers. Signals from the fluorophores were separated using a sequential imaging protocol. All images were captured at 300 ppl resolution and were manipulated to adjust whole image RGB color intensity using Adobe Photoshop (version 20.0).
Morphometric analysis
For E13.5- E16.5 specimens, presence or absence of axons was determined in the 10 μm stromal band extending from the basal surface of KRT5 or CDH1-stained urogenital sinus (UGS). For P9 and P50 specimens, axon density was determined in a 10 μm stromal band extending from the basal surface of CDH1-stained prostatic ductal epithelium (this region encompasses the majority of periductal smooth muscle). Axon density was quantified in the periurethral (Box 1), proximal (Box 2), and distal (Box 3) regions of dorsal and ventral prostate (Figure 1). The periurethral region spanned from the basal surface of urethral epithelium to the rhabdosphincter internal boundary and axon densities were calculated exclusively for ducts with patent openings to the urethra and not for other ducts in this region. The proximal region extended 650 μm from the external rhabdosphincter boundary. The distal region began 650 μm from the external rhabdosphincter and extended to the distal-most portion of prostatic ducts. Immunostained axon pixels were quantified using the thresholding feature (MaxEntropy thresholding) of ImageJ (Version 1.52e11)(Kapur et al. 1985). Image pixel density was scaled to 2.23 pixels/micron. For each age and sampled region, three non-adjacent, near-mid sagittal, technical replicate tissue sections (10 μm) were averaged per mouse and 4–10 biological replicate mice were evaluated per axon subtype. Three of these biological replicates were evaluated in the transverse orientation and the remaining in sagittal. Mice were derived from at least three separate litters per time point.
Fig. 1. Prostate TH+, VAChT+ and CGRP+ axon density can be quantified and normalized in a 10 μm periductal band.
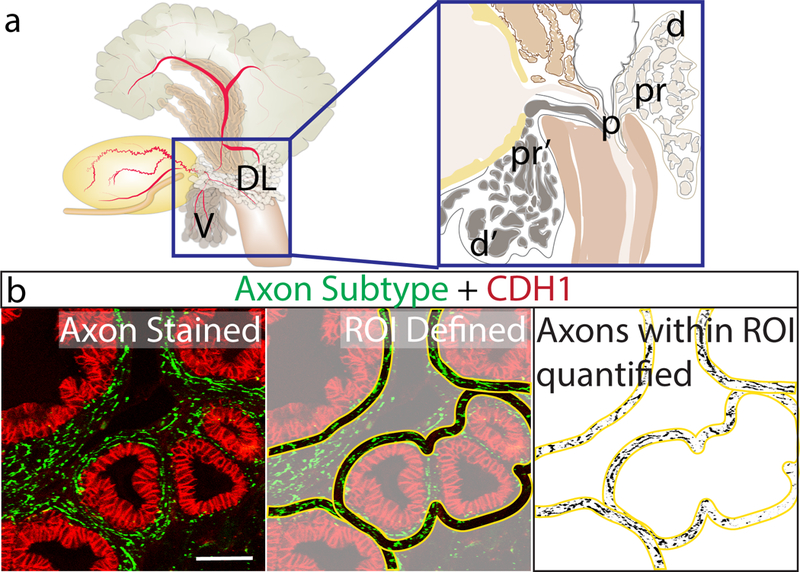
(A) Axons were quantified in sagittal sections of ventral and dorsal mouse prostate. Areas of interest included: p = periurethral in dorsal and ventral prostate, pr = proximal, d=distal in dorsal prostate, pr’= proximal ventral, d’= distal in ventral prostate. The periurethral region spanned from the basal surface of the urethral epithelium to the rhabdosphincter internal boundary. Axon densities were calculated exclusively for ducts with patent openings to the urethra and not for other ducts in this region. The proximal region extended 650 μm from the external rhabdosphincter boundary. The distal region began 650 μm from the external rhabdosphincter and extended to the distal-most portion of prostatic ducts. (B) Sections were immunohistochemically stained for markers of several axon subtypes (green) and e-cadherin to visualize prostatic and urethral epithelium (red). Stained sections were imaged using confocal microscopy, imported into ImageJ, and 10 μm bands (yellow) were traced around all prostate ducts and relative axon pixel densities were quantified using MaxEntropy auto-thresholding. Scale bar is 100 μm
Statistical Analysis
Results are reported as mean ± SE, where n= number of mice. Statistical analysis was done using GraphPad Prism 7 and a difference between means was considered significant at p ≤ 0.05. Bartlett’s test was used to determine homogeneity of variance with p ≤ 0.05 indicating inequality of variance. The Shapiro-Wilk test was used to assess normality of the residuals with p ≤ 0.05 indicating non-normal data. Data that did not meet the criteria for homogeneity of variance or normality was transformed using either a base-10 log transformation (VAChT regional density in dorsal and ventral prostate) or data was taken to the (1/3) power (TH+ regional density in dorsal and ventral prostate, TH+ temporal comparison in dorsal prostate, VAChT+ temporal comparison in dorsal and ventral prostate, CGRP+ regional density in ventral prostate). All axon density comparisons were assessed using one-way ANOVA followed by Tukey’s honestly significant difference post hoc test to identify which groups differed significantly p ≤ 0.05.
RESULTS
TH+, VAChT+, and CGRP+ axons innervate the prostate prior to prostatic bud formation
The first objective was to determine when TH+, VAChT+, and CGRP+ axons appear in the developing prostate. Mouse prostate is specified at E14.5 from a urethral expansion known as the urogenital sinus (UGS) and prostatic buds are first detected at E16.5 (Lin et al. 2003; Keil et al. 2012). We therefore evaluated axon patterns in the UGS at 24-h intervals beginning at E13.5 and continuing until E16.5. UGS tissue sections were immunostained with antibodies against TH+, VAChT+, and CGRP+ and against KRT5 to visualize epithelial cells. Axons were quantified in 10 μm peri-prostatic stromal bands radiating from the basilar epithelial boundary of the UGS (Figure 1). None of the examined axon subtypes are detected in this region at E13.5 (Figure 2). TH+, VAChT+, and CGRP+ axons are first observed in the peri-prostatic stroma at E14.5 (Figure 2). Taken together, TH+, VAChT+, and CGRP+ axons innervate prior to prostatic bud formation and during prostate specification.
Fig. 2. TH+, VAChT+, and CGRP+ axons innervate the prostate before prostate bud formation.
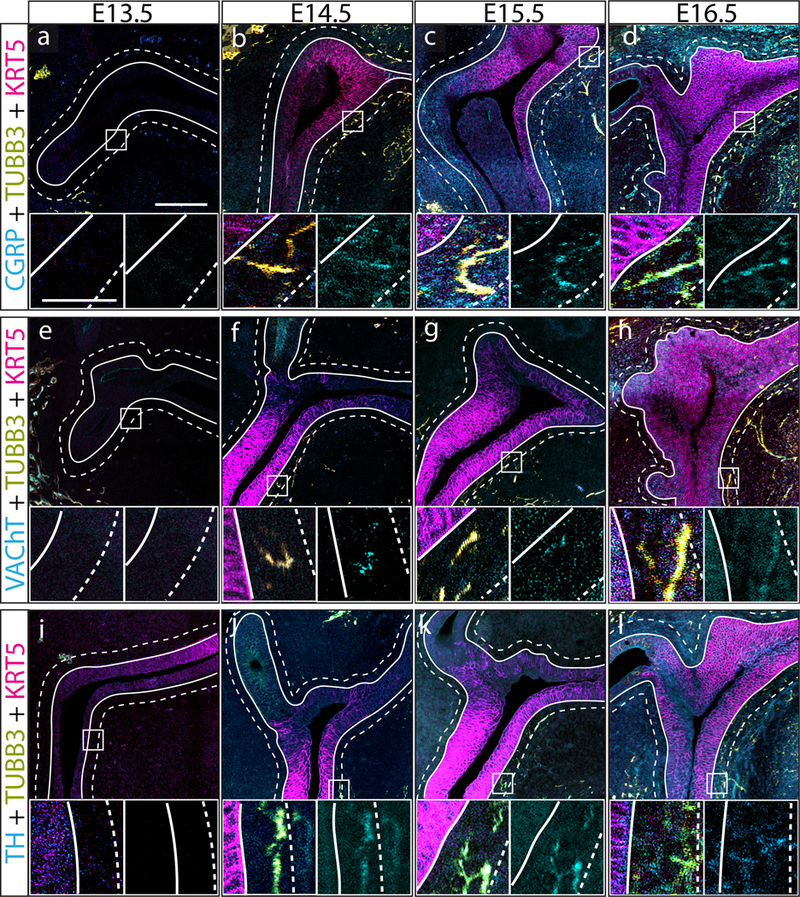
(a-l) E13.5, E14.5, E15.5, and E16.5 mouse prostate tissue sections were immunostained with antibodies against TH, VAChT+, or CGRP+ (cyan), the pan neuronal marker beta 3-tubulin (TUBB3, yellow), and keratin 5 (KRT5, magenta) to visualize prostatic and urethral epithelium. Panels are organized as follows: (top) wide field scan of the UGS showing all three-color channels, (lower left) region of interest showing axon subtype and TUBB3 channel overlap (white pixels), (lower right) region of interest showing isolated axon subtype channel. Axon density was quantified in 10 μm periductal spaces radiating outward from the basal surface of prostatic/urethral epithelium. Innervation of the developing was defined as the presence of TUBB3 and neuronal subtype channel overlap within 10 μm of UGS epithelium. The grouping of TH+ axons in the top right corner of panel j are likely associated with a microganglion. Three litter independent mice and three non-adjacent sections from each UGS were analyzed for each developmental stage. Large image scale bar is 100 μm. Inset image scale bar is 25 μm
TH+, VAChT+, and CGRP+ axon densities increase during fetal stages, peak during neonatal development, and are maintained in the adult mouse prostate
We examined the density of immunolabeled axons in a 10 μm stromal band around prostatic ducts (periductal stroma, Figure 1) to determine whether periductal densities of TH+, CGRP+, and VAChT+ axons change as prostate development progresses from the budding to branching stages and into sexual maturity. We found that periductal TH+, VAChT+, and CGRP+ axons are sparse during fetal periods, progressively increase in density through P9 (during branching morphogenesis) and are maintained at a similar level until at least P50 in dorsal prostate (Figure 3). This same pattern was present in ventral prostate (Supplementary Figure 1). Figure 3, 4, and Supplementary figure 1 and 2 detail some axons containing varicose, small swellings that are more highly immunolabeled. These varicose axons are more directly relevant to understanding function because these swellings typically indicate sites of neurotransmission to communicate with neighboring cell types. We observed varicose TH+ and VAChT+ axons within the 10 μm region of interest at P9 and P50, indicating that direct regulation of smooth muscle is feasible at these times.
Fig. 3. TH+, VAChT+, and CGRP+ axon densities increase during fetal stages of dorsal prostate development, peak during neonatal development, and are maintained in adulthood.
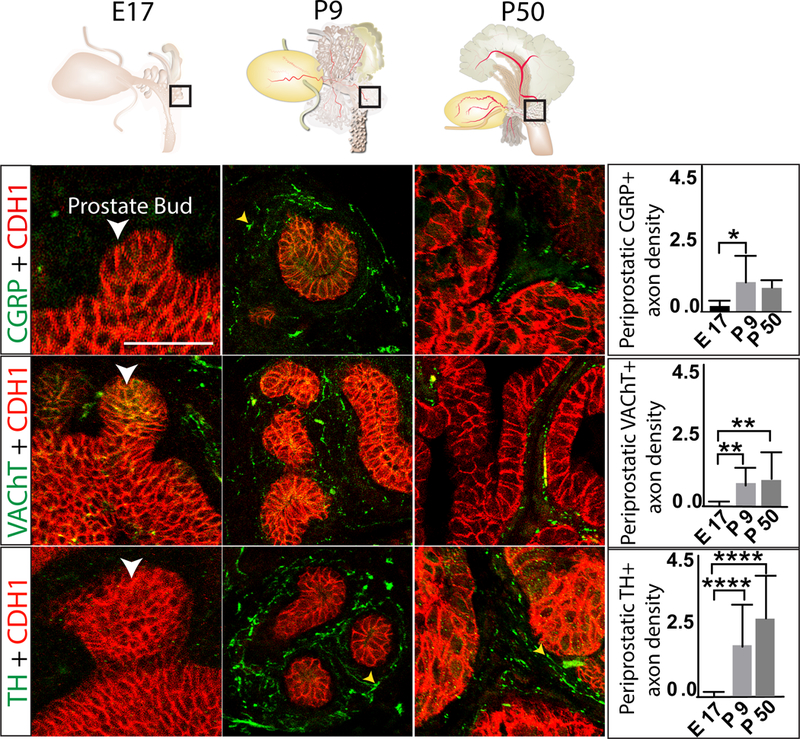
E17.5, P9, and P50 mouse dorsal prostate tissue sections were immunostained with antibodies against TH+, VAChT+, or CGRP (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red). Regions selected for analysis are schematized at the top of the figure and representative images are shown at the bottom left. Axon pixel densities were quantified in 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results graphed at the right. Varicose axons are identified with a yellow arrowhead. Results are mean ± SE of seven mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (ANOVA = p < 0.05, Tukey Post-Hoc = *p<0.05, **p<0.01 and ****p<0.0001) between groups. White arrowheads indicate prostatic buds. Scale bar is 50 μm
Fig. 4. TH+ and VAChT+ axon densities are uniform along the proximal-distal axis of dorsal prostate while CGRP+ axons are denser in the periurethral and proximal versus distal region.
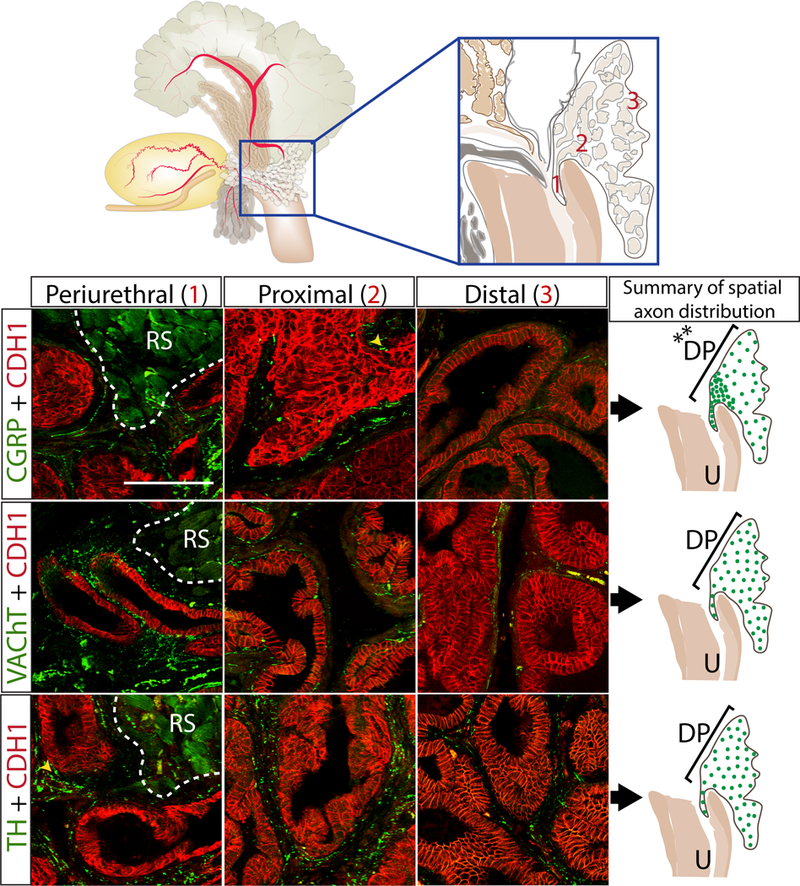
P50 mouse dorsal prostate tissue sections were immunostained with antibodies against TH+, VAChT+, or CGRP+ (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium (red)). Axon pixel densities were quantified in the 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results are shown pictorially on the right. Varicose axons are identified with a yellow arrowhead. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. An asterisk indicates a significant difference (**p<0.01) between regions. RS identifies the rhabdosphincter. Scale bar is 50 μm
TH+ and VAChT+ axon density is uniform along the proximal-distal axis of dorsal and ventral prostate while CGRP+ axons are more dense in the periurethral and proximal regions
We used our quantification method to evaluate axon density within the periurethral, proximal, and distal regions (Figure 4) of adult mouse dorsal and ventral prostate (P50) to determine whether axon density is uniform. If shown to be non-uniform, in future studies of prostate innervation it will be important to control for proximal-distal location. In dorsal prostate, we found that VAChT+ and TH+ axon densities do not differ across proximal-distal regions, while CGRP+ axon rank order densities were: periurethral > proximal > distal (Figure 4). In ventral prostate, we found similarly that VAChT+, and TH+ axon densities did not differ across the proximal-distal regions, while CGRP+ axons rank order densities were: periurethral = proximal > distal (Supplementary Figure 2). Graphs quantifying dorsal and ventral TH+, VAChT+, and CGRP+ axon densities across the periurethral, proximal, and distal axis can be found in Supplementary figures 3 and 4.
CGRP+ and VAChT+ axons, but not TH+ axons, innervate prostatic neuroendocrine cells
During analysis of axon development and density, we observed that after E17.5, a small population of CGRP+ and VAChT+ axons cross the epithelium to reach the lumen, while TH+ axons are never observed within prostatic epithelium. This sensory and cholinergic epithelial innervation was periodically observed crossing the epithelium at E17.5 and consistently observed in the periurethral and proximal region at P50. Because prostatic neuroendocrine cells are thought to migrate into UGS epithelium from the neural crest or differentiate from endodermal progenitors at this stage (Marker et al. 2003; Szczyrba et al. 2017; Toivanen and Shen 2017), we tested whether CGRP+ and VAChT+ axons innervate neuroendocrine cells. We identified neuroendocrine cells at E17.5 and P50 by immunolabeling for synaptophysin and found that they are innervated by CGRP+ and VAChT+ axons but not TH+ axons (Figure 5). TH+ axons were observed encircling blood vessels and VAChT+ axons were observed in the rhabdosphincter, as previously described (Georgas et al. 2015)(data not shown).
Fig. 5. CGRP+ and VAChT+ axons innervate neuroendocrine cells in the periurethral region whereas TH+ axons do not.
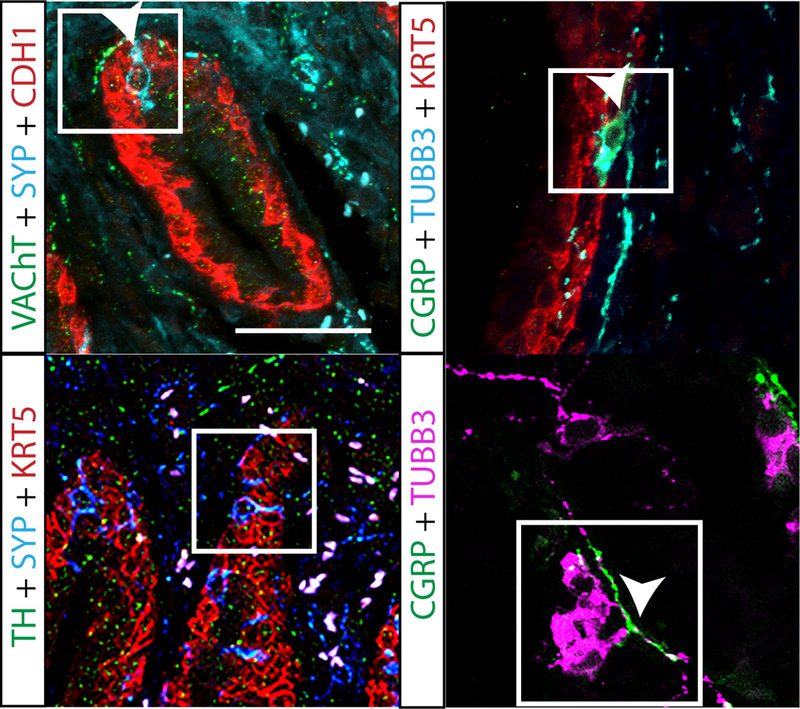
P50 mouse prostate tissue sections were immunostained with antibodies against TH+, VAChT+, or CGRP+ (green), e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red) and synaptophysin (SYP), to stain neuroendocrine cells (cyan). Note that CGRP is also expressed in neuroendocrine cells. Neuroendocrine cells were most commonly found in the periurethral region and cells with axons touching the neuroendocrine cell were considered to innervate that cell. Box indicates neuroendocrine cells and arrow heads mark axons. White arrowheads indicate innervated neuroendocrine cells. Neuroendocrine cell innervation results were consistent across three litter independent mice. Scale bar is 25 μm
DISCUSSION:
Here we identified the timing of noradrenergic, cholinergic, and peptidergic axon investment in prostate, how noradrenergic, cholinergic, and peptidergic axon density changes during development, and whether axon density is uniform along the proximo-distal axis within dorsal and ventral prostate lobes. As the role of axons in prostate disease becomes more apparent, it is important to understand peripheral axon development and density in normal, healthy animals. Results of this study could provide a valuable baseline reference for determining the impact of prostatic diseases and exposure to various drugs and environmental contaminants on prostatic axon patterns.
Pathways responsible for axon pathfinding and patterning have been identified in endoderm-derived lung, gut and bladder but not in prostate (Tollet et al. 2001; Harding et al. 2011; Aven and Ai 2013). The principal reason for this study was to pinpoint the critical window in which axons first appear in the developing prostate, thereby supporting future efforts to identify neurotrophins and other relevant neuronal survival or guidance factors. It is possible such factors are expressed later in life to recruit axons to inflamed, benign hyperplastic or neoplastic prostatic tissues. Our observation that critical windows for investment into the prostate are the same for noradrenergic, cholinergic, and sensory axons (E13.5-E14.5) raises the possibility that each axon type is recruited by similar mechanisms. Signaling mechanisms may begin to differ sometime after development, a notion supported by the fact that axon subtype densities differ among prostate diseases which arise from distinct mechanisms. For example, prostate cancer increases autonomic axon density while prostatitis increases sensory axon density (Satish Batra et al. 1990; Pontari and Ruggieri 2004; Watanabe et al. 2011; Magnon et al. 2013). Further, nerve growth factor (NGF) abundance decreases during prostate cancer progression (Pflug et al. 1992) while it increases during prostatitis (Pontari and Ruggieri 2004). Identities and concentrations of factors recruiting sensory axons into the mouse prostate may differ from those recruiting autonomic axons.
The next question we addressed is how noradrenergic, cholinergic, and peptidergic axon density changes during prostate development. Axon investment into the developing mouse prostate generally follows the time course of smooth muscle development, which is first detected in the mouse UGS at E14.5 (Vezina et al. 2008) and increases in abundance during the prostatic budding and postnatal branching stages (Thomson et al. 2002). This study reported axons followed a similar time course to smooth muscle development, TH+, VAChT+, and CGRP+ axon densities increase during fetal stages, peak during neonatal development, and are maintained in the adult mouse prostate. Whether prostatic smooth muscle maturation drives axon development or vice versa is an interesting subject for future research, as sympathetic axons are known to contract prostate smooth muscle.
We were interested in mapping the density of noradrenergic, cholinergic, and peptidergic axons along the proximo-distal axis at sexual maturity. Axon density quantification is challenged by histological heterogeneity among and within prostatic lobes. Ductal diameter, smooth muscle content, stromal: epithelial ratios, and abundance of glandular folding differ as a function of age and distance from the urethra (Bianchi-Frias et al. 2010; Oliveira et al. 2016). As a result, we developed an objective quantification method that enables comparison of axon density between regions of differing histomorphology. While previous research has predominantly focused on proximal and distal regions of mouse prostate, recent studies identify the periurethral region, spanning from the basal surface of urethral epithelium to the internal boundary of the rhabdosphincter, as a region where aging- and inflammation-mediated changes may drive prostate related urinary dysfunction (Nicholson and Ricke 2011). This study found TH+ and VAChT+ axon density is uniform along the proximal-distal axis of dorsal and ventral prostate while CGRP+ axons are more dense in the periurethral and proximal regions. CGRP+ axons contain many receptors and channels that influence excitability and growth, including transient receptor potential channels, neurotrophins, and estrogen receptors (Lanlua et al. 2001; Premkumar 2014). Therefore, regional differences in axon density across organs have the potential to influence function in many different ways. For example, in bladder, sensory axons are most dense in the neck region where they facilitate the guarding reflex, a stimulation of external urethral sphincter contraction in response to low bladder pressures to prevent bladder leaking (Danziger and Grill 2017). Overactive bladder syndrome, characterized by frequent urination, has been linked to abnormal elevations of peptidergic axon density (de Groat and Yoshimura 2009). Sensory axons in lung are most dense in bronchioles where their release of neuropeptides onto underlying target vessels and glands potentiate asthma (Lamb and Sparrow 2002; Kummer et al. 2008; Branchfield et al. 2016). CGRP+ axons in prostate are particularly dense in the periurethral and proximal region where their function is not completely known, but could be linked to detection of local pH changes, infection, or inflammatory responses (White et al. 2013).
Lastly, this study identified CGRP+ and VAChT+ axons penetrating the epithelium. As a result, we were interested in determining whether noradrenergic, cholinergic, and peptidergic axons innervate neuroendocrine cells, a specialized cell type that resides in the epithelium. This study found that both CGRP+ and VAChT+ axons innervate neuroendocrine cells while TH+ axons do not. Because CGRP+ and VAChT+ axons innervate neuroendocrine cells almost immediately after neuroendocrine cells are first detected in the developing prostate (E17), it is possible that nerve-neuroendocrine cell interactions facilitate prostate growth and development. This notion is supported by previous observations that prostatic neuroendocrine cell or nerve depletion reduces prostate size (Cheng et al. 2013).
In summary, we found that autonomic (TH+ and VAChT+) and sensory (CGRP+) axons innervate the mouse prostate during the same phase of prostate development. The density of each axon population continues to increase until it peaks during the period of branching morphogenesis and sexual maturity. CGRP+ axon density is greatest in the periurethral compared to distal region, while TH+ and VAChT+ axon density is uniform across regions. CGRP+ and VAChT+ axons also penetrate epithelium to potentially regulate neuroendocrine and epithelial cells. Futures studies will focus on factors controlling axon patterning and development and determining the impact of environmental chemical exposure on prostate peripheral axons.
Supplementary Material
Axon densities in P50 mouse prostate tissue sections were determined using the procedure described in Methods and Fig. 1 legend. Axon pixel densities were quantified in the 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results graphed. (a) CGRP+ axon density in the periurethral and proximal region is significantly greater than in the distal region. (b) VAChT+ axon density is uniform across the periurethral, proximal, and distal regions. (c) TH+ axon density is uniform across the periurethral, proximal, and distal regions. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (**p<0.01) between regions. RS identifies the rhabdosphincter. Scale bar is 50 μm
Axon densities in P50 mouse prostate tissue sections were determined using the procedure described in Methods and Fig. 1 legend. Axon pixel densities were quantified in the 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results graphed. (a) The CGRP+ axon density in the periurethral and proximal region is significantly greater than in the distal region. (b) VAChT+ axon density is uniform across the periurethral, proximal and distal regions. (c) TH+ axon density is uniform across the periurethral, proximal and distal regions. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (*p<0.05, and **p<0.01) between regions
E17.5, P9, and P50 mouse prostate tissue sections were immunostained with antibodies against TH+, VAChT+, and CGRP+ (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red). Axon pixel densities were quantified in the 10 μM periductal spaces radiating outward from the basilar surface of prostatic epithelium and results are shown pictorially on the right. Yellow arrowheads identify varicose axons. Results are mean ± SE of seven mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (*p<0.05, and **p<0.01) between regions. Scale bar is 50 μm
P50 mouse prostate tissue sections were immunostained with antibodies against TH+, VAChT+, or CGRP+ (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red). Regions selected for analysis are schematized at the top of the figure and representative images are shown at the bottom left. Axon pixel densities were quantified in the 10 μM periductal spaces radiating outward from the basal surface of prostatic epithelium and results are shown pictorially at the right. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (**p<0.01) between regions. Scale bar is 50 μm
List of primary antibodies used in this study. The antibody target, vendor and catelog number, RRID number, host species, dilution, and proof of specificity is listed.
Acknowledegements:
This work was supported by National Institutes of Health Grants RO1ES001332, U54DK104310, T32ES007015, U01DK110807, and U01DK110807-S1.
Footnotes
Conflict of Interest
The authors report no conflict of interest.
References
- Aven L, Ai X. 2013. Mechanisms of respiratory innervation during embryonic development. Organogenesis. 9(3):194–198. doi: 10.4161/org.24842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S, Christensson PI, Hartley-Asp B. 1990. Characterization of muscarinic cholinergic receptors in membrane preparations from rat prostatic adenocarcinoma. Prostate. 17(4):261–268. [DOI] [PubMed] [Google Scholar]
- Satish Batra, Christensson P-I, Hartley‐Asp B 1990. Characterization of muscarinic cholinergic receptors in membrane preparations from rat prostatic adenocarcinoma. The Prostate. 17(4):261–268. doi: 10.1002/pros.2990170402. [DOI] [PubMed] [Google Scholar]
- Baumgarten HG, Falck B, Holstein AF, Owman C, Owman T. 1968. Adrenergic innervation of the human testis, epididymis, ductus deferens and prostate: a fluorescence microscopic and fluorimetric study. Z Zellforsch Mikrosk Anat. 90(1):81–95. [DOI] [PubMed] [Google Scholar]
- Belle M, Godefroy D, Couly G, Malone SA, Collier F, Giacobini P, Chédotal A. 2017. Tridimensional Visualization and Analysis of Early Human Development. Cell. 169(1):161–173.e12. doi: 10.1016/j.cell.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. 2010. The Effects of Aging on the Molecular and Cellular Composition of the Prostate Microenvironment. PLOS ONE. 5(9):e12501. doi: 10.1371/journal.pone.0012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchfield K, Nantie L, Verheyden JM, Sui P, Wienhold MD, Sun X. 2016. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 351(6274):707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine M, Raz S, Zeigler M. 1975. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 47(2):193–202. [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Zhou Z, Nikitin AY. 2013. Detection and organ-specific ablation of neuroendocrine cells by synaptophysin locus-based BAC cassette in transgenic mice. PLoS ONE. 8(4):e60905. doi: 10.1371/journal.pone.0060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger ZC, Grill WM. 2017. Sensory feedback from the urethra evokes state-dependent lower urinary tract reflexes in rat. J Physiol (Lond). 595(16):5687–5698. doi: 10.1113/JP274191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell J, Lyman Y. 1937. A study of the secretory nerves of, and the action of certain drugs on the prostate gland. Am J Physiol. 118:64–70. [Google Scholar]
- Georgas KM, Armstrong J, Keast JR, Larkins CE, McHugh KM, Southard-Smith EM, Cohn MJ, Batourina E, Dan H, Schneider K, et al. 2015. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 142(10):1893–1908. doi: 10.1242/dev.117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. 2009. Afferent Nerve Regulation of Bladder Function in Health and Disease. Handb Exp Pharmacol.(194):91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburg GC, Hinck L. 2011. Navigating breast cancer: axon guidance molecules as breast cancer tumor suppressors and oncogenes. J Mammary Gland Biol Neoplasia. 16(3):257–270. doi: 10.1007/s10911-011-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, et al. 2011. The GUDMAP database – an online resource for genitourinary research. Development. 138(13):2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch J, Mukouyama Y-S. 2015. Spatiotemporal mapping of vascularization and innervation in the fetal murine intestine. Dev Dyn. 244(1):56–68. doi: 10.1002/dvdy.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Sahoo P, Wong A. 1985. A New Method for Gray-Level Picture Thresholding Using the Entropy of the Histogram”. Graphical Models and Image Processing. 29(3):273–285. [Google Scholar]
- Keast JR, Smith-Anttila CJA, Osborne PB. 2015. Developing a functional urinary bladder: a neuronal context. Front Cell Dev Biol. 3. doi: 10.3389/fcell.2015.00053. [accessed 2018 Jun 4]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4555086/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil KP, Mehta V, Abler LL, Joshi PS, Schmitz CT, Vezina CM. 2012. Visualization and quantification of mouse prostate development by in situ hybridization. Differentiation. 84(3):232–239. doi: 10.1016/j.diff.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC. 2015. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol. 308(2):R79–R95. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Lips KS, Pfeil U. 2008. The epithelial cholinergic system of the airways. Histochem Cell Biol. 130(2):219–234. doi: 10.1007/s00418-008-0455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JP, Sparrow MP. 2002. Three-Dimensional Mapping of Sensory Innervation with Substance P in Porcine Bronchial Mucosa. Am J Respir Crit Care Med. 166(9):1269–1281. doi: 10.1164/rccm.2112018. [DOI] [PubMed] [Google Scholar]
- Lanlua P, Decorti F, Gangula PR, Chung K, Taglialatela G, Yallampalli C. 2001. Female steroid hormones modulate receptors for nerve growth factor in rat dorsal root ganglia. Biol Reprod. 64(1):331–338. [DOI] [PubMed] [Google Scholar]
- Lin T-M, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. 2003. Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 76(1):171–181. doi: 10.1093/toxsci/kfg218. [DOI] [PubMed] [Google Scholar]
- M, Keast J 1995. Immunohistochemical properties and spinal connections of pelvic autonomic neurons that innervate the rat prostate gland. Cell Tissue Res. 281(3):533–542. [DOI] [PubMed] [Google Scholar]
- Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. 2013. Autonomic nerve development contributes to prostate cancer progression. Science. 341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. 2003. Hormonal, cellular, and molecular control of prostatic development. Dev Biol. 253(2):165–174. [DOI] [PubMed] [Google Scholar]
- McVary KT, Razzaq A, Lee C, Venegas MF, Rademaker A, McKenna KE. 1994. Growth of the rat prostate gland is facilitated by the autonomic nervous system. Biol Reprod. 51(1):99–107. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, MacGlashan DM, Braun A, Undem BJ. 2008. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol. 586(Pt 6):1595–1604. doi: 10.1113/jphysiol.2007.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson TM, Ricke WA. 2011. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 82(4–5):184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DSM, Dzinic S, Bonfil AI, Saliganan AD, Sheng S, Bonfil RD. 2016. The mouse prostate: a basic anatomical and histological guideline. Bosn J Basic Med Sci. 16(1):8–13. doi: 10.17305/bjbms.2016.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owman C, Sjöstrand NO. 1965. Short adrenergic neurons and catecholamine-containing cells in vas deferens and accessory male genital glands of different mammals. Zeitschrift für Zellforschung. 66(2):300–320. doi: 10.1007/BF00344342. [DOI] [PubMed] [Google Scholar]
- Pennefather JN, Lau WA, Mitchelson F, Ventura S. 2000. The autonomic and sensory innervation of the smooth muscle of the prostate gland: a review of pharmacological and histological studies. J Auton Pharmacol. 20(4):193–206. [DOI] [PubMed] [Google Scholar]
- Pflug BR, Onoda M, Lynch JH, Djakiew D. 1992. Reduced Expression of the Low Affinity Nerve Growth Factor Receptor in Benign and Malignant Human Prostate Tissue and Loss of Expression in Four Human Metastatic Prostate Tumor Cell Lines. Cancer Res. 52(19):5403–5406. [PubMed] [Google Scholar]
- Pontari MA, Ruggieri MR. 2004. Mechanisms In Prostatitis/Chronic Pelvic Pain Syndrome. J Urol. 172(3):839–845. doi: 10.1097/01.ju.0000136002.76898.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS. 2014. Transient Receptor Potential Channels as Targets for Phytochemicals. ACS Chem Neurosci. 5(11):1117–1130. doi: 10.1021/cn500094a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz S, Zeigler M, Caine M. 1973. The Effect of Progesterone on the Adrenergic Receptors of the Urethra1. British Journal of Urology. 45(2):131–135. doi: 10.1111/j.1464-410X.1973.tb12129.x. [DOI] [PubMed] [Google Scholar]
- Sjöstrand NO. 1965. The adrenergic innervation of the vas deferens and the accessory male genital glands. Acta physiol scand. 257. [Google Scholar]
- Szczyrba J, Niesen A, Wagner M, Wandernoth PM, Aumüller G, Wennemuth G. 2017. Neuroendocrine Cells of the Prostate Derive from the Neural Crest. J Biol Chem. 292(5):2021–2031. doi: 10.1074/jbc.M116.755082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AA, Timms BG, Barton L, Cunha GR, Grace OC. 2002. The role of smooth muscle in regulating prostatic induction. Development. 129(8):1905–1912. [DOI] [PubMed] [Google Scholar]
- Toivanen R, Shen MM. 2017. Prostate organogenesis: tissue induction, hormonal regulation and cell type specification. Development. 144(8):1382–1398. doi: 10.1242/dev.148270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollet J, Everett AW, Sparrow MP. 2001. Spatial and temporal distribution of nerves, ganglia, and smooth muscle during the early pseudoglandular stage of fetal mouse lung development. Dev Dyn. 221(1):48–60. doi: 10.1002/dvdy.1124. [DOI] [PubMed] [Google Scholar]
- Umans BD, Liberles SD. 2018. August 21 Neural Sensing of Organ Volume. Trends Neurosci. doi: 10.1016/j.tins.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S, Pennefather J, Mitchelson F. 2002. Cholinergic innervation and function in the prostate gland. Pharmacol Ther. 94(1–2):93–112. [DOI] [PubMed] [Google Scholar]
- Vezina CM, Allgeier SH, Moore RW, Lin T-M, Bemis JC, Hardin HA, Gasiewicz TA, Peterson RE. 2008. Dioxin Causes Ventral Prostate Agenesis by Disrupting Dorsoventral Patterning in Developing Mouse Prostate. Toxicol Sci. 106(2):488–496. doi: 10.1093/toxsci/kfn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, McKenna KE, McVary KT, Lee C. 1991. Requirement of innervation for maintenance of structural and functional integrity in the rat prostate. Biol Reprod. 44(6):1171–1176. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Inoue M, Sasaki K, Araki M, Uehara S, Monden K, Saika T, Nasu Y, Kumon H, Chancellor MB. 2011. Nerve growth factor level in the prostatic fluid of patients with chronic prostatitis/chronic pelvic pain syndrome is correlated with symptom severity and response to treatment. BJU International. 108(2):248–251. doi: 10.1111/j.1464-410X.2010.09716.x. [DOI] [PubMed] [Google Scholar]
- White CW, Xie JH, Ventura S. 2013. Age-related changes in the innervation of the prostate gland. Organogenesis. 9(3):206–215. doi: 10.4161/org.24843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Keast JR. 2008. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 507(2):1169–1183. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- Zahalka AH, Arnal-Estapé A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, Frenette PS. 2017. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 358(6361):321–326. doi: 10.1126/science.aah5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Axon densities in P50 mouse prostate tissue sections were determined using the procedure described in Methods and Fig. 1 legend. Axon pixel densities were quantified in the 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results graphed. (a) CGRP+ axon density in the periurethral and proximal region is significantly greater than in the distal region. (b) VAChT+ axon density is uniform across the periurethral, proximal, and distal regions. (c) TH+ axon density is uniform across the periurethral, proximal, and distal regions. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (**p<0.01) between regions. RS identifies the rhabdosphincter. Scale bar is 50 μm
Axon densities in P50 mouse prostate tissue sections were determined using the procedure described in Methods and Fig. 1 legend. Axon pixel densities were quantified in the 10 μm periductal spaces radiating outward from the basal surface of prostatic epithelium and results graphed. (a) The CGRP+ axon density in the periurethral and proximal region is significantly greater than in the distal region. (b) VAChT+ axon density is uniform across the periurethral, proximal and distal regions. (c) TH+ axon density is uniform across the periurethral, proximal and distal regions. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (*p<0.05, and **p<0.01) between regions
E17.5, P9, and P50 mouse prostate tissue sections were immunostained with antibodies against TH+, VAChT+, and CGRP+ (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red). Axon pixel densities were quantified in the 10 μM periductal spaces radiating outward from the basilar surface of prostatic epithelium and results are shown pictorially on the right. Yellow arrowheads identify varicose axons. Results are mean ± SE of seven mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (*p<0.05, and **p<0.01) between regions. Scale bar is 50 μm
P50 mouse prostate tissue sections were immunostained with antibodies against TH+, VAChT+, or CGRP+ (green) and e-cadherin (CDH1, to visualize prostatic and urethral epithelium, red). Regions selected for analysis are schematized at the top of the figure and representative images are shown at the bottom left. Axon pixel densities were quantified in the 10 μM periductal spaces radiating outward from the basal surface of prostatic epithelium and results are shown pictorially at the right. Results are mean ± SE of ten mice per group and three non-adjacent tissue sections per mouse. Asterisks indicate significant differences (**p<0.01) between regions. Scale bar is 50 μm
List of primary antibodies used in this study. The antibody target, vendor and catelog number, RRID number, host species, dilution, and proof of specificity is listed.


