Abstract
Dengue is a mosquito-borne disease of global public health importance caused by four genetically and serologically related viruses (DENV-1 to DENV-4). Efforts to develop effective vaccines and therapeutics for dengue have been slowed by the paucity of preclinical models that mimic human disease. DENV-2 models in interferon receptor deficient AG129 mice were an important advance but only allowed testing against a single DENV serotype. We have developed complementary AG129 mouse models of severe disseminated dengue infection using strains of the other three DENV serotypes. Here we used the adenosine nucleoside inhibitor NITD-008 to show that these models provide the ability to perform comparative preclinical efficacy testing of candidate antivirals in vivo against the full-spectrum of DENV serotypes. Although NITD-008 was effective in modulating disease caused by all DENV serotypes, the variability in protection among DENV serotypes was greater than expected from differences in activity in in vitro testing studies emphasizing the need to undertake spectrum of activity testing to help in prioritization of candidate compounds for further development.
Keywords: Dengue virus, flavivirus, animal model, antiviral testing, preclinical development
1. Introduction
Dengue is a globally important mosquito-borne disease caused by four serologically and genetically related flaviviruses (DENV-1 to DENV-4). The disease is of global importance, with approximately 3 billion people living in endemic countries and an estimated 390 million human infections annually that result in approximately 90 million cases of clinical disease (1–3). Disease severity can vary considerably. The most common presentation is a flu-like illness lasting several days with symptoms that can include fever, headache, nausea and vomiting, rash, joint and muscle pain. In a minority of patients, the disease progresses to more severe forms including dengue hemorrhagic fever and dengue shock syndrome, both of which can be life-threatening. Thus, there is a clear need for effective prevention and treatment options for this disease.
A live attenuated tetravalent DENV vaccine has recently been licensed in 19 countries following extensive clinical trials in dengue endemic areas (4, 5). However, the efficacy of the vaccine against disease caused by DENV-1 and DENV-2 and, in participants who were DENV-naïve at study entry was relatively modest, and due to safety concerns in younger children, it is recommended that minimum age for vaccination is nine years old. Further, the World Health Organization (WHO) has recommended that the vaccine be used only in countries where dengue is highly endemic (6). Although this vaccine should substantially reduce the clinical burden of dengue over time, further vaccine development efforts are clearly warranted. In addition, treatment of clinical dengue continues to center on supportive care as there are no licensed antiviral drugs (7). In fact, only five compounds have been evaluated for treatment of dengue in clinical trials with four of these (chloroquine, lovastatin, prednisolone and celgosovir) being classified as non-specific inhibitors rather than DENV specific antivirals. None of these compounds showed efficacy (8). Consequently, there is also an urgent need for effective small molecule therapeutics. Unfortunately, progress has been slowed in part by a paucity of small animal models that show pathologic features similar to those seen in human disease that can be used for early stage preclinical efficacy testing. This situation is changing due to sustained model development efforts and so there is now a much greater range of models available (9, 10). However, the majority of these employ challenge virus of only one DENV serotype. One of the models that has been used extensively, particularly in early stage studies, is the AG129 (interferon α/β and γ receptor deficient) mouse (11–14). It was originally shown to be susceptible to lethal neurologic infection following challenge with DENV-2 strain New Guinea C (15) and then its clinical relevance increased with the development of DENV-2 strains such as D2S10, D2Y98P and others able to cause lethal disseminated disease (16–18). To further extend the utility of the model, we have developed AG129 mouse models of DENV-3 (19), DENV-4 (20, 21) and more recently DENV-1 (22) disease that use non-mouse-adapted DENV strains to produce a rapid, disseminated disease with high lethality. Comparative studies in these models should be of value in understanding similarities and differences in the pathologic basis of the disease caused by different DENV serotypes. In addition, for the first time they allow comparative efficacy testing for candidate antivirals against all four DENV serotypes in vivo. To demonstrate proof-of-concept of their utility in this role we compared the efficacy of the adenosine nucleoside inhibitor NITD-008 (13) against DENV infection using all four DENV serotypes.
2. Methods and materials
2.1. Antiviral Compound
NITD-008 was provided by the National Institute for Allergy and Infectious Disease (NIAID) and formulated daily in vehicle as described previously for animal studies (13).
2.2. Cells and viruses
Vero and C6/36 cells were obtained from the American Type Culture Collection (Manassas, VA) and maintained as described previously (19).
DENV-1 strain Western Pacific 74 (WP 74) was isolated from a human dengue case on the island of Nauru in 1974 (23). DENV-2 strain D2S10 was a kind gift from Eva Harris (University of California, Berkley); DENV-2 strain NGC and DENV-4 strain TVP-376 were kindly provided by Robert Putnak (Walter Reed Army Institute of Research). DENV-3 strain C0360/94 and DENV-4 strain 703–4 are low passage Thai human isolates that have been described recently (19, 20). For these studies, stock cultures of all viruses were prepared in C6/36 cells supplemented with 2% FBS and quantified by plaque (DENV-1, DENV-2 and DENV-4) or focus (DENV-3) formation assays in Vero cells using standard methods (19, 20).
2.3. Yield reduction assays
In vitro antiviral activity was assessed by yield reduction assay. Briefly, Vero cells were seeded in 12-well plates, and monolayers were inoculated with DENV strains (M.O.I of 0.1) for 30 minutes at ambient temperature, then washed with PBS, and NITD-008 (final concentration of 0.2, 0.5, 1.0, 2.0, 5.0 or 10.0 μM) added to wells in triplicate. Plates were incubated for 48 hours at 37°C, and the supernatants were harvested and virus titer assayed as described above. To determine the percentage infectivity, individual titers of NITD-008 treated samples were normalized relative to the titer of control samples (not treated with NITD-008). Non-linear regression was applied using log(inhibitor) vs normalized response function in GraphPad Prism 7.0 for PC to determine the concentration of NITD-008 required to reduce viral titers by 50% (IC50).
2.4. In vivo antiviral efficacy testing in AG129 mice
All animal procedures were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee and studies were carried out in strict compliance with the recommendations of the Guide for the Care and Use of Laboratory Animals (National Research Council).
AG129 mice were bred and maintained at UTMB. Groups of 6–8 week-old animals (n=12) were inoculated by intraperitoneal (i.p.) injection with 7.0 log10 plaque or focus forming units of respective DENVs, or served as naïve controls (N=6). Half of the animals in each group were treated by oral gavage with NITD-008 (20mg/kg in 0.1ml) twice daily for 4 days beginning immediately after virus inoculation; remaining animals were treated with an equal volume of vehicle using the same regimen. On days 2 and 3 post inoculation (p.i.) blood was collected from 3 mice/group by retro-orbital bleed to quantitate viremia. Animals were weighed daily and monitored for morbidity for 28 days. Animals with severe disease or weight loss of >20% of initial body weight were euthanized and recorded as being dead on the following day for statistical analysis. Two independent studies were undertaken for each DENV and the results combined for analysis.
2.5. Virus quantitation by RT-PCR
RNA was extracted from serum samples and the level of viremia determined by RT-PCR using our previously described methods (24). Results are presented as log10 genome equivalents per ml serum (log10 GE/ml).
2.6. Statistics
Incidence data were compared by Fisher’s exact test. Student’s t-test was used for comparisons involving group mean values. All p values are two-tailed with values <0.05 being considered significant. Statistical analysis was performed using GraphPad Prism 6.0 for PC.
3. Results
3.1. In vitro activity of NITD-008 against DENV-1, DENV-2, DENV-3 and DENV-4
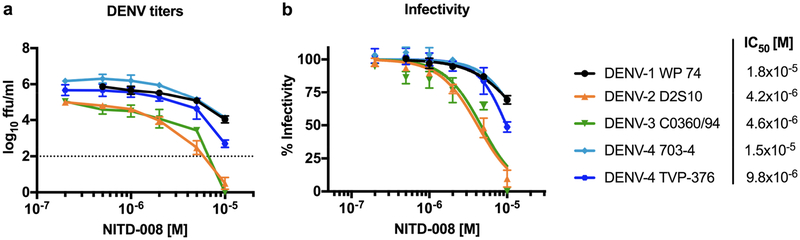
We initially evaluated the activity of NITD-008 in vitro in yield reduction assays using DENV strains representing all four DENV serotypes that were to be used in the in vivo efficacy testing studies. The results showed that only the highest concentration tested, 1×10−5 M, completely inhibited DENV-2 D2S10 and DENV-3 C0360/94 infectivity (Fig 1a). However, DENV-1 WP74 and both DENV-4 strains 703–4 and TVP-376 were only partially inhibited (50–75% infectivity) by NITD-008 (Fig 1b). Further the IC50 of NITD-008 against each DENV was calculated from the inhibition curve as follows: DENV-1 WP74: 1.8×10−5, DENV-2 D2S10: 4.2×10−6, DENV-3 C0360/94: 4.6×10−6, DENV-4 703–4: 1.5×10−5, and DENV-4 TVP-376: 9.8×10−6 M showing that NITD-008 is most active against DENV-2 and DENV-3 in vitro.
Figure 1. In vitro activity of NITD-008 against DENV.
Activity was measured by yield reduction assay using DENV-1, −2, −3, and −4 strains in Vero cells. (A) Mean viral titers of each virus after NITD-008 treatment are shown as ffu/ml; DENV-1, −3, and −4 n=3, DENV-2: n=9; dashed line indicates limit of detection. (B) Titers were normalized to show mean infectivity percentage of each strain. Nonlinear regression inhibition curves were used to determine the IC50.
3.2. Impact of NITD-008 on outcome of dengue disease in AG129 mice
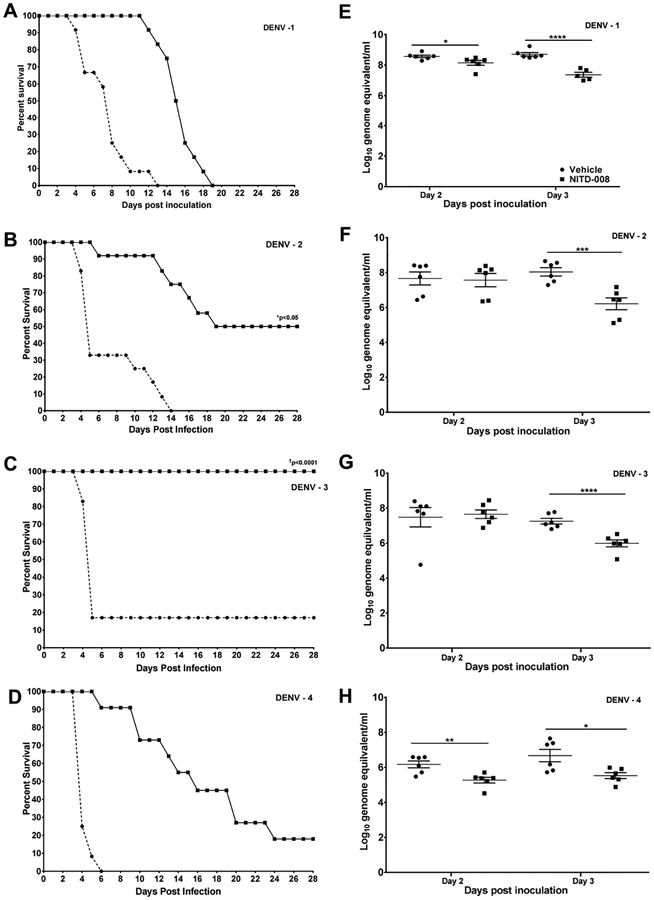
In in vivo testing studies, AG129 mice inoculated with DENV-1 strain WP 74, DENV-2 strain D2S10, DENV-3 strain C0360/94, or DENV-4 strain 703–4 and treated with vehicle developed rapid, progressive disease with high lethality (Figure 2A–D). In animals treated with NITD-008 the course of disease was altered for all four DENV serotypes, although the impact of treatment varied (Figure 2A–D). In DENV-3 inoculated mice, NITD-008 treatment prevented lethality in all animals compared to 83% lethality in vehicle controls (p<0.0001; Table 1). NITD-008 treatment of DENV-2 infected mice did not provide complete protection from lethality, but did significantly increase the number of animals that survived to the end of the study from 0% to 50% (p=0.014), and in mice that developed lethal infection the mean day of death (MDD) was significantly extended compared vehicle treated controls (14.2 ± 1.9 vs 7.0 ±1.0 days; p= 0.002; Table 1). In contrast, NITD-008 treatment failed to significantly increase survival in mice infected with either DENV-1 or DENV-4 compared to controls; although, for both viruses the MDD was significantly extended in the treated animals (14.7 ± 2.0 vs 4.3 ± 0.2 days DENV-4; 14.7 ± 3.6 vs 7.5 ± 2.5 days DENV-1 each p<0.0001; Table 1). Control animals not inoculated with virus but treated with NITD-008 using the same dosing regimen remained healthy through the observation period (data not shown).
Figure 2: Impact of NITD-008 on Dengue caused by the four virus serotypes in AG129 mice.
Survival curves and viremia levels in 6–8 week old AG129 mice inoculated with 7.0 log10 pfu DENV-1 WP 74 (A and E), DENV-2 D2S10 (B and F), DENV-3 C0360/94 (C and G) or DENV-4 703–4 (D and H) and treated twice daily for 4 days with NITD-008 (20mg/kg in 0.1ml) or vehicle by oral gavage beginning immediately after challenge. Survival was significantly increased in DENV-2 and DENV-3 challenged animals (*p<0.05 and p<0.0001 respectively; Fischer’s exact test). Viremia was measured in half of the animals from each group on day 2 p.i. and the remaining animals on day 3 p.i. and quantitated by RT-PCR. *p<0.05, **p<0.01, ***p<0.005 and ****p<0.001 (Student’s t-test compared to vehicle controls).
Table 1.
Efficacy of NITD-008 against dengue virus infection in AG129 mice
| Group | Virus | Dead (%) |
p value | Mean Day of Death (± SD)a |
p value | Survivors with morbidity (%)b |
Total with Signs (%) |
|---|---|---|---|---|---|---|---|
| NITD-008 | DENV 1 WP 74 |
12/12 (100) |
NS | 14.7 ± 3.6 | <0.0001 | - | 12/12 (100) |
| Vehicle | DENV 1 WP 74 |
12/12 (100) |
7.5 ± 2.5 | - | - | 12/12 (100) |
|
| NITD-008 | DENV 2 D2S10 |
6/12 (50) |
0.014 | 14.2 ± 1.9 | 0.002 | 2/6 (33) |
8/12 (67) |
| Vehicle | DENV 2 D2S10 |
12/12 (100) |
7.0 ± 1.0 | - | - | 12/12 (100) |
|
| NITD-008 | DENV 3 C0360/94 |
0/12 (0) |
<0.0001 | - | - | 10/12 (83) |
10/12 (83) |
| Vehicle | DENV 3 C0360/94 |
10/12 (83) |
4.8 ± 0.4 | - | 1/2 (50) |
11/12 (92) |
|
| NITD-008 | DENV 4 703–4 |
9/11 (82) |
NS | 14.7 ± 2.0 | <0.0001 | 2/2 (100) |
11/11 (100) |
| Vehicle | DENV 4 703–4 |
12/12 (100) |
4.3 ± 0.2 | - | - | 12/12 (100) |
|
| NITD-008 | DENV 4 TVP-376 |
5/6 (83) |
NS | 16.8 ± 3.4 | <0.01 | 1/1 (100) |
6/6 (100) |
| Vehicle | DENV 4 TVP-376 |
6/6 (100) |
4.2 ± 0.4 | - | - | 6/6 (100) |
Calculated using only animals that died
morbidity defined as weight loss exceeding 10% of weight at challenge
3.3. Impact of NITD-008 treatment on viremia in DENV infected AG129 mice
Vehicle-treated control mice infected with all strains of DENV experienced high levels of viremia in serum on both days 2 and 3 p.i. as measured by RT-PCR (Figure 2E–H). Previous studies have established that for these DENV strains there is an approximately 10:1 ratio for genome copies to infectious virus particles. NITD-008 treatment did not significantly reduce the number of animals with detectable viral RNA for any of the DENV strains tested on either day. Further, viral RNA levels in drug-treated DENV-2 and DENV-3 infected animals were comparable to those in controls on day 2 p.i., but were significantly lower on day 3 p.i. (6.2 ± 0.8 vs 8.0 ± 0.6 log10 GE/ml, p<0.005 DENV-2; 6.0 ± 0.5 vs 7.3 ± 0.4 log10 GE/ml, p<0.001 DENV-3). Interestingly, viral RNA levels in NITD-008 treated DENV-1 strain WP 74 and DENV-4 strain 703–4 treated mice were significantly lower than controls on both days 2 (8.1 ± 0.4 vs 8.6 ± 0.2 log10 GE/ml p<0.05 DENV-1; 5.2 ± 0.4 vs 6.1 ± 0.5 log10 GE/ml p<0.01 DENV-4,) and 3 p.i (7.4 ± 0.4 vs 8.7 ± 0.3 log10 GE/ml p<0.0001 DENV-1; 5.5 ± 0.4 vs 6.7 ± 0.9 log10 GE/ml p<0.05 DENV-4), although NITD-008 treatment failed to significantly increase survival in mice infected with either of these viruses.
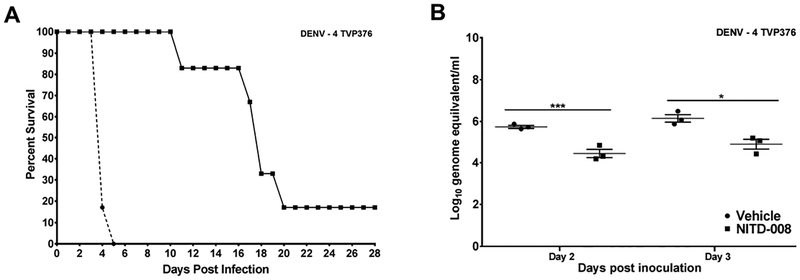
3.4. Evaluation of NITD-008 against DENV-4 TVP-376 infection in AG129 mice
The availability of a second DENV-4 strain, TVP-376, able to produce disseminated, lethal disease in AG129 mice (21) allowed us to evaluate further whether the limited efficacy of NITD-008 against DENV-4 703–4 infection was strain specific or represented a more broadly applicable reduced in vivo activity against DENV serotype 4 viruses. In studies using the same antiviral treatment regimen used previously, results with DENV-4 strain TVP-376 closely paralleled those observed with DENV-4 strain 703–4. NITD-008 treatment failed to significantly increase survival (Figure 3) but did significantly extend the MDD in animals that experienced lethal infection (16.8 ± 3.4 vs 4.2 ± 0.2 days p<0.01; Table 1). Further, the viremia in treated animals was significantly reduced on both days 2 (4.4 ± 0.3 vs 5.7 ± 0.1 log10 GE/ml p<0.005) and 3 p.i. (4.9 ± 0.4 vs 6.1 ± 0.4 log10 GE/ml p<0.05) (Figure 3).
Figure 3: Impact of NITD-008 on Dengue caused by DENV-4 strain TVP376 in AG129 mice.
Survival curve (A) and viremia levels (B) in 6–8 week old AG129 mice inoculated with 7.0 log10 pfu DENV-4 TVP 376 and treated twice daily for 4 days with NITD-008 (20mg/kg in 0.1ml) or vehicle by oral gavage beginning immediately after virus inoculation. Animals were monitored for 4 weeks. Viremia measured in half of the animals from each group on day 2 p.i. and the remaining animals on day 3 p.i. and quantitated by RT-PCR. *p<0.05, and ***p<0.005 (Student’s t-test compared to vehicle controls).
4. Discussion
We have described AG129 mouse models of disseminated infection with high lethality caused by DENV-1, DENV-3 and DENV-4 strains that complement the established DENV-2 strain D2S10 model(19–22). These models are of significance for basic dengue research and particularly for preclinical therapeutic and vaccine testing, because they enable comparative in vivo efficacy testing against all four DENV serotypes. To demonstrate their value in antiviral testing, we compared the effect of treatment with the adenosine nucleoside inhibitor NITD-008 against DENV infection in AG129 mice using all four DENV serotypes. NITD-008 was developed at the Novartis Institute for Tropical Diseases in Singapore. The compound showed in vitro activity against DENV strains representing all four DENV serotypes. Further, in in vivo studies AG129 mice were completely protected from lethality due to DENV-2 D2S10 when animals were treated with doses of ≥10mg/kg twice daily for 3 days beginning on the day of infection (13). Unexpected side-effects observed in extended treatment studies prevented the compound’s development as a clinical therapeutic (25). However, despite the lack of clinical potential, the compound was an ideal candidate for these proof-of-concept studies and was available from NIAID through their program on Preclinical Services for the Development of Interventional Agents for Infectious Diseases.
In initial studies, we examined the antiviral activity of NITD-008 in vitro in yield reduction assays with DENV strains representing all four serotypes and including the strains used in the in vivo studies. We found that the compound was active against all four serotypes with IC50 values that ranged from 4–18 μM. These values are higher than those reported by Yin et al. (13), which is unsurprising given differences in the virus strains, cell lines and assays used. While we did not find substantial differences in activity among the DENV serotypes, our results indicated that the compound had the greatest in vitro activity against DENV-2 and DENV-3.
For in vivo studies, we used an NITD-008 dose (20mg/kg) and treatment schedule that we anticipated would provide complete protection against lethality in AG129 mice challenged with DENV-2 strain D2S10 based on previous reports (13). We found that this regimen provided varying protection against the different DENV serotypes. Initially, this was observed in the impact on viremia levels. Both DENV-2 and DENV-3-infected animals experienced high titer viremia that was significantly reduced by NITD-008 treatment on day 3, but not day 2 p.i. In contrast, in animals infected with DENV-1 or either of the two DENV-4 strains NITD-008 treatment significantly reduced viremia levels on both day 2 and day 3 p.i. As the infection progressed, NITD-008 treatment provided complete protection against lethality in DENV-3 infected animals and significantly reduced lethality in DENV-2 infected animals. Surprisingly, given the impact on viremia for DENV-1 and both DENV-4 strains, NITD-008 treatment failed to significantly reduce lethality in DENV-4-infected mice although for both serotypes the time to lethality was significantly extend compared to that in controls.
The failure of NITD-008 to provide complete protection against lethality due to DENV-2 D2S10 infection in our studies was unexpected as Yin et al. (13) saw complete protection in their studies using similar treatment conditions. One major difference between the studies was that in the previously published studies, animals were only evaluated until 11 days p.i. compared to 28 days here. In our studies 5/6 DENV-2 D2S10-infected NITD-008 treated animals died after day 11 p.i. This suggests that the present studies are consistent with the previous results and that NITD-008 treatment reduced virus replication sufficiently to extend the MDD but did not completely clear the virus so that once the treatment regimen was completed replication rebounded leading to disease resurgence and death. Results in NITD-008 treated mice infected with DENV-1 or either of the DENV-4 strains were similar, with most treated animals dying after day 11 p.i. It is possible that extending the period of drug treatment beyond day 3 p.i could improve protection and prevent lethality with all DENVs but this is beyond the proof-of-principle studies reported here. Importantly, these results strongly support the use of an observation period of at least 21–28 days for antiviral studies to provide information about possible disease rebound after the antiviral treatment has finished. We recognize that in a clinical setting, the re-emergence of signs of disease would trigger additional treatment, but in preclinical evaluation studies, the ability to prevent rebound provides an additional criterion for comparative antiviral activity evaluations that could be important in helping to prioritize candidate compounds for further development.
In summary, these studies show that newly available DENV-1, DENV-3, and DENV-4 models of severe dengue in AG129 mice provide an important resource for preclinical evaluation of experimental therapeutics to treat dengue. Our results demonstrate the feasibility of spectrum-of-activity testing in vivo using viruses of multiple DENV serotypes. This is another important step forward in early stage preclinical testing that will help to identify the best candidate antiviral agents reducing both development costs and the time to licensure of urgently needed therapeutics for this disease.
Development of AG129 mouse models of severe dengue for all four virus serotypes makes spectrum-of-activity testing possible.
Studies with a test antiviral showed differences in efficacy between serotypes in vivo not anticipated from in vitro activity.
These models have the potential to accelerate the development of new antiviral agents for dengue.
Acknowledgements
This study was supported in part by funds from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Health, Department of Health and Human Services under contracts N01 AI 30065 and HHSN272201000401 HHSN27200009 A71. V.V.S. was supported in part by a NIAID T32 postdoctoral fellowship AI 07536.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.WHO. Dengue: guidelines for diagnosis, treatment, prevention, and control. New ed. Geneva: TDR: World Health Organization; 2009. 147 p. p. [PubMed] [Google Scholar]
- 2.Simmons CP, Farrar JJ, Nguyen v V, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–32. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med. 2015;373(13):1195–206. [DOI] [PubMed] [Google Scholar]
- 5.Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372(2):113–23. [DOI] [PubMed] [Google Scholar]
- 6.Dengue vaccine: WHO position paper - July 2016. Wkly Epidemiol Rec. 2016;91(30):349–64. [PubMed] [Google Scholar]
- 7.Simmons CP, McPherson K, Van Vinh Chau N, Hoai Tam DT, Young P, Mackenzie J, et al. Recent advances in dengue pathogenesis and clinical management. Vaccine. 2015;33(50):7061–8. [DOI] [PubMed] [Google Scholar]
- 8.Kaptein SJ, Neyts J. Towards antiviral therapies for treating dengue virus infections. Curr Opin Pharmacol. 2016;30:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Chan KW, Watanabe S, Kavishna R, Alonso S, Vasudevan SG. Animal models for studying dengue pathogenesis and therapy. Antiviral Res. 2015;123:5–14. [DOI] [PubMed] [Google Scholar]
- 10.Sarathy VV, Milligan GN, Bourne N, Barrett AD. Mouse models of dengue virus infection for vaccine testing. Vaccine. 2015;33(50):7051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang J, Schul W, Butters TD, Yip A, Liu B, Goh A, et al. Combination of alpha-glucosidase inhibitor and ribavirin for the treatment of dengue virus infection in vitro and in vivo. Antiviral research. 2011;89(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe S, Rathore AP, Sung C, Lu F, Khoo YM, Connolly J, et al. Dose- and schedule-dependent protective efficacy of celgosivir in a lethal mouse model for dengue virus infection informs dosing regimen for a proof of concept clinical trial. Antiviral Res. 2012;96(1):32–5. [DOI] [PubMed] [Google Scholar]
- 13.Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, et al. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A. 2009;106(48):20435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Yip A, Seah PG, Blasco F, Shi PY, Herve M. Modulation of inflammation and pathology during dengue virus infection by p38 MAPK inhibitor SB203580. Antiviral research. 2014;110C:151–7. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73(1):783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. Journal of virology. 2006;80(20):10208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zellweger RM, Prestwood TR, Shresta S. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe. 2010;7(2):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan GK, Ng JK, Trasti SL, Schul W, Yip G, Alonso S. A non mouse-adapted dengue virus strain as a new model of severe dengue infection in AG129 mice. PLoS Negl Trop Dis. 2010;4(4):e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarathy VV, White M, Li L, Gorder SR, Pyles RB, Campbell GA, et al. A lethal murine infection model for dengue virus 3 in AG129 mice deficient in type I and II interferon receptors leads to systemic disease. J Virol. 2015;89(2):1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milligan GN, Sarathy VV, Infante E, Li L, Campbell GA, Beatty PR, et al. A Dengue Virus Type 4 Model of Disseminated Lethal Infection in AG129 Mice. PLoS One. 2015;10(5):e0125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarathy VV, Infante E, Li L, Campbell GA, Wang T, Paessler S, et al. Characterization of lethal dengue virus type 4 (DENV-4) TVP-376 infection in mice lacking both IFN-alpha/beta and IFN-gamma receptors (AG129) and comparison with the DENV-2 AG129 mouse model. J Gen Virol. 2015;96(10):3035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milligan GN, Sarathy VV, White MM, Greenberg MB, Campbell GA, Pyles RB, et al. A lethal model of disseminated dengue virus type 1 infection in AG129 mice. J Gen Virol. 2017;98(10):2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Repik PM, Dalrymple JM, Brandt WE, McCown JM, Russell PK. RNA fingerprinting as a method for distinguishing dengue 1 virus strains. Am J Trop Med Hyg. 1983;32(3):577–89. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs J, Chu H, O’Day P, Pyles R, Bourne N, Das SC, et al. Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, et al. Ten years of dengue drug discovery: progress and prospects. Antiviral research. 2013;100(2):500–19. [DOI] [PubMed] [Google Scholar]