Abstract
Premise of the study:
Ecological differentiation (ED) between sexual and asexual organisms may permit the maintenance of reproductive polymorphism. Several studies of sexual/asexual ED in plants have shown that the geographic ranges of asexuals extend beyond those of sexuals, often in areas of higher latitude or elevation. But very little is known about ED at fine scales, wherein coexistence of sexuals and asexuals may be permitted by differential niche occupation.
Methods:
We used 149 populations of sexual and apomictic lineages in the genus Boechera (rock cress) collected across a portion of this mustard’s vast range. We characterized reproductive mode, ploidy, and species identity or hybrid parentage of each individual, and then used a multi-pronged statistical approach to 1) identify ED between sexuals and asexuals; 2) investigate the impacts of two confounding factors, polyploidy and hybridization, on ED; and 3) determine the environmental variables underlying ED.
Key results:
We found that sexuals and asexuals are significantly ecologically differentiated across the landscape, despite fine-scale interdigitation of these two reproductive forms. Asexual reproduction was strongly associated with greater disturbance, reduced slope, and greater environmental variability. Although ploidy had little effect on the patterns observed, hybridization has a unique impact on the relationships between asexual reproduction and specific environmental variables.
Conclusions:
Ecological differentiation along the axes of disturbance, slope, and climatic variability, as well as the effects of heterozygosity, may contribute to the maintenance of sexuality and asexuality across the landscape, ultimately impacting the establishment and spread of asexual lineages.
Keywords: apomixis, asexual reproduction, Boechera, Brassicaceae, ecological differentiation, environmental variability, geographic parthenogenesis, hybridization
INTRODUCTION
Classic evolutionary theory predicts that, in species with polymorphic reproductive systems, sexual and asexual reproduction cannot coexist stably (Maynard Smith, 1971; Marshall and Brown, 1981). Upon initiation of asexual reproduction, holding all else constant, the two-fold cost of sex enables asexual organisms to produce twice as many offspring as sexuals, leading ultimately to extinction of the latter (Maynard Smith, 1978). However, empirical research has revealed numerous systems in which sexuals and asexuals coexist, including animals (Vrijenhoek and Lerman, 1982; Case, 1990; Halkett et al., 2005; Lehto and Haag, 2010; Neiman et al., 2011), fungi (Saleh et al., 2012; Castel et al., 2014), and plants (Meirmans et al., 2003; Krahulcová et al., 2009). Additionally, multiple theoretical models predict conditions that permit persistence of sexuals in the face of asexual reproductive assurance, which enables rapid asexual population expansion following invasion of a sexual population. These conditions include population structure, which increases the time necessary for asexual fixation across subdivided populations (Hartfield et al., 2012), and the Hill-Robertson effect, where associations between deleterious loci result in the increase of modifiers associated with recombination (Peck, 1993; Hartfield et al., 2010).
Coexistence may also be maintained via ecological differentiation (“ED”) between reproductive strategies, where sexual and asexual organisms occupy different niches (Case and Taper, 1986; Rispe and Pierre, 1998; Burt, 2000; Doncaster et al., 2000; Agrawal, 2009). In animal systems, sexual/asexual reproductive variation is associated with environmental factors such as temperature (Dedryver et al., 2001), xeric conditions (Kearney, 2003), disturbance (Ben-Ami and Heller, 2007), and temporal dynamics (Halkett et al., 2005). For example, sexually-produced eggs may enable winter survival in aphids and Daphnia Müller (Rispe and Pierre, 1998). To understand which specific variables affect coexistence, however, these environments must be dissected further. In Daphnia, multiple winter environmental characteristics such as photoperiod, drought, and low food availability may influence the transition to sexual reproduction (see, e.g., Stross and Hill, 1965). Additionally, multiple plant-feeding insects specialize on different hosts (a form of environmental differentiation), supporting the coexistence of sexuals and asexuals. For example, sexual individuals of the aphid Rhopalosiphum padi L. use C3 Poaceae as summer hosts, transitioning to Prunus padus L. in the autumn, while asexual individuals use mostly C4 Poaceae year-round (Gilabert et al., 2014). Cumulatively, ED appears to play a key role in the maintenance of multiple forms of reproductive polymorphism in nature, and may permit the establishment of asexual lineages or populations by preventing competition with other lineages.
In plants, there are fewer studies of sexual-asexual ecological differentiation, and they have focused almost entirely on large-scale geographic patterns. At this scale, several key environmental characteristics have been shown to differentiate sexuals from apomicts (asexuals that reproduce via clonal seed). Apomictic Ranunculus kuepferi Greuter & Burdet commonly occurs in previously glaciated sites in the Alps, while sexual individuals are found only at lower latitudes, in areas that were unglaciated (Cosendai and Hörandl, 2010). Similarly, sexual Townsendia hookeri Beaman is restricted to sites beyond the boundaries of the Cordilleran and Laurentide ice sheets, while apomictic populations are found in both previously glaciated and unglaciated areas (Thompson and Whitton, 2006). Apomictic taxa have also invaded distant new habitats, suggesting an association between asexuality and disturbance or novel habitats (Amsellem et al., 2001; Chapman et al., 2004). These studies suggest that at broad scales, asexuality is promoted by glaciation or environmental characteristics associated with glaciation and novel habitats. But, to our knowledge, only one previous study has examined ED at a fine scale. Verduijn and colleagues (2004) focused on contrasting microenvironments between sexual and asexual members of a single population of dandelions, finding that the two forms occurred in different microhabitats.
The lack of focus on sexual/asexual dynamics at a fine geographic scale has hindered our understanding of the ecology of asexual formation and spread, a fundamental gap in the ecology of sex. Additionally, none of these studies have been able to identify the specific factors driving sexual-asexual ED, due to the confounding effects of polyploidy as well as fundamental differences in reproductive assurance; in each of the previously mentioned plant systems (Townsendia, Ranunculus, dandelion), asexuals are polyploid and by definition do not require mates, while sexuals are diploid and outcrossing, thus requiring mates. Thus our understanding of ecological factors driving sexual/asexual coexistence has been limited by a focus on broad geographic scales, and on taxa in which confounding factors may impact the observed patterns.
Apomixis is tightly linked not only with polyploidy but with hybridization. Apomictic lineages are often derived from hybridization events (Asker and Jerling, 1992). It is thus possible that sexual/asexual ED is due not to reproductive mode itself, but instead is driven by correlated factors. Numerous examples exist in the literature of ED between polyploids and diploids (Kirchheimer et al., 2016; Sonnleitner et al., 2016), and hybrids and their progenitors (Rieseberg et al., 2003).To our knowledge, no studies have examined ED among all combinations of asexuality and these associated traits. How might correlated factors influence our expectations of ED? Hybrid plants combine parental traits in novel ways, displaying traits that are unique to one parent, intermediate between parents, a combination of assorted characteristics of each parent, or entirely novel (Rieseberg and Ellstrand, 1993; Yakimowski and Rieseberg, 2014). Considering ecological niche as a trait, we may expect hybrid/progenitor ED to follow any of these predictions. Further, the increased heterozygosity associated with hybridization, which provides more genetic variability to hybrids, may permit expansion into new habitats, similar to theory proposed for polyploids (Stebbins, 1985).
Assuming that hybridization between sexual lineages results in the formation of apomicts, asexual and sexual lineages will briefly coexist immediately following the creation of new asexuals. The establishment of exclusively asexual populations requires that asexuals either outcompete sexuals or disperse outside of the site of origin, which may occur via differences in reproductive assurance, or dispersal or establishment potential. Indeed, the latter has been shown in Crataegus, where asexual fruits are larger than those produced by sexual trees, driving asexual range expansion (Coughlan et al., 2014). In mixed sexual/asexual populations where sexuals reproduce via outcrossing, asexual pollination of sexual ovules is predicted to result either in the spread of asexual alleles into formerly sexual genotypes, or the production of unfit hybrids, leading to asexual expansion and the loss of sexual lineages (Whitton et al., 2017). However, this is unlikely to be a factor in taxonomic groups with a high rate of self-fertilization. Alternatively, environmental associations with reproductive mode may permit asexual spread and establishment in niches that are unsuited to sexuals, as seen in invasive Rubus (Amsellem et al., 2001) and hawkweed (Chapman et al., 2004). Although any of these processes may occur individually or in combination with one another, which environmental variables are associated with the spread of asexual lineages beyond their site of origin remains unknown.
The genus Boechera Á. Löve & D. Löve (“rock cress,” formerly Arabis L.; Brassicaceae) offers an ideal system for studying the impacts of asexual reproduction free from confounding factors. Consisting of at least 70 sexual species, Boechera has long been considered taxonomically difficult due to shifts to apomixis, the presence of multiple ploidal levels, and frequent hybridization among taxa (Al-Shehbaz and Windham, 2010). The range of the genus includes much of North America, from northern Mexico to the Canadian territories, and east to Greenland (Al-Shehbaz and Windham, 2010). The genus occupies a wide range of ecological conditions (Rushworth et al., 2011), allowing study of ecological and evolutionary mechanisms across diverse native habitats. Although some major clades within western Boechera have been identified, phylogenetic relationships among these clades remain largely unresolved (Alexander et al., 2013), consistent with recent rapid divergence. Most of the sexual diploid species that have been genetically characterized are largely self-fertilizing (Roy, 1995; Schranz et al., 2005; Beck et al., 2012; Li et al., 2017). Nonetheless, some low level of outcrossing must occur, as interspecific hybridization events have resulted in hundreds of unique hybrid combinations (Li et al., 2017). Throughout the Boechera literature, these hybridization events are strongly associated with apomixis (Schranz et al., 2005; Beck et al., 2012; Li et al., 2017). These two traits have historically prevented reliable identification of most Boechera specimens; polyploidy is likewise prevalent in the genus, with triploidy the most common higher ploidy level (Al-Shehbaz and Windham, 2010).
Our focal species for this study is the highly selfing Boechera retrofracta Graham (Á. Löve & D. Löve), the widest-ranging species in the genus, which occurs in all Canadian provinces and territories west from Quebec, and in the United States is common from Utah to California, and north to Alaska. Across its range, B. retrofracta occupies mostly sagebrush and open forest habitats from 300 – 3300m in elevation, and includes sexual diploids, apomictic diploids, and apomictic triploids, while hybridizing with many other Boechera species (Al-Shehbaz and Windham, 2010).
Boechera is the only genus of flowering plants in which diploid apomicts are prevalent, providing a rare opportunity to disentangle asexuality from the effects of polyploidy (Beck et al., 2012). Additionally, the prevalence of self-fertilization in sexually reproducing Boechera species ensures that both sexual and asexual Boechera can reproduce without mates, removing the disparity in reproductive assurance often hypothesized to result in ED. Self-fertilization reduces heterozygosity by half with each generation, resulting in highly homozygous sexual genotypes, whereas the strong association between hybridization and apomixis in Boechera results in the apomicts having fixed heterozygous genotypes. The presence of non-hybrid asexual Boechera resulting from within-species outcrossing events further permits disentangling of the impacts of heterozygosity versus hybridization per se on sexual/asexual ED.
Here we study over 100 populations of sexual and asexual Boechera to characterize sexual/asexual ED, and ask if ED facilitates coexistence of these two forms of reproduction across populations. We first use a recently published multilocus genotyping database to identify the species or hybrid parentage of each individual with high accuracy. We find that Boechera retrofracta hybridizes extensively and outcrosses periodically, forming numerous inter- and intraspecific (i.e., hybrid and non-hybrid) apomictic lineages.We examine sexual-asexual ED among all individuals in the study area, and additionally control for polyploidy to address its unique impact on observed patterns. We next focus on sexual B. retrofracta, sexual members of the closely related Boechera stricta (Graham) Al-Shehbaz, their asexual hybrid, and asexual non-hybrid B. retrofracta, to disentangle the effects of hybridization on patterns of sexual/asexual ED. Lastly, we compare ecological characteristics of purely asexual vs. mixed sexual/asexual populations to pinpoint variables that may facilitate asexual spread across the landscape. This comprehensive approach allows us to determine the environmental characteristics that shape sexual/asexual coexistence across populations of Boechera, free from the confounding effects of ploidy, hybridization, and reproductive assurance, a question that has thus far gone unaddressed in plants.
MATERIALS AND METHODS
Sampling and growth procedures—
Seeds from 149 Boechera sites across central Idaho and western Montana were collected between 2005 and 2009 (Appendix S1; see the Supplemental Data with this article) and used for all subsequent analyses. Populations were defined as groups of individuals separated by at least 1km, and collections ranged in size from 1–32 maternal seed families per population (mean=6.85). Our focus was on Boechera retrofracta, which has been segregated from Boechera holboellii (Hornem.) Á. Löve & D. Löve, formerly known as Arabis holboellii Hornem. (Windham and Al-Shehbaz, 2006). We targeted individuals with morphological traits attributed to B. retrofracta and potential hybrids involving B. retrofracta and B. stricta, which is morphologically and genetically distinct from our focal species (Alexander et al., 2013). Seeds were grown on wet filter paper in Petri dishes until seed leaves emerged and then transferred to a soil mixture of Fafard 4P Mix and Sunshine MVP (Sun Gro Horticulture, Agawam, Massachusetts, USA) and grown in the Duke University Research Greenhouses. One individual was chosen at random to represent each seed family.
DNA extraction and PCR—
Multilocus genotypes for each individual were generated via DNA extraction and PCR. Leaf tissue was collected from a total of 1020 greenhouse-grown individuals. DNA extractions were performed on either fresh or silica-dried samples, by one of two methods: a KoAC protocol (Lee and Mitchell-Olds, 2011), or a CTAB protocol (Doyle and Dickson,1987) modified as described in Beck et al. (2012). Thirteen multiplexed fluorescent-labeled microsatellite markers (Clauss et al., 2002; Dobeš et al., 2004a; Song et al., 2006) were amplified using the protocols of Beck et al. (2012); one individual did not amplify well and was removed from further analyses. Loci were selected to maximize allelic diversity in multiple species across the genus, which permitted locus amplification in interspecific hybrids (Appendix S2). Reactions were run on an Applied Biosystems 3730xl DNA Analyzer (Applied Biosystems, Waltham, Massachusetts, USA) and analyzed using Genemarker (SoftGenetics, State College, Pennsylvania, USA).
Species identification—
Boechera species and hybrids were identified via comparison of allelic profiles to those in the Boechera Microsatellite Website (BMW), a 15-locus microsatellite database of >4000 herbarium specimens representing approximately 80 sexual diploid species and 400 unique hybrid parental combinations, considered asexual taxa (Li et al., 2017). BMW calculates an allelic similarity score for each multilocus genotype, corrected for missing data, and offers putative matches based on this score. Trials of known samples using BMW resulted in 96% correct identification to species (Li et al., 2017). Following BMW analysis, identifications were confirmed with morphological traits (Appendix S3). We used principal components analysis (PCA) of genetic data converted to Lynch distance (briefly, a measure of dissimilarity calculated as twice the number of bands two genotypes have in common, divided by the total number of bands in the two genotypes; Lynch, 1990) in the R package Polysat (Clark and Jasieniuk, 2011), to visually assess genetic intermediacy of putative crosses between B. stricta and B. retrofracta and within B. retrofracta.
Apomict and polyploid identification—
Several methods were used to determine ploidy level and reproductive mode in our collections. Ploidy level was first estimated using the maximum allele number across loci in an individual, as in Beck et al. (2012). For 132 genotypes, one or two leaves from mature plants were also analyzed for ploidy level with a Partec PAS flow cytometer (Partec, Munster, Germany) using an internal standard of Brassica rapa L. as described in Schranz et al. (2005). Hybridization among species of different genome sizes occasionally led to flow cytometry-based ploidy estimates that were inconsistent with inferences from maximum allele number. To confirm the accuracy of maximum allele number ploidy determinations, chromosome squashes were performed on 16 individuals (four putative triploid apomicts and 12 putative diploid apomicts; Appendix S4). In all cases, chromosome squashes confirmed the accuracy of maximum allele number inference over inferences based on flow cytometry (Appendix S5).
Because nearly all sexual species in Boechera are highly self-fertilizing, whereas apomixis is associated with outcrossing events, heterozygosity is often used to diagnose reproductive mode in the genus with high accuracy (Roy and Rieseberg, 1989; Sharbel and Mitchell-Olds, 2001; Schranz et al., 2006; Kantama et al., 2007; Beck et al., 2012; Li et al., 2017). To validate the application of a heterozygosity cut-off as in Li et al. (2017), we screened 7–10 offspring per each of 17 putatively apomictic lines (as determined by the heterozygosity cut-off), verifying that offspring were genetically identical to the parent (results not shown). Reproductive mode was thus inferred from observed heterozygosity in the remainder of our dataset: individuals heterozygous at ≥50% of loci were considered apomictic, and those heterozygous at <50% of loci were inferred to reproduce via sex. This threshold is consistent with the bimodal distribution of heterozygosity across the genus (Li et al., 2017).
Environmental variables—
Environmental variables were harvested from the WorldClim database (Hijmans et al., 2005) using the R package raster (Hijmans, 2013). BIO1–BIO11 are temperature-related; BIO12–BIO19 relate to precipitation. The scale of these data is 1km2, which may not entirely capture the conditions experienced by sessile organisms in environments with substantial topographic relief (Kirchheimer et al., 2016). Latitude, longitude, and three topographical variables (elevation, within-population slope, and distance to nearest road [a proxy for disturbance]) were extracted using Google Earth. Prior to harvesting environmental data, we merged populations that occurred within 4km of each other and at similar elevations, and removed populations with <4 individuals, reducing our dataset from 149 populations (N=1020) to 96 populations (N=955). Before statistical analyses, all variables except latitude and longitude were standardized to a mean of 0 and standard deviation of 1 to enable comparison on a common scale. Environmental variables are highly correlated with each other, which was accounted for either through principal components analysis (PCA) or through removal of highly correlated variables (see “Statistical analyses”).
Focal comparisons—
To elucidate the impacts of asexuality, polyploidy, and hybridization on ED, we subdivided the dataset into four focal comparisons, which we denote “FC1” through “FC4”. The statistical process described in the following section (“Statistical analyses”) was repeated for each FC, following removal of small populations, defined (as above) as those containing <4 individuals.
To identify variables broadly underlying sexual/asexual ED, FC1 contrasted all apomictic individuals (regardless of ploidy) vs. all sexuals (955 individuals, 96 populations). FC2 isolated the impacts of polyploidy on the pattern observed through two different methods: first, DAPC and MANCOVA compared sexual diploids, asexual diploids, and asexual triploids; second, for simplicity, we dropped the triploids and included only diploid sexuals and asexuals in our GLM (812 individuals, 73 populations). To identify the effects of hybridization, FC3 (592 individuals, 67 populations) contrasted non-hybrid B. retrofracta apomicts (“asexual Br”), hybrid B. retrofracta × B. stricta apomicts (“Br × Bs”), and the two sexual parental species (B. retrofracta—“Br”—and B. stricta—“Bs”). Only 10 polyploids were identified in the FC3 dataset, and these were not removed from statistical analyses. Finally, to identify variables that may underlie the initial establishment of asexual populations, FC4 examined ED between purely asexual vs. mixed sexual/asexual populations (750 individuals, 75 populations: 51 mixed sexual/asexual, 24 purely asexual).
Statistical analyses—
Summary statistics were calculated for the entire dataset, and a series of statistical analyses were subsequently implemented for each FC. Observed and expected heterozygosity and GIS (Nei, 1987), an analogue of the inbreeding coefficient FIS, were calculated for each population in GenoDive v2.0b27 (Meirmans and Van Tienderen, 2004, Appendix S1). GIS was only calculated for sexual accessions in populations with >1 sexual individual.
For each FC, the following statistical process was performed in R version 3.3.1 (R Core Team, 2016). We first conducted PCA to control for correlations among all standardized environmental and topographical variables, using the “DAPC” function (“Discriminant Analysis of Principal Components”) in the R package adegenet (Jombart, 2008). DAPC uses a combination of PCA and discriminant function analysis (“DFA”) to create synthetic variables that describe the greatest axes of differentiation between previously defined groups. These groups differed for each FC as follows: FC1, sexual and asexual individuals; FC2, sexual diploids, asexual diploids, and asexual triploids; FC3, each of the four focal taxa (asexual Br, asexual Br × Bs, sexual Br, and sexual Bs); FC4, mixed sexual/asexual populations vs. pure asexual populations. DFA was used to identify the axes describing ED between sexuals and asexuals, and determine which environmental variables contributed most to ED. To verify statistical significance of ED between groups, we used multivariate analysis of covariance (MANCOVA) to compare PCA values for PCs 1–5 as in Duncan et al. (2013), with population as a covariate. Predictor variables were as follows: for FC1, sex/asex; for FC2, diploid sexual vs. diploid asexual vs. triploid asexual; for FC3, Br vs. Bs vs Br × Bs vs asexual Br; and for FC4, mixed sexual/asexual vs. pure asexual. Univariate analyses of individual PCs were also conducted (not shown).
To determine the role of specific environmental variables contributing to ED, two approaches were used. First, loadings of each environmental variable to discriminant functions were identified in the DFA portion of DAPC. To investigate the possibility of additional important variables, we next implemented generalized linear models (GLMs) using the glm command in the R package lme4 (Bates et al., 2014) with population-level data. In FCs 1–3, sex vs. asex was the response variable; for FC4, the response was pure asexual vs. mixed sexual-asexual. Note that, for simplicity, these response variables differ from the categories used in other statistical analyses. We selected uncorrelated climate variables as predictors, identified using PCA loadings, to emphasize the greatest amount of variation in climate when possible. P-values were corrected for multiple comparisons when necessary, using a sequential Bonferroni correction (Holm, 1979).
By definition, correlations may vary among the FCs because the datasets and correlation structure differ. This necessitated the selection of relevant environmental variables for each GLM that minimized correlation. For each of the FCs, the following variables had correlation coefficients below 0.7 and were retained: FC1 and FC2—annual mean temperature (BIO1), annual temperature range (BIO7), total annual precipitation (BIO12), precipitation seasonality (BIO15), slope, elevation, and disturbance; FC3—annual mean temperature (BIO1), isothermality (BIO3), temperature seasonality (BIO4), precipitation seasonality (BIO15), slope, elevation, and disturbance; and FC4—isothermality (BIO3), annual temperature range (BIO7), total annual precipitation (BIO12), precipitation seasonality (BIO15), slope, and disturbance. Isothermality represents the range of annual temperatures in a given location on a given day; high values of isothermality correspond to a more temperate environment, while low values correspond to locations where seasonal variation is high.
RESULTS
Multiple sexual and apomictic taxa in a small geographic area—
Of our total of 1020 individuals, 983 could be confidently identified to species or hybrid combination; those that could not be definitively identified were excluded from subsequent analyses (Appendix S1). Four sexual species were present in the dataset (464 B. retrofracta individuals, 112 B. stricta, 22 Boechera pendulocarpa (A. Nelson) Windham & Al-Shehbaz, and six Boechera williamsii (Rollins) Dorn, its first report outside of Wyoming). We identified 16 well-supported hybrid species combinations representing various configurations of B. retrofracta, B. stricta, B. pendulocarpa, B. williamsii, Boechera sparsiflora (Nutt.) Dorn, Boechera fecunda (Rollins) Dorn, and Boechera exilis (A. Nelson) Dorn. (Appendix S6). In addition, a small number of hybrids were tentatively identified as involving Boechera puberula (Nutt.) Dorn, Boechera microphylla (Nutt.) Dorn, Boechera subpinnatifida (S. Watson) Al-Shehbaz, or Boechera paupercula (Greene) Windham & Al-Shehbaz (Appendix S1).
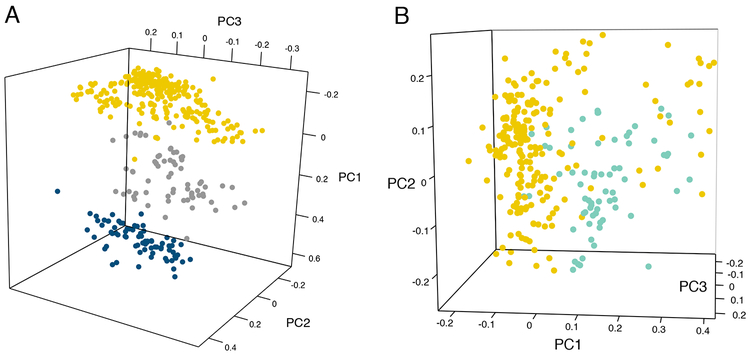
Average homozygosity of sexual B. retrofracta was 0.94 (i.e., 94% of loci are homozygous across our sample; range: 0.58–1.0), and average GIS for sexual B. retrofracta populations was 0.665, confirming that B. retrofracta is highly self-fertilizing like most sexual Boechera species (Beck et al., 2012). Boechera retrofracta morphology is also consistent with self-fertilization, as flowers are small and white, typical of the selfing syndrome (Al-Shehbaz and Windham, 2010). Highly heterozygous, within-species apomicts were present in B. retrofracta, B. pendulocarpa, and B. stricta. Examination of these individuals determined that they were morphologically consistent with their species designation. PCA visualizations of genetic data for 114 putative B. retrofracta × B. stricta hybrids and heterozygous asexual B. retrofracta accessions were likewise consistent with their hypothesized origins, indicated by genetic intermediacy between the putative parents (Fig. 1).
Fig. 1.
Principal components analysis of microsatellite data depicting genetic intermediacy of asexual Boechera between putative parental taxa. (A) PCA of B. stricta (dark blue), B. retrofracta (gold), and B. retrofracta × B. stricta hybrids (gray), which are both morphologically and genetically intermediate between the two parental species. The first three axes of variation explain 66.7% of the variation. (B) PCA of homozygous B. retrofracta (gold) and heterozygous asexual B. retrofracta (green). Heterozygous B. retrofracta are genetically intermediate between clusters of homozygous B. retrofracta, consistent with outcrossing between divergent lineages of B. retrofracta resulting in asexuality. The first three axes of variation explain 53.2% of the variation.
No evidence for geographic structure of reproductive mode—
Over half (575/1020; 56%) of the individuals sampled for this study reproduce asexually. Consistent with earlier studies, we find that apomictic individuals are most often diploid or triploid, with 300 diploid and 275 triploid asexuals sampled. Two putative tetraploids were removed from further analyses. Asexuals were widespread across the study area, occurring in 113 of 149 populations, and co-occurring with sexual reproduction in 55 populations (36.9% of all populations). By comparison, 38.9% (58/149) of populations were entirely asexual, while 24.1% (36/149) consisted of only sexual individuals (Table 1). A G-test showed that these ratios deviated from those expected by chance (G=6.04, d.f.=2, P=0.049), with pure asexual and mixed sexual/asexual populations overrepresented.
Table 1.
Summary of reproduction and polyploidy in populations contained in this study. Percent of total populations in parentheses.
| Reproduction | Individuals | Populations | Diploid populations | Triploid populations | Mixed ploidy populations |
|---|---|---|---|---|---|
| sexual | 444 | 36 (24.1) | 36 (48.6) | 0 (0) | 0 (0) |
| apomictic | 575 | 58 (38.9) | 21 (28.4) | 21 (36.2) | 16 (29.6) |
| mixed | - | 55 (36.9) | 17 (23.0) | 0 (0) | 38 (70.4) |
Sexuals and asexuals are ecologically differentiated—
PCA of environmental variables showed overlap among all groups in each of the analyses, with the first five principal components explaining >94% of the variation for each of the focal comparisons (data not shown). Nonetheless, sexuals and asexuals were ecologically differentiated (variable means summarized in Appendix S7 and PC means summarized in Appendix S8), and significantly so (MANCOVA for FC1, P<2.20e–16; all MANCOVA results summarized in Table 2). Comparing diploid sexuals, diploid asexuals, and triploid asexuals (FC2), MANCOVA again showed that the groups were ecologically differentiated (P<2.20e–16). Contrasts showed that sexuals differ from both diploid apomicts (P=6.60e–16) and triploid apomicts (P=6.60e–16), and that diploid and triploid apomicts differ from one another (P=7.82e–08). Sexual Br, sexual Bs, their hybrids, and non-hybrid Br (FC3), also differ in ecological preference (P<2.20e–16). Contrasts showed that the two sexual species differ significantly (P=1.10e–15), and both apomictic groups differed from sexual Br (hybrid, P=1.10e–15; non-hybrid, P=1.10e–15) as well as from each other (P=1.12e–09). Mixed sexual/asexual populations were also significantly ecologically differentiated from pure asexual populations (P<2e–16).
Table 2.
Results from MANCOVA showing significant ecological differentiation for climatic variables among groups, including a covariate for population. (A) FC1: comparison of all sexuals with all asexuals. (B) FC2: full model comparing sexuals (“2xS”), diploid asexuals (“2xA”), and triploid asexuals (“3xA”), followed by contrasts. (C) FC3: full model comparing sexual Boechera retrofracta (“sexual Br”), sexual B. stricta (“sexual Bs”), non-hybrid asexual B. retrofracta (“asexual Br”), and hybrid asexual B. stricta × B. retrofracta (“Br × Bs”), followed by contrasts. (D) FC4: full model comparing pure asexual with mixed sexual/asexual populations.
| Model | Wilks’ λ | approx F | d.f. (n,d) | P value | |
|---|---|---|---|---|---|
| A | full | 0.85 | 32.87 | 5,949 | <2.20e-16 |
| B | full | 0.85 | 33.81 | 5,949 | <2.20e-16 |
| 2xS vs. 2xA | 0.89 | 17.34 | 5,696 | 6.60e-16 | |
| 2xA vs. 3xA | 0.92 | 8.60 | 5,498 | 7.82e-08 | |
| 2xS vs. 3xA | 0.83 | 29.28 | 5,698 | 6.60e-16 | |
| C | full | 0.29 | 64.78 | 15, 1720.2 | <2.20e-16 |
| sexual Br vs. sexual Bs | 0.23 | 294.09 | 5,431 | 1.10e-15 | |
| sexual Br vs. asexual Br | 0.77 | 26.05 | 5, 188 | 1.10e-15 | |
| Br × Bs vs. asexual Br | 0.77 | 11.30 | 5, 188 | 1.12e-15 | |
| Br × Bs vs. sexual Bs | 0.31 | 90.56 | 5,202 | 1.10e-15 | |
| Br × Bs vs. sexual Br | 0.77 | 27.30 | 5,455 | 1.10e-15 | |
| D | full | 0.88 | 19.79 | 5,743 | <2e-16 |
Disturbance and slope differentiate asexuals and sexuals—
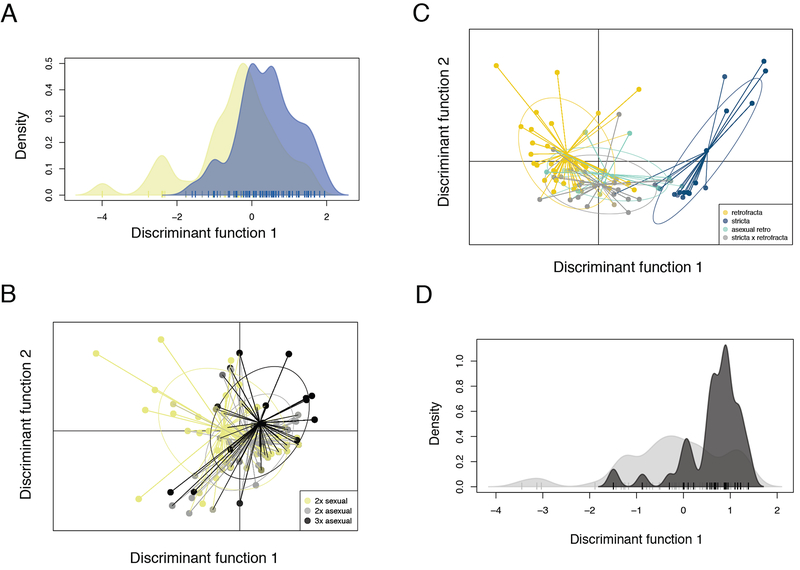
Visualization of DAPC discriminant functions show broad overlap between sexual and asexual niches, with some ecological differentiation apparent nonetheless (Fig. 3A). Overall, diploid asexuals show more environmental overlap with triploid asexuals than diploid sexuals do with either group (Fig. 3B).
Fig. 3.
Visualization of ecological differentiation in four different focal comparisons via discriminant analysis of principal components (DAPC). (A) Asexuals (blue) and sexuals (yellow) co-occur in multiple populations, but are moderately ecologically differentiated. (B) Diploid asexuals (gray) and triploid asexuals (black) overlap substantially in ecological space compared with diploid sexuals (yellow); (C) Boechera retrofracta (gold) is ecologically well-differentiated from B. stricta (blue), while asexual B. retrofracta (green) and asexual hybrid B. retrofracta × B. stricta (gray) occupy environments intermediate between the two species. (D) Purely asexual populations (black) are concentrated in a narrow range of ecological conditions, compared with mixed sexual/asexual populations (gray).
Discriminant function loading values show that two variables—slope and disturbance—contributed heavily to axes differentiating sexuals from asexuals in all FCs, with asexuals occurring in flatter, more disturbed sites (Appendix S7, and Appendix S9). In montane environments, slope and distance to nearest road might be correlated, as roads are generally built in accessible locations; however, the correlation between these variables in the full dataset was only 0.01. Compared to sexuals, asexuals on average occur in areas with greater yearly drought stress (indicated by lower precipitation in both the wettest month and wettest quarter), and areas of greater temperature stress (indicated by lower minimum temperature in the coldest month; Appendix S7). Asexuals overall occur in locations slightly further south than sexuals (asexual mean latitude 44.71±0.02 compared to the sexual mean 44.87±0.02, about 10 km; Appendix S7).
To examine ED of apomicts and sexuals on a finer scale, we compared sexual Bs, sexual Br, apomictic Br, and apomictic Br × Bs hybrids (FC3). The two sexual species occupied non-overlapping regions of environmental space along discriminant function 1, while the two apomicts spanned the interspecies gap (Fig. 3C). Slope and disturbance were again heavily loaded in FC3, as was latitude (Appendix S9, section C). Both hybrid and non-hybrid apomicts occurred substantially closer to roads than sexual Bs or Br, indicating an association between asexuality and disturbance (Appendix S7, section C). However, both forms of asexuals occurred in locations with intermediate slopes between the two sexual species, as well as intermediate latitudes (Br, high slope and high latitude; Bs, low slope and low latitude; Appendix S7, section C).
Pure asexual populations were concentrated in a small range of environmental conditions compared with mixed sexual/asexual populations (Fig. 3D). Slope and disturbance were also heavily loaded in the comparison of pure asexual and mixed sexual/asexual populations, with pure asexual populations occurring in more disturbed (asexual, 19.40 ± 6.46m from nearest road vs. mixed, 244.34 ± 68.81m from nearest road) and flatter locations (asexual slope, 0.20 ± 0.03 vs mixed slope, 0.23 ± 0.02).
Apomicts occur in areas of greater environmental variability—
We next used GLMs to evaluate the impact of ploidy level and hybridization on environmental associations with reproductive mode. In the full dataset, Moran’s I showed no evidence of spatial autocorrelation, although this was not the case in FC3 (observed Index=0.11, expected Index=−0.02, P=0.01) or FC4 (observed Index=0.33, expected Index=−0.01, P=3.40e–14). Thus some of the signal observed in these subsets may be underlain by geographic structure. In GLMs for FC1, FC2, and FC3, slope was the most significant predictor of reproductive mode, with apomicts occurring in locations of lower slope (flatter topography) than sexuals, and closer to roads, consistent with a positive relationship between apomixis and disturbance. GLMs also indicated a strong positive association between asexual reproduction and temperature and precipitation seasonality, with asexual parentage (hybrid vs. non-hybrid) affecting the patterns observed. A comparison of mixed sexual/asexual and pure asexual populations also found that increased temperature variation predicted pure asexuality.
FC1 included all individuals regardless of species or ploidy level. Of the seven variables incorporated, four had a significant relationship with reproductive mode after correcting for multiple comparisons (BIO7, BIO12, BIO15, slope, disturbance; Table 3A). The prevalence of sex increased as a function of slope (0.70 ± 0.10, Z=7.07, P=6.32e–12) and disturbance (0.28 ± 0.08, Z=3.29, P=2.97e–03), and decreased with increased temperature range (BIO7, −1.13 ± 0.17, Z=−6.53, P=2.02e–10) and greater seasonality of precipitation (BIO15, −0.78 ± 0.20, Z=−3.97, P=2.18e–04). Cumulatively, these relationships suggest that apomixis in Boechera is predicted not only by slope and disturbance, but also by increased variability in temperature and precipitation.
Table 3.
Results of GLM (family=binomial) predicting apomixis/sex for interspecific hybrids, with significant P-values in bold. (A) Triploids included (FC1, N=96 populations) for an overall sexual vs. asexual comparison; (B) Analysis limited to diploid sexuals and diploid asexuals (FC2, N=73 populations).
| Variable | Estimate | Std error | Z | P value |
| BIO1 | 0.581 | 0.481 | 1.208 | 0.227 |
| BIO7 | −1.132 | 0.173 | −6.527 | 2.02e-10 |
| BIO12 | −0.619 | 0.273 | −2.266 | 0.053 |
| BIO15 | −0.778 | 0.196 | −3.967 | 2.18e-04 |
| slope | 0.698 | 0.099 | 7.067 | 6.32e-12 |
| elevation | −0.426 | 0.403 | −1.055 | 0.291 |
| disturbance | 0.275 | 0.084 | 3.293 | 2.97e-03 |
| B. | ||||
| Variable | Estimate | Std error | Z | P value |
| BIO1 | 2.563 | 0.730 | 3.509 | 8.98e-04 |
| BIO7 | −1.065 | 0.241 | −4.428 | 1.90e-05 |
| BIO12 | −0.187 | 0.439 | −0.426 | 0.67 |
| BIO15 | −0.853 | 0.299 | −2.850 | 0.009 |
| slope | 0.595 | 0.122 | 4.893 | 2.97e-06 |
| elevation | 0.960 | 0.565 | 1.698 | 0.053 |
| disturbance | 0.274 | 0.112 | 2.446 | 0.029 |
In analyses controlling for ploidy (FC2), significance was retained for BIO7, BIO15, slope, and distance to nearest road (Table 3B). Controlling for ploidy resulted in one new significant effect—annual mean temperature (BIO1; Z=3.51, P=8.98e–04), with asexuals occurring at lower mean temperatures. All relationships between apomixis and environmental variables maintained directionality, with the exception of elevation, which was non-significant.
In FC3 (isolating the effects of interspecific hybridization from heterozygosity), reproductive mode was predicted by BIO1 (annual mean temperature, Z=3.83, P=3.78e–04), BIO3 (isothermality, Z=,−2.47 P=0.03), BIO4 (temperature seasonality, Z=−4.39, P=1.13e–05), BIO15 (precipitation seasonality, Z=−4.36, P=5.12e–05), slope (Z=3.79, P=3.02e–04), and disturbance (Z=3.88, P=4.24e–04; Table 4). Although both hybrid and non-hybrid asexuals occur in more disturbed locations than either sexual species, asexual means for slope, elevation, and annual mean temperature were intermediate between those of the sexual species (Appendix S7, section C). Temperature and precipitation seasonality exhibited more complex relationships. Non-hybrid asexual Br occurs in more environmentally extreme locations (i.e., those with greater temperature and precipitation seasonality, as well as sites with lower values of isothermality) than either sexual species or hybrid asexuals. In contrast, mean precipitation seasonality for hybrids is intermediate between their parental species, while mean temperature seasonality for hybrids is lower than either parental species; isothermality values are highest for hybrids as well. Cumulatively, these relationships suggest that hybrids have reduced robustness to variation in temperature.
Table 4.
Results of GLM (family=binomial) predicting apomixis/sex for FC3, with significant P-values in bold (N=67 populations).
| Variable | Estimate | Std error | Z | P value |
|---|---|---|---|---|
| BIO1 | 3.269 | 0.853 | 3.834 | 3.78e-04 |
| BIO3 | −0.551 | 0.223 | −2.468 | 0.027 |
| BIO4 | −1.132 | 0.258 | −4.390 | 1.13e-05 |
| BIO15 | −0.591 | 0.136 | −4.363 | 5.12e-05 |
| slope | 0.508 | 0.134 | 3.790 | 3.02e-04 |
| elevation | 1.737 | 0.731 | 2.376 | 0.053 |
| disturbance | 0.786 | 0.203 | 3.876 | 4.24e-04 |
The FC4 GLM comparing pure asexual and mixed sexual/asexual populations indicated an association between temperature range and asexuality, with pure asexual populations occurring in sites associated with greater range in temperatures (estimate=1.49, Z=2.449, P=0.01; Table 5). Surprisingly, slope and disturbance were not significant in this model, despite vastly different mean values for disturbance (mixed sexual/asexual 244.34 ± 68.81m vs. pure asexual 19.40 ± 6.46m) that may suggest disturbance facilitates the establishment of pure asexual populations.
Table 5.
Results of GLM (family=binomial) predicting pure asexual vs. mixed sexual/asexual populations, with significant P-values in bold (FC4, N=75 populations).
| Variable | Estimate | Std error | Z | P value |
|---|---|---|---|---|
| BIO3 | −0.147 | 0.429 | −0.342 | 0.732 |
| BIO7 | 1.493 | 0.610 | 2.449 | 0.014 |
| BIO12 | 2.188 | 0.921 | 2.449 | 0.053 |
| BIO15 | 1.265 | 0.653 | 1.939 | 0.530 |
| slope | −0.437 | 0.343 | −1.273 | 0.203 |
| disturbance | −2.595 | 2.246 | −1.155 | 0.248 |
DISCUSSION
Across our study area, ecological differentiation between sexuals and asexuals is widespread in Boechera. This pattern is characterized by differences in disturbance, slope, and environmental variability, with asexual individuals tending to occur in flatter, more disturbed, and more variable environments than their sexual counterparts. Although these relationships are mostly unaffected by polyploidy, hybridization does have an impact, with hybrid asexuals present in environments that are intermediate between parental habitat, novel from parental habitat, or similar to the habitat of one parent or the other. Variability in temperature also differs between pure asexual and mixed sexual/asexual populations, suggesting that differences in temperature might permit the initial establishment and spread of asexual Boechera. Collectively, these results offer one of very few examples of ecological differentiation maintaining coexistence of sexual and asexual plants across the landscape. Furthermore, this is the only study, to our knowledge, that has disentangled the confounding effects of polyploidy and hybridization on sexual/asexual ED.
Rampant apomixis and hybridization across the landscape—
With over half of our accessions identified as apomicts, and 379 (65%) of those representing interspecific hybrids (comprising 16 unique species combinations), this study offers further evidence for the widespread nature of apomixis and hybridization in Boechera. The pervasiveness of these phenomena is consistent with the findings of Beck et al. (2012) and Li et al. (2017), which together documented hundreds of novel asexual hybrid combinations in Boechera. These observations provide strong support for the hypothesis that apomixis has arisen repeatedly in the genus (Sharbel and Mitchell-Olds, 2001; Dobeš et al., 2004b; Kiefer and Koch, 2012; Aliyu et al., 2013). Given this evidence for multiple origins of asexuality in Boechera, it may be unsurprising that we find no coarse geographic patterns in the distribution of sexuals and asexuals. Instead, sexuals and asexuals co-occur within approximately one-third of the populations surveyed. This co-occurrence could be due in part to a single older origin of an asexual lineage followed by mutation accumulation and dispersal; support for this process comes from one recent study that examined multiple lineages from a single pair of parental species and reported close genetic relationships among daughter lineages, consistent with one origin followed by subsequent mutation (Lovell et al., 2017).
Alternatively, asexual lineages may be the result of migration events, and unrelated to the sexual genotypes with which they co-occur. Our results mostly support the latter process. For example, in the present study, hybrids involving Boechera sparsiflora are distributed across 23 populations, but sexual representatives of this species are not present in the dataset at all. Although sampling error may have introduced this pattern (rare sexual B. sparsiflora individuals might occur in the study area, but just were not sampled), this is unlikely to be the full explanation, as the northern range limit of B. sparsiflora is far south of our study area. This pattern of apomicts ranging beyond their sexual progenitors suggests two potential scenarios: first, that apomictic plants may have more effective dispersal or colonization abilities in comparison with sexuals; or second, that apomictic hybrids originated concurrently with range contractions of sexual congeners. Sexual populations of Townsendia hookeri, the Easter daisy, occur beyond both the northern and southern limits of apomictic populations, and patterns of diversity suggest that glacial activity produced the large latitudinal gap between sexual populations (Thompson and Whitton, 2006). Montane glaciers were present in the Rockies of central Idaho during the Pleistocene (Porter et al., 1983; Young et al., 2011), and these similarly may have fragmented distributions of widespread sexual species. Boechera hybrid apomicts may have formed in glacial refugia, where sexual species came into secondary contact (Dobeš et al., 2004b). As glaciers retreated, asexuals could either have dispersed into newly available habitat more quickly than their sexual progenitors, or dispersed at the same rate but outcompeted sexual lineages. Further analyses are needed to determine the processes that have led to the presence of apomictic hybrids in locales distant from their sexual parental species.
Environmental variation, slope, and disturbance underlie sexual/asexual ecological differentiation—
In all four focal comparisons, our results show that asexual reproduction occurs in areas associated with disturbance, low slopes, and substantial variation in both temperature and seasonal precipitation (Tables 3–5, Appendix S9). Asexuality in both animals and plants has long been associated with “marginal” or “extreme” ecology (Glesener and Tilman, 1978; Hörandl, 2006). Glesener and Tilman (1978) hypothesized that asexuality is associated with environmental uncertainty (higher elevation or latitude, extreme drought conditions, greater disturbance) after reviewing data available for asexual animal populations and their sexual progenitors. Indeed, asexual plants are routinely found at higher elevations or latitudes than sexual plants (Bierzychudek, 1985; Hörandl, 2006). In high elevation habitats, organisms may be subjected to lower overall temperatures, large daily temperature differentials, and substantial variation in monthly precipitation. In the Frank Church-River of No Return Wilderness, where many of our populations are located, low temperatures may reach −34°C or lower, while high temperatures above 37°C have been recorded; the average temperature differential on a given summer day is 22°C. Average annual precipitation is similarly highly variable, with total rain and melted snow along the Salmon River estimated to be 7 – 10 inches, and 50 – 60 inches in western areas of the Wilderness (Finklin, 1988). In an area in which climatic variation is substantial even during the course of a single day, the association of asexual Boechera with high variability is consistent with a link between asexuality and unforgiving environments.
Disturbance is likewise considered an element of environmental uncertainty, and has historically been associated with asexuality (Glesener and Tilman 1978). In our study area, comprising the mountainous and heavily forested Lemhi and Custer counties in Idaho and Beaverhead County in Montana, anthropogenic disturbance is the exception. At one site in this region, the vegetative community has been shown to be intact over the last several thousand years (Mehringer et al., 1977). In this area roads are a prime example of recent anthropogenic disturbance of pristine habitat, as the presence of roadways is rare across the majority of Boechera populations.
In marginal habitats, asexuals may escape biotic pressures such as competitors, pathogens, or herbivores, offering another mechanism for ED (Glesener and Tilman, 1978). Few empirical examples of this phenomenon exist, but decades of research show a strong association between sex and pathogen infection in the New Zealand mudsnail, with sexuals occupying high infection risk sites (Jokela and Lively, 1995; Lively and Jokela, 2002; King et al., 2009) and asexuals occurring in low-risk areas (Gibson et al., 2016), showing evidence for niche differentiation between sexuals and asexuals that is mediated by biotic interactions. Likewise, Verhoeven and Biere (2013) found that soil microbes occurring in northern apomictic dandelion populations were less pathogenic than those from southern sexual populations; asexual populations also experienced reduced weevil infestation. Thus biotic interactions may substantially influence sexual/asexual niche differentiation and alter the geographic distribution of each reproductive mode.
Slope is significant in three of our focal comparison GLMs (Tables 3 and 4) and heavily weighted in all discriminant functions (Appendix S9), although Br × Bs hybrids in FC3 are found at slopes intermediate between their parental species. Several factors could potentially explain the importance of slope to ED. For example, soil density or composition could differ between locations that vary in drainage capability, or asexuals may require more water than sexuals during the growing season (water availability will be higher in flat locations). Consistent with this hypothesis is evidence that facultatively asexual Boechera produce more sexual ovules in stressful environments (Mateo de Arias, 2013).
When compared to mixed sexual/asexual populations, purely asexual populations are found in more disturbed and less sloped environments, and in those with greater range in temperature, although only temperature range is significant in our GLM. This suggests that, beyond mere association with asexual reproduction, these factors may facilitate the spread of asexual genotypes beyond their site of origination, consistent with the link between asexuality and invasion (see, e.g., Amsellem et al., 2001; Chapman et al., 2004). Although a recent study found that asexuals and sexuals do not differ in their proclivity for invasion (Dellinger et al., 2016), numerous counter examples exist. In one recent example of invasive asexuality, the marbled crayfish (a polyploid hybrid asexual) has spread to multiple freshwater systems around the world, and expanded its range 100-fold during a 10-year invasion in Madagascar (Gutekunst et al., 2018). Doubtless, reproductive assurance plays an important role in the invasiveness of asexual animals, and likewise, for apomictic plants that are descended from outcrossing sexuals. However, it is possible that several other factors may allow asexual invasion, including epigenetic variation, permissive or beneficial environmental conditions, or increased heterozygosity due to polyploidy or hybridization.
Disentangling the influence of confounding factors on sexual/asexual ED—
In flowering plants, apomixis nearly always co-occurs with hybridization and polyploidy (Asker and Jerling, 1992). Each of these features will uniquely impact morphological traits that, in turn, affect reproductive isolation and gene flow, with consequences for niche occupation and range limits. For example, most asexuals enjoy reproductive assurance, unlike their sexual progenitors, which is a likely cause of range expansion in many taxa (Hörandl, 2006). Another example is that of recently-derived polyploids, which are instantly reproductively isolated from their progenitors because of meiotic failure in inter-ploidy hybrids (Coyne and Orr, 2004). Without novel available niches, neopolyploids may be driven to extinction; those polyploids that survive are likely to be ecologically differentiated from their homoploid relatives.
We find that ecological differentiation is significant both with and without controlling for polyploidy (Table 2). These results suggest that polyploidy has a minimal impact on ED itself, a conclusion that is further bolstered by the maintenance of significance and directionality in the specific environmental variables associated with sexual/asexual ED, with a minor impact on the relationship between elevation and asexuality. The relatively slight effect of polyploidy in our study system is contrast to results in other taxa (Bierzychudek, 1985; Lo et al., 2013; Schinkel et al., 2016; Sonnleitner et al., 2016). This also conflicts with previous work in Boechera, which detected little niche differentiation between sexual and asexual accessions across the full geographic range, and attributed ecological differences to polyploidy (Mau et al., 2015). This disparity in results could arise from a number of factors. First, unlike Mau et al., our study takes advantage of large sample sizes focused on a small portion of the geographic and taxonomic range of Boechera, which may provide greater statistical power to detect ecological divergence between sexual and apomictic genotypes. Second, the taxonomy of Boechera is complex, and hybrid parentage and identity of the numerous apomictic Boechera taxa have been nearly impossible to establish based on morphology alone (Windham and Al-Shehbaz, 2006). Our fine-scale investigation of ED was only possible because of a recently published method for precise Boechera identification (Li et al., 2017). Third, Mau et al. (2015) highlighted B. retrofracta as one of the few ecologically differentiated Boechera species; patterns observed in our B. retrofracta-heavy dataset may thus differ from taxonomically broader datasets. Lastly, it is possible that scale affects the patterns observed—fine-scale ED may not operate in the same way across the full geographic range of the genus, due to evolutionary or stochastic processes that play out over vast time scales. This may reflect the often-observed disparity between micro- and macroevolutionary patterns in evolutionary biology as a whole (see, for example, Rabosky and Matute, 2013).
Previous studies have implicated hybridization as an important driver of ED (Kearney, 2005; Coughlan et al., 2014), and hybridization appears to be an important driver of sexual-asexual ED in Boechera as well. Although hybridization can have detrimental effects on fitness, the introduction of new genetic material may increase evolutionary amplitude (Arnold and Martin, 2010; Yakimowski and Rieseberg, 2014). Shifts in habitat preference may occur in hybrids (Rieseberg et al., 2003), which may even permit invasion of new habitats (Schierenbeck and Ellstrand, 2009; Hovick and Whitney, 2014). In a review of hybrid plant morphology, Rieseberg and Ellstrand (1993) found that most plant traits were either intermediate between the parents (45%) or indistinguishable from one parent or the other (45%). Alternatively, hybrid traits may be more extreme than either parent, which has been shown to scale with the genetic distance between progenitor species (Stelkens and Seehausen, 2009). Even if transgressive segregation does not occur, interspecific hybridization will result in novel trait combinations with potentially profound evolutionary and ecological implications (Seehausen, 2004).
Our focused comparison of two sexual species, their asexual hybrid, and asexual non-hybrids reveals complex patterns of ecological differentiation. Considering environmental preference a trait of an organism, we find that hybrid Boechera meet each of the patterns predicted in the hybridization literature—hybrids occur in environments that are intermediate between their parents (slope and precipitation seasonality), similar to one parent or the other (precipitation in wettest quarter and mean diurnal range), or more extreme than either parent (disturbance and isothermality; Appendix S7, section C). Cumulatively, our results suggest that hybridization has a unique impact on ED that differs from the effects of asexuality.
Why do the effects of hybridization differ between the full dataset and the Br/Bs comparison? Several explanations are possible. First, particular taxa and their niche preferences may simply produce different patterns. If FC3 included a different set of species, or consisted of a greater proportion of the ~80 sexual Boechera species and their hybrids, our conclusions would likely have differed. This issue underscores the importance of taxon identification in any study of asexual organisms, as a given result will vary based on the taxonomic understanding of the study group. Second, ED may depend on hybrid age; older hybrids could have established ecological niches that differ from their parental species, while recently derived hybrids may simply have lacked time to do so (Mau et al., 2015). The age of hybridization events could well differ between our full and reduced dataset; hybridization event(s) giving rise to the Br × Bs apomicts may have been recent, while FC1 may contain sufficient numbers of older hybrids that GP is apparent. Evidence for both recent (Koch et al., 2003; Dobeš et al., 2004a; Kiefer et al., 2009) and older Pleistocene hybridization events (Dobeš et al., 2004b) suggest a range of ages for apomictic lineages in Boechera. Further work is needed to accurately age the formation of hybrid lineages across the genus, and assess the relative frequency of recent vs. older hybridization events and their respective impacts on ED.
In our comparison of non-hybrid and hybrid asexuals with their parental species, our results show that it is non-hybrid asexuals that occur in the most variable environments, rather than hybrids. Reproductive assurance does not differ between sexual and asexual rock cresses, and other associated traits are more likely to cause this observation. Chief among candidate associated traits is heterozygosity, which is low in selfing organisms and high in those produced via hybridization or outcrossing. In most studies of sexual/asexual ED, the effects of heterozygosity cannot be disentangled from those of hybridization or polyploidy. Indeed, although the masking of deleterious alleles accumulated via Muller’s ratchet could facilitate rapid adaptation or increase tolerance of stressful environments, these phenomena may be caused by polyploidy (Chao et al., 2013), hybridization (reviewed in Abbott et al., 2013), or simply by disparities in heterozygosity (Gutekunst et al., 2018). Increased heterozygosity via outcrossing is sufficient to mask deleterious alleles, as evidenced by the vast increase in yield and improved drought resistance of hybrid maize (Crow, 1998). The results presented here are consistent with enhanced tolerance of environmental variability conferred by heterosis, although further studies are required to confirm the consequences of heterozygosity on fitness in rock cress.
CONCLUSIONS
Here we address a critical gap in our understanding of the ecology of sex: What environmental variables underlie ecological differentiation between sexual and asexual plants? Although addressed in studies spanning the entire range of several plant species, to our knowledge, only one study asks this question at a densely sampled focal geographic scale, and no previous work has been able to isolate the effects of asexuality from those of polyploidy, hybridization, and reproductive assurance. The intermediate scale of this work enables robust statistical power to detect patterns across a large number of populations, without the confounding influence of large-scale climatic variation or substantial differences in biogeographic history. Our extensive sampling of the mustard genus Boechera showed that sexuals and asexuals are ecologically differentiated across our study area, comprising 149 populations occurring across a swath of Boechera’s native range. Asexuality is associated with increased disturbance and environmental variability, as well as flatter topography; hybridization affects these associations. It is possible that these environmental conditions promote the establishment of asexual populations, although further study is needed. This work has important implications for the study of sexual/asexual coexistence in plants, and the genetically and empirically tractable system Boechera offers a unique opportunity for further investigation.
Supplementary Material
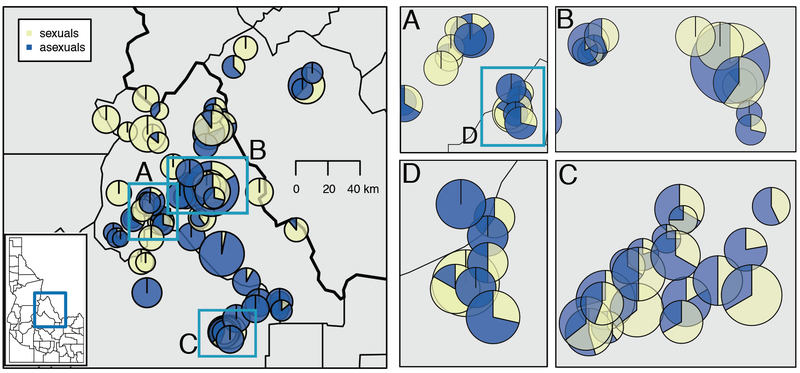
Fig. 2.
Map of 96 Boechera populations analyzed statistically in this study, located in central Idaho and western Montana. Piecharts, scaled for population size, represent individual populations, with sexuals shown in yellow and asexuals in blue; geographic scale is indicated (main map). (A–D) show individual populations in areas of high population density. Inset D is within the area of inset A.
ACKNOWLEDGEMENTS
The authors thank James Beck, Gideon Bradburd, Jenn Coughlan, Tanya Duncan, Marc Johnson, Chris Muir, Mohamed Noor, Mark Rausher, Carl Rothfels, Seema Sheth, and John Willis for helpful discussion, and Rob Colautti, Cheng-Ruei Lee, Evan Raskin, and Maggie Wagner for field assistance. We additionally thank two anonymous reviewers and Associate Editor Carol Goodwillie for their thoughtful comments which greatly improved this manuscript. This work was supported by funding from the National Science Foundation to CAR (in the form of a Graduate Research Fellowship and DEB-1311269) and MDW (DEB-0816560), and from the National Institutes for Health to TM-O (R01 GM086496).
Footnotes
DATA ACCESSIBILITY
Original data is archived in Dryad under doi:10.5061/dryad.1jn5887
LITERATURE CITED
- Abbott R, Albach D, Ansell S, Arntzen JW, Baird SJE, Bierne N, Boughman J, et al. 2013. Hybridization and speciation. Journal Of Evolutionary Biology 26: 229–246. [DOI] [PubMed] [Google Scholar]
- Agrawal AF 2009. Spatial heterogeneity and the evolution of sex in diploids. American Naturalist 174 Suppl 1: S54–70. [DOI] [PubMed] [Google Scholar]
- Al-Shehbaz IA, AND Windham MD. 2010. Boechera. In Flora of North America Editorial Committee [ed.], Flora of North America North of Mexico, vol. 7, 348–412, New York, New York, USA and Oxford, England, UK. [Google Scholar]
- Alexander PJ, Windham MD, Beck JB, Al-Shehbaz IA, Allphin L, AND Bailey CD. 2013. Molecular Phylogenetics and Taxonomy of the Genus Boechera and Related Genera (Brassicaceae: Boechereae). Systematic Botany 38: 192–209. [Google Scholar]
- Aliyu OM, Seifert M, Corral JM, Fuchs J, AND Sharbel TF. 2013. Copy Number Variation in Transcriptionally Active Regions of Sexual and Apomictic Boechera Demonstrates Independently Derived Apomictic Lineages. The Plant Cell 25: 3808–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsellem L, Noyer J, AND Hossaert-McKey M. 2001. Evidence for a switch in the reproductive biology of Rubus alceifolius (Rosaceae) towards apomixis, between its native range and its area of introduction. American Journal Of Botany 88: 2243–2251. [PubMed] [Google Scholar]
- Arnold ML, AND Martin NH. 2010. Hybrid fitness across time and habitats. Trends In Ecology & Evolution 25: 530–536. [DOI] [PubMed] [Google Scholar]
- Asker SE, AND Jerling L. 1992. Apomixis in Plants. CRC Press, Inc., Boca Raton, Florida, USA. [Google Scholar]
- Bates D, Maechler M, Bolker B, AND Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4, version R package version 1.1–7. website: http://CRAN.R-project.org/package=lme4. [Google Scholar]
- Beck JB, Alexander PJ, Allphin L, Al-Shehbaz IA, Rushworth C, Bailey CD, AND Windham MD. 2012. Does hybridization drive the transition to asexuality in diploid Boechera? Evolution 66: 985–995. [DOI] [PubMed] [Google Scholar]
- Ben-Ami F, AND Heller J. 2007. Temporal patterns of geographic parthenogenesis in a freshwater snail. Biological Journal Of The Linnean Society 91: 711–718. [Google Scholar]
- Bierzychudek P 1985. Patterns in Plant Parthenogenesis. Experientia 41: 1255–1264. [DOI] [PubMed] [Google Scholar]
- Burt A 2000. Perspective: Sex, Recombination, and the Efficacy of Selection - Was Weismann Right? Evolution 54: 337–351. [DOI] [PubMed] [Google Scholar]
- Case T 1990. Patterns of coexistence in sexual and asexual species of Cnemidophorus lizards. Oecologia 83: 220–227. [DOI] [PubMed] [Google Scholar]
- Case T, AND Taper M. 1986. On the coexistence and coevolution of asexual and sexual competitors. Evolution: 366–387. [DOI] [PubMed] [Google Scholar]
- Castel M, Mailleret L, Andrivon D, Ravigne V, AND Hamelin FM. 2014. Allee effects and the evolution of polymorphism in cyclic parthenogens. American Naturalist 183: E75–88. [DOI] [PubMed] [Google Scholar]
- Chao D-Y, Dilkes B, Luo H, Douglas A, Yakubova E, Lahner B, AND Salt DE. 2013. Polyploids Exhibit Higher Potassium Uptake and Salinity Tolerance in Arabidopsis. Science 341: 658–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Robson B, AND Pearson M. 2004. Population genetic structure of a colonising, triploid weed, Hieracium lepidulum. Heredity 92: 182–188. [DOI] [PubMed] [Google Scholar]
- Clark LV, AND Jasieniuk M. 2011. polysat: an R package for polyploid microsatellite analysis. Molecular Ecology Resources 11: 562–566. [DOI] [PubMed] [Google Scholar]
- Clauss M, Cobban H, AND Mitchell-Olds T. 2002. Cross-species microsatellite markers for elucidating population genetic structure in Arabidopsis and Arabis (Brassicaeae). Molecular Ecology 11: 591–601. [DOI] [PubMed] [Google Scholar]
- Cosendai A-C, AND Hörandl E. 2010. Cytotype stability, facultative apomixis and geographical parthenogenesis in Ranunculus kuepferi (Ranunculaceae). Annals Of Botany 105: 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan JM, Stefanović S, AND Dickinson TA. 2014. Relative resource allocation to dispersal and competition demonstrates the putative role of hybridity in geographical parthenogenesis. Journal of Biogeography 41: 1603–1613. [Google Scholar]
- Coyne J, AND Orr H. 2004. Speciation. Sinauer Associates, Sunderland, MA, USA. Crow, J. F. 1998. 90 Years Ago: The Beginning of Hybrid Maize. Genetics 148: 923–928. Dedryver, C.-A., M. Hullé, J.-F. Le Gallic, M. C. Caillaud, AND J.-C. Simon. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coexistence in space and time of sexual and asexual populations of the cereal aphid Sitobion avenae. Oecologia 128: 379–388. [DOI] [PubMed] [Google Scholar]
- Dellinger AS, Essl F, Hojsgaard D, Kirchheimer B, Klatt S, Dawson W, Pergl J, et al. 2016. Niche dynamics of alien species do not differ among sexual and apomictic flowering plants. New Phytologist 209: 1313–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobeš C, Mitchell-Olds T, AND Koch M. 2004a. Intraspecific diversification in North American Boechera stricta (= Arabis drummondii), Boechera × divaricarpa, and Boechera holboellii (Brassicaceae) inferred from nuclear and chloroplast molecular markers—an integrative approach. American Journal Of Botany 91: 2087–2101. [DOI] [PubMed] [Google Scholar]
- Dobeš C, Mitchell-Olds T, AND Koch M. 2004b. Extensive chloroplast haplotype variation indicates Pleistocene hybridization and radiation of North American Arabis drummondii, A. x divaricarpa, and A. holboellii (Brassicaceae). Molecular Ecology 13: 349–370. [DOI] [PubMed] [Google Scholar]
- Doncaster C, Pound G, AND Cox S. 2000. The ecological cost of sex. Nature 404: 281–285. [DOI] [PubMed] [Google Scholar]
- Doyle J, AND Dickson E. 1987. Preservation of plant samples for DNA restriction endonuclease analysis. Taxon 36: 715–722. [Google Scholar]
- Duncan TM, AND Rausher MD. 2013. Morphological and genetic differentiation and reproductive isolation among closely related taxa in the Ipomoea series Batatas. American Journal Of Botany 100: 2183–2193. [DOI] [PubMed] [Google Scholar]
- Finklin AI 1988. Climate of the Frank Church-River of No Return Wilderness, Central Idaho USDA Forest Service INT-240, Ogden, Utah, USA. [Google Scholar]
- Gibson AK, Xu JY, AND Lively CM. 2016. Within-population covariation between sexual reproduction and susceptibility to local parasites. Evolution 70: 2049–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert A, Simon J-C, Dedryver C-A, AND Plantegenest M. 2014. Do ecological niches differ between sexual and asexual lineages of an aphid species? Evolutionary Ecology 28: 1095–1104. [Google Scholar]
- Glesener R, AND Tilman D. 1978. Sexuality and the components of environmental uncertainty: clues from geographic parthenogenesis in terrestrial animals. American Naturalist: 659–673. [Google Scholar]
- Gutekunst J, Andriantsoa R, Falckenhayn C, Hanna K, Stein W, Rasamy J, AND Lyko F. 2018. Clonal genome evolution and rapid invasive spread of the marbled crayfish. Nature Ecology and Evolution 2: 567–573. [DOI] [PubMed] [Google Scholar]
- Halkett F, Plantegenest M, Prunier-Leterme N, Mieuzet L, Delmotte F, AND Simon J. 2005. Admixed sexual and facultatively asexual aphid lineages at mating sites. Molecular Ecology 14: 325–336. [DOI] [PubMed] [Google Scholar]
- Hartfield M, Otto SP, AND Keightley PD. 2010. The role of advantageous mutations in enhancing the evolution of a recombination modifier. Genetics 184: 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfield M, Otto SP, AND Keightley PD. 2012. The maintenance of obligate sex in finite, structured populations subject to recurrent beneficial and deleterious mutation. Evolution 66: 3658–3369. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ 2013. raster: Geographic data analysis and modeling, version R package version 2.1–66. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, AND Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology 25: 1965–1978. [Google Scholar]
- Holm S 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6: 65–70. [Google Scholar]
- Hörandl E 2006. The complex causality of geographical parthenogenesis. New Phytologist 171: 525–538. [DOI] [PubMed] [Google Scholar]
- Hovick SM, AND Whitney KD. 2014. Hybridisation is associated with increased fecundity and size in invasive taxa: meta-analytic support for the hybridisation-invasion hypothesis. Ecology Letters 17: 1464–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela J, AND Lively CM. 1995. Parasites, sex, and early reproduction in a mixed population of freshwater snails. Evolution 49: 1268–1271. [DOI] [PubMed] [Google Scholar]
- Jombart T 2008. adegenet: an R package for the multivariate analysis of genetic markers. Bioinformatics 24: 1403–1405. [DOI] [PubMed] [Google Scholar]
- Kantama L, Sharbel TF, Schranz ME, Mitchell-Olds T, de Vries S, AND de Jong H. 2007. Diploid apomicts of the Boechera holboellii complex display large-scale chromosome substitutions and aberrant chromosomes. Proceedings Of The National Academy Of Sciences, USA 104: 14026–14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M 2003. Why is sex so unpopular in the Australian desert? Trends In Ecology & Evolution 18: 605–607. [Google Scholar]
- Kearney M 2005. Hybridization, glaciation and geographical parthenogenesis. Trends In Ecology & Evolution 20: 495–502. [DOI] [PubMed] [Google Scholar]
- Kiefer C, AND Koch MA. 2012. A Continental-Wide Perspective: The Genepool of Nuclear Encoded Ribosomal DNA and Single-Copy Gene Sequences in North American Boechera (Brassicaceae). PLOS ONE 7: e36491–36416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer C, Dobeš C, Sharbel TF, AND Koch MA. 2009. Phylogeographic structure of the chloroplast DNA gene pool in North American Boechera - A genus and continental-wide perspective. Molecular Phylogenetics And Evolution 52: 303–311. [DOI] [PubMed] [Google Scholar]
- King KC, Delph LF, Jokela J, AND Lively CM. 2009. The Geographic Mosaic of Sex and the Red Queen. Current Biology 19: 1438–1441. [DOI] [PubMed] [Google Scholar]
- Kirchheimer B, Schinkel CCF, Dellinger AS, Klatt S, Moser D, Winkler M, Lenoir J, et al. 2016. A matter of scale: apparent niche differentiation of diploid and tetraploid plants may depend on extent and grain of analysis. Journal of Biogeography 43: 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Dobeš C, AND Mitchell-Olds T. 2003. Multiple hybrid formation in natural populations: Concerted evolution of the internal transcribed spacer of nuclear ribosomal DNA (ITS) in North American Arabis divaricarpa (Brassicaceae). Molecular Biology and Evolution 20: 338–350. [DOI] [PubMed] [Google Scholar]
- Krahulcová A, Rotreklová O, Krahulec F, Rosenbaumová R, AND Plačková I. 2009. Enriching Ploidy Level Diversity: the Role of Apomictic and Sexual Biotypes of Hieracium subgen. Pilosella (Asteraceae) that Coexist in Polyploid Populations. Folia Geobotanica 44: 281–306. [Google Scholar]
- Lee C-R, AND Mitchell-Olds T. 2011. Quantifying effects of environmental and geographical factors on patterns of genetic differentiation. Molecular Ecology 20: 4631–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto MP, AND Haag CR. 2010. Ecological differentiation between coexisting sexual and asexual strains of Daphnia pulex. Journal of Animal Ecology 79: 1241–1250. [DOI] [PubMed] [Google Scholar]
- Li F-W, Rushworth CA, Beck JB, AND Windham MD. 2017. Boechera microsatellite website: an online portal for species identification and determination of hybrid parentage. Database 2016: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively CM, AND Jokela J. 2002. Temporal and spatial distributions of parasites and sex in a freshwater snail. Evolutionary Ecology Research 4: 219–226. [Google Scholar]
- Lo EYY, Stefanovic S, AND Dickinson TA. 2013. Geographical parthenogenesis in Pacific Northwest hawthorns (Crataegus; Rosaceae). Botany 91: 107–116. [Google Scholar]
- Lovell JT, Williamson RJ, Wright SI, McKay JK, AND Sharbel TF. 2017. Mutation Accumulation in an Asexual Relative of Arabidopsis. PLOS Genetics 13: e1006550–1006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M 1990. The Similarity Index and DNA Fingerprinting. Molecular Biology and Evolution 7: 478–484. [DOI] [PubMed] [Google Scholar]
- Marshall D, AND Brown A. 1981. The evolution of apomixis. Heredity 47: 1–15. [Google Scholar]
- Mateo de Arias M 2013. Effects of Plant Stress on Facultative Apomixis in Boechera (Brassicaceae). Doctor of Philosophy, Utah State University, Logan, UT, USA. [Google Scholar]
- Mau M, Lovell JT, Corral JM, Kiefer C, Koch MA, Aliyu OM, AND Sharbel TF. 2015. Hybrid apomicts trapped in the ecological niches of their sexual ancestors. Proceedings Of The National Academy Of Sciences, USA 112: E2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J 1971. What use is sex? Journal Of Theoretical Biology 30: 319–335. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J 1978. The Evolution of Sex. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Mehringer P, Arno S, AND Petersen K. 1977. Postglacial History of Lost Trail Pass Bog, Bitterroot Mountains, Montana. Arctic And Alpine Research 9: 345–368. [Google Scholar]
- Meirmans P, AND Van Tienderen P. 2004. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes 4: 792–794. [Google Scholar]
- Meirmans P, Vlot E, Den Nijs J, AND Menken S. 2003. Spatial ecological and genetic structure of a mixed population of sexual diploid and apomictic triploid dandelions. Journal Of Evolutionary Biology 16: 343–352. [DOI] [PubMed] [Google Scholar]
- Nei M 1987. Molecular Evolutionary Genetics. Columbia University Press, New York. [Google Scholar]
- Neiman M, Paczesniak D, Soper DM, Baldwin AT, AND Hehman G. 2011. Wide variation in ploidy level and genome size in a New Zealand freshwater snail with coexisting sexual and asexual lineages. Evolution 65: 3202–3216. [DOI] [PubMed] [Google Scholar]
- Peck J 1993. Frequency-dependent selection, beneficial mutations, and the evolution of sex. Proceedings of the Royal Society of London B: Biological Sciences 254: 87–92. [DOI] [PubMed] [Google Scholar]
- Porter SC, Pierce KL, AND Hamilton TD. 1983. Late Wisconsin mountain glaciation in the Western United States In Porter SC[ed.], Late Quaternary Environments in the western United States, vol. 1, 71–111. University of Minnesota Press, Minneapolis. [Google Scholar]
- R Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rabosky DL, AND Matute DR. 2013. Macroevolutionary speciation rates are decoupled from the evolution of intrinsic reproductive isolation in Drosophila and birds. Proceedings Of The National Academy Of Sciences, USA 110: 15354–15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg L, Raymond O, Rosenthal D, Lai Z, Livingstone K, Nakazato T, Durphy J, et al. 2003. Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301: 1211–1216. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, AND Ellstrand NC. 1993. What can morphological and molecular markers tell us about plant hybridization. Critical Reviews In Plant Sciences 12: 213–241. [Google Scholar]
- Rispe C, AND Pierre J-S. 1998. Coexistence between cyclical parthenogens, obligate parthenogens, and intermediates in a fluctuating environment. Journal Of Theoretical Biology 195: 97–110. [DOI] [PubMed] [Google Scholar]
- Roy B 1995. The Breeding Systems of Six Species of Arabis (Brassicaceae). American Journal Of Botany 82: 869–877. [Google Scholar]
- Roy B, AND Rieseberg L. 1989. Evidence for apomixis in Arabis. Journal of Heredity 80: 506–508. [Google Scholar]
- Rushworth CA, Song B-H, Lee C-R, AND Mitchell-Olds T. 2011. Boechera, a model system for ecological genomics. Molecular Ecology 20: 4843–4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh D, Xu P, Shen Y, Li C, Adreit H, Milazzo J, Ravigne V, et al. 2012. Sex at the origin: an Asian population of the rice blast fungus Magnaporthe oryzae reproduces sexually. Molecular Ecology 21: 1330–1344. [DOI] [PubMed] [Google Scholar]
- Schierenbeck KA, AND Ellstrand NC. 2009. Hybridization and the evolution of invasiveness in plants and other organisms. Biological Invasions 11: 1093–1105. [Google Scholar]
- Schinkel CCF, Kirchheimer B, Dellinger AS, Klatt S, Winkler M, Dullinger S, AND Hörandl E. 2016. Correlations of polyploidy and apomixis with elevation and associated environmental gradients in an alpine plant. AoB PLANTS 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz M, Dobes C, Koch M, AND Mitchell-Olds T. 2005. Sexual reproduction, hybridization, apomixis, and polyploidization in the genus Boechera (Brassicaceae). American Journal Of Botany 92: 1797–1810. [DOI] [PubMed] [Google Scholar]
- Schranz ME, Kantama L, de Jong H, AND Mitchell-Olds T. 2006. Asexual reproduction in a close relative of Arabidopsis: a genetic investigation of apomixis in Boechera (Brassicaceae). New Phytologist 171: 425–438. [DOI] [PubMed] [Google Scholar]
- Seehausen O 2004. Hybridization and adaptive radiation. Trends In Ecology & Evolution 19: 198–207. [DOI] [PubMed] [Google Scholar]
- Sharbel T, AND Mitchell-Olds T. 2001. Recurrent polyploid origins and chloroplast phylogeography in the Arabis holboellii complex (Brassicaceae). Heredity 87: 59–68. [DOI] [PubMed] [Google Scholar]
- Song B, Clauss M, Pepper A, AND Mitchell-Olds T. 2006. Geographic patterns of microsatellite variation in Boechera stricta, a close relative of Arabidopsis. Molecular Ecology 15: 357–369. [DOI] [PubMed] [Google Scholar]
- Sonnleitner M, Hülber K, Flatscher R, Escobar García P, Winkler M, Suda J, Schönswetter P, AND Schneeweiss G. 2016. Ecological differentiation of diploid and polyploid cytotypes of Senecio carniolicus sensu lato (Asteraceae) is stronger in areas of sympatry. Annals Of Botany 117: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins G 1985. Polyploidy, hybridization, and the invasion of new habitats. Annals Of The Missouri Botanical Garden 72: 824–832. [Google Scholar]
- Stelkens R, AND Seehausen O. 2009. Genetic distance between species predicts novel trait expression in their hybrids. Evolution 63: 884–897. [DOI] [PubMed] [Google Scholar]
- Stross RG, AND Hill JC. 1965. Diapause Induction in Daphnia Requires Two Stimuli. Science 150: 1462–1464. [DOI] [PubMed] [Google Scholar]
- Thompson SL, AND Whitton J. 2006. Patterns of recurrent evolution and geographic parthenogenesis within apomictic polyploid Easter daises (Townsendia hookeri). Molecular Ecology 15: 3389–3400. [DOI] [PubMed] [Google Scholar]
- Verduijn M, van Dijk P, AND Van Damme J. 2004. Distribution, phenology and demography of sympatric sexual and asexual dandelions (Taraxacum officinale s.l.): geographic parthenogenesis on a small scale. Biological Journal Of The Linnean Society 82: 205–218. [Google Scholar]
- Verhoeven KJF, AND Biere A. 2013. Geographic parthenogenesis and plant-enemy interactions in the common dandelion. BMC Evolutionary Biology 13: 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijenhoek R, AND Lerman S. 1982. Heterozygosity and developmental stability under sexual and asexual breeding systems. Evolution: 768–776. [DOI] [PubMed] [Google Scholar]
- Whitton J, Sears CJ, AND Maddison WP. 2017. Co-occurrence of related asexual, but not sexual, lineages suggests that reproductive interference limits coexistence. Proceedings of the Royal Society of London B: Biological Sciences 284: 20171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham MD, AND Al-Shehbaz IA. 2006. New and Noteworthy Species of Boechera (Brassicaceae) I: Sexual Diploids. Harvard Papers In Botany: 1–28. [Google Scholar]
- Yakimowski SB, AND Rieseberg LH. 2014. The role of homoploid hybridization in evolution: a century of studies synthesizing genetics and ecology. American Journal Of Botany 101: 1247–1258. [DOI] [PubMed] [Google Scholar]
- Young NE, Briner JP, Leonard EM, Licciardi JM, AND Lee K. 2011. Assessing climatic and nonclimatic forcing of Pinedale glaciation and deglaciation in the western United States. Geology 39: 171–174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.