Abstract
Background
To report the clinical experience of eye sparing surgery (ESS) and adjuvant carbon-ion or proton radiotherapy (CIRT or PRT) for orbital malignancies.
Methods
An analysis of the retrospective data registry from the Shanghai Proton and Heavy Ion Center for patients with orbital tumors was conducted. The 2-year local progression-free, regional recurrence-free, distant metastasis-free, progression-free, and overall survival (LPFS, RRFS, DMFS, PFS, OS) rates as well as associated prognostic indicators were analyzed. Radiotherapy-induced acute and late toxicities were summarized.
Results
Between 7/2014 to 5/2018, 22 patients with orbital malignancies of various pathologies received ESS followed by CIRT (18), PRT (1), or PRT + CIRT boost (3). With a median follow-up of 20.25 (range 3.8–38.8) months, the 2-year OS, PFS, LPFS, RRFS, and DMFS rates were 100, 57.9, 92.9, 93.3, and 72.8%, respectively. No acute severe (i.e., ≥grade 3) toxicity was observed. Two patients experienced severe visual impairment as late toxicities.
Conclusion
With few observed acute and late toxicities, particle radiotherapy following ESS provided effective local control with infrequent severe toxicities for patients with orbital malignancies.
Keywords: Particle radiotherapy, Orbital malignancies, Eye-sparing surgery
Background
Orbital tumors are relatively rare with an incidence of 3.4/106 person-years [1]; however, its management poses a major challenge to oncologists due to the complexities in the pathologies of the tumors and their proximity to the critical organs at risk (OARs).
Orbital malignancies can arise from any of the orbital structures such as extra-ocular muscles, fat, glands, vessels, nerves, and ocular adnexa. Extensive resection inevitably causes vision damage and disfigurement. Eye-sparing surgery (ESS) is the current preferred primary treatment for nearly all types of neoplasm of epithelial or mesenchymal origin [2]; nevertheless, sufficient margins are difficult to achieve especially for locally advanced diseases. Limited resection poses a high risk of local recurrence.
Multidisciplinary approach including surgery followed by adjuvant radiotherapy or chemoradiation is usually needed for orbital malignancies. Intensity-modulated radiotherapy (IMRT) has been used adjuvantly after surgery or in definitive settings for unresectable cases; however, radiation-induced toxicity limits the doses of IMRT to tumor targets due to excessive entrance and exit doses in the beam paths [3]. Lower doses are usually insufficient for controlling the more commonly diagnosed orbital malignancies including squamous cell carcinoma, adenoid cystic carcinoma (ACC) and soft-tissue sarcoma (STS) [1, 2, 4–6].
There is an increasing interest in the use of particle radiotherapy such as carbon-ion or proton radiotherapy (CIRT or PRT) in the management of head and neck malignancies, particularly for those occurred close to critical OARs, such as orbital tumors [7]. Due to its unique physical characteristic of Bragg Peak, particle radiotherapy allows for providing a high-dose coverage to the tumor with relatively low entrance and minimal exit doses [8, 9]. The use of intensity-modulated particle therapy (IMPT) technology may further improve dose distribution and reduce adverse-effects without compromising efficacy in the treatment of cancers within complex anatomical scenario thereby improves the therapeutic ratio [10, 11].
Carbon-ion beam has higher linear energy transfer (LET) and relative biological effectiveness (RBE) as compared to those of photon or proton [12–15]. The advantages in both physical and biological characteristics of carbon ion, in theory, make it more suitable in the management of conditions with both anatomic limitations and the radio-resistance such as ACC, melanoma, and sarcoma of the orbit. However, data describing clinical outcomes after particle radiotherapy especially CIRT for tumors of the orbit or ocular adnexa is lacking.
The Shanghai Proton and Heavy Ion Center (SPHIC) started to provide IMPT using pencil beam scanning (PBS) technology in 5/2015 [16]. In this article, we report the outcomes in terms of efficacy and safety of a group of patients with orbital tumors treated with adjuvant particle radiotherapy after ESS.
Methods
Pretreatment evaluation
Pretreatment evaluations included a complete history and physical examination (H&P), complete blood count (CBC), serum electrolytes, and MRI or CT (if MRI was contraindicated) of the head and neck region. PET-CT was performed if clinically indicated.
All patients were staged with the AJCC staging system (7th or 8th edition depend on the date of diagnosis). All protocols were registered to the institutional review board (IRB) of the SPHIC. All cases were discussed in the multidisciplinary tumor clinic of SPHIC to confirm the indication of adjuvant particle radiotherapy before inclusion into the institutional cancer registry and planning.
IMPT and chemotherapy
All patients were immobilized with AlphaCradle® and thermoplastic masks in supine position. Plain CT for simulation from the vertex to the inferior margin of clavicular heads were performed at 1.5-mm slice thickness. MRI-CT fusion was performed for all patients prior to target delineation. The gross tumor volume (GTV) was defined as the tumor discovered on clinical examination or imaging studies for patients with incomplete surgical resection. We define clinical target volume (CTV) covering post-surgical GTV (CTV-G) after R2 resection/biopsy to deliver prescribed doses as GTV plus 1-3 mm margin (depend on the proximity to OARs). CTV for patients with R1 resection or achieved complete response (CR) after chemotherapy included pretreatment tumor bed plus high-risk areas for tumor extension. An additional 3–6 mm margin was added to the CTVs to create the planning target volume (PTV) for uncertainty with regard to dose distribution and potential setup errors.
Doses of particle radiotherapy were measured by Gy-equivalents (GyE) to account for the RBE differences compared to photon. Dose constraints of critical OARs are based on TD5/5 described by Emami et al. [17] except for optic nerve (D20 < 30GyE) and temporal lobes (V40 < 7.66 cc; V50 < 4.66 cc) set forth by the National Institute or Radiation Science of Japan [18]. For patients who had previous photon-based radiation, old treatment plans were obtained. Recovery from previous radiotherapy doses was set at 70% [19]. Planning for particle radiotherapy were performed using the Siemens Syngo® treatment planning system.
CIRT and PRT were delivered with PBS technology. Two-3 beams were typically delivered from the horizontal or 45o directions. Setup accuracy was confirmed using bony landmarks on orthogonal X-ray on daily basis. Weekly CT were required to verify tumor regression/progression and anatomic changes. Chemotherapy was used at the discretion of the attending oncologists.
Follow-up
All patients were admitted and examined daily during particle radiotherapy. After the discharge, all patients were encouraged to be followed-up using the standardized institutional follow-up protocol. The first follow-up was provided within 4–6 weeks after the completion of treatment. Patients were then followed-up every 3 months in the first 2 years, every 6 months in the following 3 years, and annually thereafter. A complete H&P with a focus to the eyes, orbits, head/neck region, as well as MRI of the head area are required at each follow-up. Other studies are ordered if clinically indicated.
Data analysis
The duration of survival was calculated from the diagnosis of the disease until death or the last follow-up. The time to locoregional or distant failure was measured from the initiation of any treatment until disease progression or recurrence. Freedom from failure and OS rates were calculated using the Kaplan-Meier method. Cox regression model as was used for both uni- and multi-variate analyses to compare the difference of the survival probabilities and to define significant prognostic factors. All analyses were performed using the SPSS statistics package (Version 22.0).
Adverse events were scored by the attending radiation oncologist(s) according to the CTCAE (version 4.03). Acute toxicities included the adverse events occurred during or within 3 months after the initiation of particle radiotherapy. Late toxicity was defined as those occurred after 3 months from or persisted for > 3 months after the initiation of particle radiotherapy.
Results
Characteristics of patients and surgery
Between 11/2015 and 6/2018, 23 consecutive patients with orbital tumor were treated at SPHIC. All patients had ESS before particle radiotherapy. One patient was excluded from this analysis due to a change of diagnosis from pathology confirmation in the mid of CIRT which substantially changed treatment. The median follow-up of the remaining 22 patients was 20.25 (range 3.8–38.8) months.
Most patients (81.8%) presented with malignancies of the lacrimal gland or lacrimal sac, and 77.2% had malignancies of epithelial origin. One patient presented with locally recurrent lacrimal gland ACC had ESS twice. She also received photon-based radiotherapy (60Gy/30Fx) after the first surgery. The characteristics of the patients, their diseases, and treatment were detailed in Table 1.
Table 1.
Characteristics of patients, their disease, and treatment
| Characteristic | No. of patients (%) |
|---|---|
| Median age (range) | 46.5 (14–74) |
| Sex | |
| Male | 14 (63.6) |
| Female | 8 (36.4) |
| Tumor site | |
| Lacrimal gland | 13 (59.1) |
| Lacrimal sac | 5 (22.7) |
| Orbital bone | 1 (4.5) |
| Other | 3 (13.6) |
| Tumor histology | |
| Adenoid cystic carcinoma | 11 (50.0) |
| Adenocarcinoma | 5 (22.7) |
| Squamous cells carcinoma | 1 (4.5) |
| Melanoma | 1 (4.5) |
| rhabdomyosarcoma | 1 (4.5) |
| desmoplastic small round cell tumor | 1 (4.5) |
| alveolar soft part sarcoma | 1 (4.5) |
| chondrosarcoma | 1 (4.5) |
| T category | |
| T1 | 2 (9.1) |
| T2 | 7 (31.8) |
| T3 | 4 (18.2) |
| T4 | 9 (40.9) |
| Tumor status | |
| Primary | 21 (95.5) |
| Recurrence | 1 (4.5) |
| Surgical margin | |
| R0 | 3 (13.6) |
| R1 | 6 (27.3) |
| R2 or biopsy | 13 (59.1) |
| Interval from surgery to radiotherapy, mo | |
| Median (range) | 2.2 (1.2–6.13) |
| Radiotherapy technique | |
| PRT | 1 (4.5) |
| CIRT | 18 (81.8) |
| PRT + CIRT | 3 (13.6) |
| Radiotherapy dose (Gy BED) | |
| Median (range) | 85.05 (67.2–94.5) |
| GTV (ml) | |
| Median (range) | 16.0 (1.9–67.6) |
| CTV (ml) | |
| Median (range) | 43.4 (18.8–209.9) |
| Concurrent chemotherapy or immunotherapy | |
| Cisplatin | 2 (9.1) |
| Interferon α-2b | 1 (4.5) |
| No | 19 (86.4) |
Particle radiotherapy
PRT, CIRT, or their combination were used in 1, 18, and 3 patients with curative intention, respectively. The median time between surgery and particle therapy was 2.2 months (range 1.2–6.13).
Three patients who achieved R0 resection received PRT (56 GyE/28 fractions, 1 case) or CIRT (60 GyE/20 fractions, 2 cases), respectively. For the remaining 19 patients, 3 received PRT (56 GyE/28 fractions) followed by CIRT boost (15 GyE/3 fractions), and 16 received CIRT (60–70 GyE for primary/residual tumor and 54-62GyE for CTVs in 18–23 fractions using simultaneous integrated boost technique). Elective nodal irradiation was not performed for any patients. All 22 patients completed particle radiotherapy without break. A typical treatment plan is illustrated in Fig. 1.
Fig. 1.
Axial (a) and coronal (b) views of a post eye sparing surgery CT scan of a patient with left lacrimal gland ACC. Axial (c) and coronal (d) views of a typical intensity-modulated carbon ion radiotherapy treatment plan
Survival outcomes
With a median follow-up of 20.25 (range 3.8–38.8) months, all 22 patients were alive. One patient who had R2 resection followed-by cisplatin chemotherapy for T3N0M0 rhabdomyosarcoma of the lacrimal sac developed local recurrence at 17.8 months after CIRT. Another with R2 resected T4N0M0 ACC of the lacrimal gland developed regional recurrence after PRT + CIRT boost at 11.5 months. The 2-year local-progression-free and regional-recurrence-free survival (LPFS and RRFS) rates were 92.9 and 93.3%, respectively (Fig. 2a & b). Four patients with lacrimal gland malignancies (2 with ACC and 2 with adenocarcinoma, 2 with T2 and 2 with T4 disease) developed distant metastasis (DM) at a median time of 9.47 months (range 8.13–20.8). The 2-year DM-free survival (DMFS) rate was 72.8%, and the 2-year progression free survival (PFS) rate was 57.9% (Fig. 2c & d) for the entire cohort. None of the 13 patients with lacrimal gland malignancy developed local failure or progression (i.e., LPFS = 100%). The 2-year RRFS, DMFS and PFS were 88.9 and 53.0% and 39.3% for patients with lacrimal gland malignancies, respectively.
Fig. 2.
Local progression-free survival (LPFS) (a), regional recurrence-free survival (b), distant metastasis free-survival (DMFS) (c), and progression-free survival (PFS) (d) rate curves of the entire cohort
Acute and chronic toxicities
The characteristics of acute and late toxicities are summarized in Table 2. Nine patients (40.9%) experienced grade 1 or 2 acute toxicities induced by particle radiotherapy. No acute toxicity of grade 3 or above was observed. Seven patients (31.8%) experienced late toxicities of various grades including 3 with grade 1 dry eyes, 1 with grade 1 brain injury, 1 with grade 2 retinopathy, 1 with grade 3 visual impairment and 1 with blindness in the affected eye (grade 4). During the follow-up period, no vision impairment was observed, except for 2 patients developed grade 3 and grade 4 visual acuity reduction after CIRT. One of them experienced ipsilateral vision acuity reduction from normal to 20/200–40/200 at 6 months accompanied by optic atrophy diagnosed by MRI; the other patient developed blindness at 3 months without changes on MR scan. One patient who has affected eyeball fixation due to twice eye-sparing surgery and photon-based radiotherapy prior to re-irradiation by CIRT, and no ocular movement disorder was observed in remaining patients who received particle radiotherapy. In addition, there was no eye injury in contralateral side in all patients at present.
Table 2.
Characteristics of toxicities
Prognostic factors
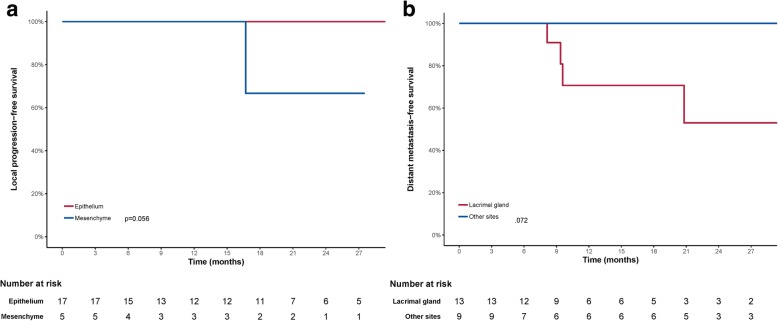
Univariate analyses using log-rank test indicated tumors with mesenchymal origins had a trend toward a worse LPFS (p = 0.056) (Table 3, Fig. 3a). In addition, tumors of lacrimal gland had a trend with worse DMFS (p = 0.072) (Table 3, Fig. 3b). When BED was taken as a continuous variable using Cox regression analysis, higher BED had a trend to associate with improved DMFS (hazard ratio, 0.884; 95% CI, 0.776–1.007 [P = 0.064]) (Table 4). However, margin status, T-classification, volume of GTV or CTV did not associate with PFS, LPFS, RRFS or DMFS in both log-rank test and Cox regression analysis (Tables 3 & 4).
Table 3.
Univariate analysis by the log-rank test
| Characteristics | PFS | LPFS | RRFS | DMFS |
|---|---|---|---|---|
| Gender | 0.910 | 0.527 | 0.480 | 0.362 |
| Age (<46.5 y vs. >46.5 y) | 0.442 | 0.527 | 0.480 | 0.847 |
| Tumor site (other vs. lacrimal gland) | 0.118 | 0.248 | 0.414 | 0.072 |
| Origin (mesenchymal vs. epithelial histology) | 0.653 | 0.056 | 0.617 | 0.254 |
| T classification (T1/2 vs. T3/4) | 0.524 | 0.386 | 0.414 | 0.784 |
| Surgical margin (R0 + R1 vs. R2) | 0.887 | 0.317 | 0.350 | 0.221 |
| BED (<85.05 GyE vs. ≥85.05 GyE) | 0.813 | 0.602 | 0.617 | 0.806 |
| GTV (< 16 ml vs.>16 ml) | 0.928 | 0.527 | 0.157 | 0.666 |
| CTV (< 43.4 ml vs.>43.4 ml) | 0.431 | 0.317 | 0.285 | 0.327 |
Fig. 3.
Local progression-free survival (a), Distant metastasis-free survival (b) curves showing malignancies of mesenchymal origin had a trend toward a worse LPFS and a trend that lacrimal gland tumor had worse DMFS
Table 4.
Univariate analysis of DMFS and PFS by Cox regression
| DMFS | PFS | |||
|---|---|---|---|---|
| Variables | P | HR (95% CI) | P | HR (95% CI) |
| Gender | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 0.378 | 2.431 (0.338–17.48) | 0.910 | 1.103 (0.202–6.036) |
| Age, y | 0.186 | 1.046 (0.979–1.118) | 0.752 | 1.001 (0.958–1.061) |
| Histology | ||||
| Epithelium | Ref | Ref | Ref | Ref |
| Mesenchyme | 0.488 | 0.031 (0.001–560.47) | 0.656 | 0.613 (0.071–5.280) |
| T classification | ||||
| T1 + T2 | Ref | Ref | Ref | Ref |
| T3 + T4 | 0.784 | 0.760 (0.107–5.415) | 0.529 | 1.731 (0.313–9.564) |
| Surgical margin | ||||
| R0 + R1 | Ref | Ref | Ref | Ref |
| R2 | 0.254 | 0.268 (0.028–2.579) | 0.887 | 0.890 (0.178–4.450) |
| BED | 0.064 | 0.884 (0.776–1.007) | 0.100 | 0.898 (0.791–1.021) |
| GTV, ml | 0.948 | 1.002 (0.948–1.059) | 0.928 | 0.998 (0.953–1.045) |
| CTV, ml | 0.978 | 1.000 (0.980–1.021) | 0.797 | 0.998 (0.980–1.016) |
Multivariate analysis using Cox regression using tumor correlation factors such as histological type, BED (continuous variable), T-classification, volume of GTV and CTV suggested a significant relationship for higher BED with improved PFS (hazard ratio, 0.732; 95% CI, 0.557–0.960 [P = 0.024]) and a trend with improved DMFS (hazard ratio, 0.717; 95% CI, 0.512–1.004 [P = 0.053]). Furthermore, larger CTV field may improve PFS (hazard ratio, 0.952; 95% CI, 0.906–1.001 [P = 0.054]) (Table 5).
Table 5.
Multivariate analysis of DMFS and PFS by Cox regression
| DMFS | PFS | |||
|---|---|---|---|---|
| Variables | P | HR (95% CI) | P | HR (95% CI) |
| T classification | ||||
| T1 + T2 | Ref | Ref | Ref | Ref |
| T3 + T4 | 0.368 | 6.673 (0.107–415.609) | 0.189 | 6.28 (0.406–97.134) |
| Surgical margin | ||||
| R0 + R1 | Ref | Ref | Ref | Ref |
| R2 | 0.233 | 0.042 (0–7.646) | 0.969 | 1.059 (0.059–18.20) |
| Histology | ||||
| Epithelium | Ref | Ref | Ref | Ref |
| Mesenchyme | 0.976 | 0 | 0.384 | 0.255 (0.012–5.548) |
| BED | 0.053 | 0.717 (0.512–1.004) | 0.023 | 0.726 (0.551–0.957) |
| GTV, ml | 0.079 | 1.199 (0.979–1.469) | 0.104 | 1.117 (0.977–1.001) |
| CTV, ml | 0.095 | 0.946 (0.887–1.010) | 0.053 | 0.953 (0.907–1.278) |
Discussion
We analyzed 22 patients with orbital tumor after ESS followed by PRT and/or CIRT. With a median follow-up of 20.3 months, the 2-year OS, PFS, LPFS, RRFS, and DMFS rates were 100, 57.9, 92.9, 93.3, and 72.8%, respectively. No acute severe (i.e., ≥grade 3) toxicity was observed. The occurrences of severe late toxicities were also infrequent. These findings suggest that particle radiotherapy after ESS could provide satisfactory local control with acceptable toxicities at 2 years for patients with orbit tumors. However, DM remains a challenge for overall disease control.
Due to the complexity of the anatomy, ocular exenteration was advocated histologically; however, disease control remained suboptimal. In a report of 39 patients with orbital malignancies received exenteration with (10 patients) or without (29 patients) adjuvant radiation, ~ 20% experienced local recurrence after a median follow-up of 34.7 weeks. The 3-year OS and recurrence/death-free survival rates were 50.5 and 47.5%, respectively [20]. Results from multiple retrospective studies revealed that 5-year LPFS ranged at 20–22% after surgery (exenteration or eye spearing) without adjuvant radiation [21, 22]. Adjuvant radiotherapy following exenteration produced substantially improved local control as compared to surgery alone. Three- or 5-year LPFS rates of 60~65% [21, 23], with similar OS rates of 60% have been reported. In a more recently published series, adjuvant IMRT after exenteration produced a 3-year LPFS and OS rates of 91 and 70%, respectively [24].
ESS provides an important opportunity for function preservation for patients with orbital tumors. Disease control and survival rates after less aggressive (i.e., eye-sparing) surgery assimilates those from exenteration when adjuvant radiotherapy was added. In 11 lacrimal gland tumor patients treated with ESS, only 1 patient declined adjuvant radiotherapy and developed local recurrence [25]. A more recently published series of 37 patients with lacrimal gland carcinoma (> 80% with T1 or T2 diseases) reported a 2-year recurrence free survival of approximately 95%. Of the 31 patients received adjuvant radiotherapy, 12 had PRT [22]. Although the composition of patients and pathologies in our series differ substantially from the above-mentioned papers, the outcomes remain encouraging. All 22 patients were alive at the time of analysis with a 2-year LPFS rate of 92.9%, although ~ 60% of patients in our series presented with T3/T4 diseases. Furthermore, patients with lacrimal gland tumors achieved 2-year LPFS and RRFS rates of 100 and 88.9%, respectively, although ~ 40% had T4 disease. Our results mimicked the most favorable 2-year outcome in terms of survival and local control despite of a less favorable clinical presentation [21, 24, 26, 27].
With effective locoregional control, DM became the most common mode of failure in patients with orbital malignancies. DM rate of 27.5% and 3-year DMFS of 70% were reported for orbital carcinomas [23]. However, DM is more challenging for lacrimal glade cancer especially adenoid cystic carcinoma [21, 23]. Skinner et al. [21] reported a 5-year DMFS rate of 65% for lacrimal gland carcinoma patients, and found that DM was not correlated with histopathology, surgical margin or type of surgery (eye-spearing vs. exenteration). These results were in line with our finds, of which DM was seen in 30.8% of lacrimal gland patients. Furthermore, the 2-year DMFS of our entire cohort and those with lacrimal gland malignancies were 71.6 and 53%, respectively. Our univariate analysis indicated a trend for DM in patients with lacrimal gland malignancies (P = 0.072). In both the univariate and multivariate analysis, factors such as histopathology, surgical margin status, or T-classification were not associated with DMFS. However, BED may have significant impact on DMFS: The higher the BED, the lower the risk of DM. Moreover, the overall PFS, largely related to DMFS, is not only significantly associated with BED but also may correlated with CTV volume (P = 0.053). These findings suggested that particle therapy may have a significant impact on disease control due to its improved conformality secondary to its physical characteristics. Well localized and more precise dose distribution enables higher dose as well as bigger CTV with OAR sparing, a feature that is important in particle radiotherapy for most head and neck cancers [10, 11]. Furthermore, CIRT not only provide advantages in dose distribution, but also biologically due to its higher linear energy transfer (LET). The value of the RBE of carbon-ion is 2–5:1 as compared with photons and protons, which is highly relevant for radio-resistant tumors such as STS [14, 15]. Our data suggested mesenchymal malignancies of the orbit may pose a higher risk of local recurrence (p = 0.056). And our previous experience with CIRT for head and neck sarcomas revealed favorable disease control with acceptable toxicity profile [28].
In spite of the improved function preservation and locoregional disease control with ESS and adjuvant IMRT, radiation-induced toxicities remained a challenge in the management of orbital tumors due to its anatomical complexity [29, 30]. Published data indicated that with doses exceeding 50Gy, conjunctival keratinization, lacrimal gland atrophy and fibrosis, corneal decompensation would occur. When doses exceeded 60Gy, symblepharon, keratoconjunctivitis, permanent dry eyes became a concern. Moreover, the probability of radiation-induced optic neuropathy may occur in 7–20% of patients [6, 17, 31, 32]. Particle radiotherapy provides distinctive advantages for tumors close to critical OARs such as orbital malignancies due to its distinctive physical characteristics. Patterns of treatment-induced adverse effects were studied extensively in a series of 20 orbital tumor patients treated with ESS followed by PRT at M.D. Anderson Cancer Center [7]. Although disease control was not the focus of the study, the authors reported one patient with local and another with regional recurrence. In addition to the 35% patients who experienced grade 3 acute dermatitis, 30% experienced grade 3 chronic toxicities of epiphora and eyelid function disorder. In addition, grade 2, 3, and 4 visual decrease were observed in 2, 2, and 1 patient, respectively. The risk of severe chronic toxicity was higher when the maximum corneal dose exceeded 36Gy (BED) [7]. Higher BED was found to associate with improved outcome in our series, indicating the advantage of particle radiotherapy for this condition which usually occur close to dose limiting OARs. CIRT with less penumbra as compared to proton may provide additional physical advantage. In addition, favorable outcomes in terms of radiation-induced toxicities were observed in our series: Only grade 1/2 acute adverse-effects were observed in 9 patients (40.9%). Approximately 22.7% of patients developed grade 1 or 2 late effects. Nevertheless, 2 patients experienced severe decrease of vision at 3 and 6 months after CIRT. In both cases the tumors were attached or close to the optic nerve or eye.
Several pitfalls of our study need to be discussed. First, because of variations in certain institutional clinical trial regimens, few patients were treated with PRT (1 cases) or PRT + CIRT boost (3 cases) although most patients received CIRT alone. Our analysis largely reflected the results after CIRT for orbital malignancies. Second, orbital tumor includes a group of heterogenous conditions from various origins with substantial different biological behaviors. Combining different pathologies would inevitably affect the uniformity of the results. Third, owing to the limited follow up time, these clinical results must be considered with caution, we will continue to follow up these patients and report clinical outcomes with longer period. Fourth, our study suffered from the nature of retrospective studies with a relatively small sample size; nevertheless, we provided the outcomes of the largest series of orbital tumors treated with particle radiotherapy in terms of disease, control, survival, and safety. Considering the rarity of the condition, nearly all published literatures were retrospective in nature from single institutions. Clearly, prospective investigations to compare efficacies from different treatment modalities or technologies are difficult to initiate without international collaboration among specialized academic centers.
Conclusion
Adjuvant particle radiotherapy following ESS provided a satisfactory OS and locoregional control at 2 years. DM remained a major form of treatment failure. No severe acute treatment-induced toxicity was observed, and severe late toxicities was observed in < 10% of cases. Long-term follow-up is needed to confirm the efficacy and safety of adjuvant particle radiotherapy, in particular CIRT, for orbital tumor after ESS.
Acknowledgements
Not applicable.
Abbreviations
- ACC
Adenoid cystic carcinoma
- BED
Biological equivalent dose
- CBC
Complete blood count
- CIRT
Carbon-ion radiotherapy
- CR
Complete response
- CT
Computed tomography
- CTCAE
Common terminology criteria for adverse events
- CTV
Clinical target volume
- CTV-G
Post-surgical GTV
- DMFS
Distant metastasis free survival
- ESS
Eye sparing surgery
- GTV
Gross tumor volume
- GyE
Gy-equivalents
- H&P
History and physical examination
- IMPT
Intensity-modulated particle therapy
- IMRT
Intensity-modulated radiotherapy
- IRB
Institutional review board
- LET
Linear energy transfer
- LPFS
Local progression-free survival
- MRI
Magnetic resonance imaging
- OARs
Organs at risk
- OS
Overall survival
- PBS
Pencil beam scanning
- PET-CT
Positron emission tomography/computed tomography
- PFS
Progression free survival
- PRT
Proton radiotherapy
- PTV
Planning target volume
- RBE
Relative biological effectiveness
- RRFS
Regional recurrence-free survival
- SPHIC
Shanghai Proton and Heavy Ion Center
- STS
Soft-tissue sarcoma
Authors’ contributions
WH: Design of the study, data acquisition, statistical analysis, results interpretation, drafting and revising the article, final approval of the version, responsible for all aspects of the work. JH: data acquisition, data analysis, drafting and revising the article, version approval, responsible for all aspects of the work. JG: data acquisition, drafting and revising the article. JY: data acquisition, revising the article, final approval of the version, responsible for all of the work. XQ: data acquisition, revising the article, version approval, responsible for all aspects of the work. LK: Conceptualization and design of the of study, data analysis and interpretation, drafting and revising the article, funding acquisition, final approval of the version, responsible for all aspects of the work. JJL: Conceptualization and design of the study, data analysis, interpretation of the results, drafting and revising the article. All authors approved the submitted version, agreement to be accountable for all aspects of the work.
Funding
This work was supported by Shanghai Municipal Commission of Health and Family Planning (Project No. 20164Y0155), Pudong New Area Science and Technology Development Foundation (Project No. PKJ2016-Y41). The funders had no role in design of the study, in the data collection, analysis, or interpretation of data, in the decision to publish, or in the writing of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This retrospective study was approved by the Ethics Committee of Shanghai Proton and Heavy Ion Center. Written informed consent was obtained for each participant before enrolling in this study.
Consent for publication
Written informed consent for publication was obtained for each patient before enrolling in this study.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weixu Hu, Email: weixu.hu@sphic.org.cn.
Jiyi Hu, Email: jiyi.hu@sphic.org.cn.
Jing Gao, Email: jing.gao@sphic.org.cn.
Jing Yang, Email: jing.yang@sphic.org.cn.
Xianxin Qiu, Email: xianxin.qiu@sphic.org.cn.
Lin Kong, Phone: +86-21-38296518, Email: lin.kong@sphic.org.cn.
Jiade J. Lu, Phone: +86-21-38296518, Email: jiade.lu@sphic.org.cn
References
- 1.Hassan WM, Bakry MS, Hassan HM, Alfaar AS. Incidence of orbital, conjunctival and lacrimal gland malignant tumors in USA from surveillance, epidemiology and end results, 1973-2009. Int J Ophthalmol. 2016;9(12):1808–1813. doi: 10.18240/ijo.2016.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallen-St Clair J, Arshi A, Tajudeen B, Abemayor E, St John M. Epidemiology and treatment of lacrimal gland tumors: a population-based cohort analysis. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1110–1116. doi: 10.1001/jamaoto.2014.2846. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal DI, Chambers MS, Fuller CD, Rebueno NC, Garcia J, Kies MS, Morrison WH, Ang KK, Garden AS. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishna Y, Coupland SE. Lacrimal sac tumors--a review. Asia Pac J Ophthalmol (Phila) 2017;6(2):173–178. doi: 10.22608/APO.201713. [DOI] [PubMed] [Google Scholar]
- 5.Sakaida H, Kobayashi M, Yuta A, Imanishi Y, Majima Y. Squamous cell carcinoma of the nasolacrimal duct. Eur Arch Otorhinolaryngol. 2009;266(3):455–458. doi: 10.1007/s00405-008-0677-x. [DOI] [PubMed] [Google Scholar]
- 6.Karcioglu ZA, Hadjistilianou D, Rozans M, DeFrancesco S. Orbital rhabdomyosarcoma. Cancer Control. 2004;11(5):328–333. doi: 10.1177/107327480401100507. [DOI] [PubMed] [Google Scholar]
- 7.Holliday EB, Esmaeli B, Pinckard J, Garden AS, Rosenthal DI, Morrison WH, Kies MS, Gunn GB, Fuller CD, Phan J, et al. A multidisciplinary orbit-sparing treatment approach that includes proton therapy for epithelial tumors of the orbit and ocular adnexa. Int J Radiat Oncol Biol Phys. 2016;95(1):344–352. doi: 10.1016/j.ijrobp.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan FM: The physics of radiation therapy, fourth edn: Lippincott Williams & Wilkins; 2009.
- 9.van de Water TA, Bijl HP, Schilstra C, Pijls-Johannesma M, Langendijk JA. The potential benefit of radiotherapy with protons in head and neck cancer with respect to normal tissue sparing: a systematic review of literature. Oncologist. 2011;16(3):366–377. doi: 10.1634/theoncologist.2010-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank SJ, Cox JD, Gillin M, Rosenthal DI, Garden AS, Ang KK, Mohan R, Palmer MB, Amin M, Zhu XR. Intensity modulated proton therapy for head-and-neck cancer: the first clinical experience. Int J Radiat Oncol Biol Phys. 2012;84:S475–S476. doi: 10.1016/j.ijrobp.2012.07.1260. [DOI] [Google Scholar]
- 11.Frank SJ, Cox JD, Gillin M, Mohan R, Garden AS, Rosenthal DI, Gunn GB, Weber RS, Kies MS, Lewin JS, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys. 2014;89(4):846–853. doi: 10.1016/j.ijrobp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujii H, Kamada T, Baba M, Tsuji H, Kato H, Kato S, Yamada S, Yasuda S, Yanagi T, Kato H, et al. Clinical advantages of carbon-ion radiotherapy. New J Phys. 2008;10:075009. doi: 10.1088/1367-2630/10/7/075009. [DOI] [Google Scholar]
- 13.Kanai T, Endo M, Minohara S, Miyahara N, Koyama-ito H, Tomura H, Matsufuji N, Futami Y, Fukumura A, Hiraoka T, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys. 1999;44(1):201–210. doi: 10.1016/S0360-3016(98)00544-6. [DOI] [PubMed] [Google Scholar]
- 14.Elsasser T, Kramer M, Scholz M. Accuracy of the local effect model for the prediction of biologic effects of carbon ion beams in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2008;71(3):866–872. doi: 10.1016/j.ijrobp.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Jones B. A simpler energy transfer efficiency model to predict relative biological effect for protons and heavier ions. Front Oncol. 2015;5:184. doi: 10.3389/fonc.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu JD, Ye M, Guo Q, Fu JF, Moyers XM, Zhang ST, Mao NY, Kong YS, Hsi X, Shahnazi XY, et al. The preliminary report of a registration clinical trial of proton and heavy ion irradiation. Zhonghua Zhong Liu Za Zhi. 2018;40(1):52–56. doi: 10.3760/cma.j.issn.0253-3766.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-Y. [DOI] [PubMed] [Google Scholar]
- 18.Koto M, et al. Skull base and upper cervical spine tumors. In: Tsujii H, Kamada T, Shirai T, et al., editors. In Carbon-Ion Radiotherapy Principles, Practices, and Treatment Planning. Heidelberg: Springer; 2014. pp. 155–161. [Google Scholar]
- 19.Nieder C, Milas L, Ang KK. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10(3):200–209. doi: 10.1053/srao.2000.6593. [DOI] [PubMed] [Google Scholar]
- 20.Aryasit O, Preechawai P, Hirunpat C, Horatanaruang O, Singha P. Factors related to survival outcomes following orbital exenteration: a retrospective, comparative, case series. BMC Ophthalmol. 2018;18(1):186. doi: 10.1186/s12886-018-0850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner HD, Garden AS, Rosenthal DI, Ang KK, Morrison WH, Esmaeli B, Pinnix CC, Frank SJ. Outcomes of malignant tumors of the lacrimal apparatus: the University of Texas MD Anderson Cancer Center experience. Cancer. 2011;117(12):2801–2810. doi: 10.1002/cncr.25813. [DOI] [PubMed] [Google Scholar]
- 22.Woo KI, Sagiv O, Han J, Frank SJ, Kim YD, Esmaeli B. Eye-preserving surgery followed by adjuvant radiotherapy for lacrimal gland carcinoma: outcomes in 37 patients. Ophthalmic Plast Reconstr Surg. 2018;34(6):570–574. doi: 10.1097/IOP.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 23.Esmaeli B, Ahmadi MA, Youssef A, Diba R, Amato M, Myers JN, Kies M, El-Naggar A. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 2004;20(1):22–26. doi: 10.1097/01.IOP.0000105518.72611.4F. [DOI] [PubMed] [Google Scholar]
- 24.Tao R, Ma D, Takiar V, Frank SJ, Fuller CD, Gunn GB, Beadle BM, Morrison WH, Rosenthal DI, Edson MA, et al. Orbital carcinomas treated with adjuvant intensity-modulated radiation therapy. Head Neck. 2016;38(Suppl 1):E580–E587. doi: 10.1002/hed.24044. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeli B, Yin VT, Hanna EY, Kies MS, William WN, Jr, Bell D, Frank SJ. Eye-sparing multidisciplinary approach for the management of lacrimal gland carcinoma. Head Neck. 2016;38(8):1258–1262. doi: 10.1002/hed.24433. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad SM, Esmaeli B, Williams M, Nguyen J, Fay A, Woog J, Selvadurai D, Rootman J, Weis E, Selva D, et al. American joint committee on Cancer classification predicts outcome of patients with lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2009;116(6):1210–1215. doi: 10.1016/j.ophtha.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Woo KI, Yeom A, Esmaeli B. Management of Lacrimal Gland Carcinoma: lessons from the literature in the past 40 years. Ophthalmic Plast Reconstr Surg. 2016;32(1):1–10. doi: 10.1097/IOP.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Gao J, Wu X, Hu J, Hu W, Kong L, Lu JJ. Salvage carbon ion radiation therapy for locally recurrent or radiation-induced second primary sarcoma of the head and neck. J Cancer. 2018;9(12):2215–2223. doi: 10.7150/jca.24313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(3):650–659. doi: 10.1016/j.ijrobp.2010.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Petsuksiri J, Frank SJ, Garden AS, Ang KK, Morrison WH, Chao KS, Rosenthal DI, Schwartz DL, Ahamad A, Esmaeli B. Outcomes after radiotherapy for squamous cell carcinoma of the eyelid. Cancer. 2008;112(1):111–118. doi: 10.1002/cncr.23143. [DOI] [PubMed] [Google Scholar]
- 31.Gordon KB, Char DH, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiat Oncol Biol Phys. 1995;31(5):1123–1139. doi: 10.1016/0360-3016(95)00062-4. [DOI] [PubMed] [Google Scholar]
- 32.Batth SS, Sreeraman R, Dienes E, Beckett LA, Daly ME, Cui J, Mathai M, Purdy JA, Chen AM. Clinical-dosimetric relationship between lacrimal gland dose and ocular toxicity after intensity-modulated radiotherapy for sinonasal tumours. Br J Radiol. 2013;86(1032):20130459. doi: 10.1259/bjr.20130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.