To the Editor:
Primary graft dysfunction (PGD), a form of severe acute lung injury, is the main cause of early morbidity and mortality after lung transplantation. Pulmonary hypertension (PH), either WHO group I or III, is a significant risk factor for PGD (1). We previously demonstrated that genetic variation in long pentraxin-3 (PTX3), a phylogenetically conserved acute phase reactant that modulates inflammatory responses, as well as elevated post-transplant plasma PTX3 levels were associated with an increased risk of PGD after lung transplantation(2, 3). A recent study demonstrated elevated plasma PTX3 levels in patients with PAH. Furthermore, PAH-directed therapies resulted in decreased plasma PTX3 levels(4). We therefore performed a cohort study to evaluate the impact of pre-transplant plasma PTX3 levels on the relationship between pre-existing PH and PGD after lung transplantation.
The cohort included WHO Group I and III PH patients enrolled in the prospective, multicenter Lung Transplant Outcomes Group study between 2003 and 2010. We collected clinical data and plasma samples from subjects prior to lung transplantation. The Institutional Review Boards at each site approved our study and informed consent was obtained from each subject.
As an exposure variable for PGD risk, mean pulmonary arterial pressure (mPAP) was evaluated as a continuous variable measured at the time of right heart catheterization at the outset of lung transplantation or at the time of lung transplant evaluation. PGD was defined as grade 3 PGD at 48 or 72 hours after transplantation as previously described (1). Plasma PTX3 levels were measured prior to lung transplantation using a commercially available ELISA (R & D Systems, Inc., Minneapolis, MN). Laboratory personnel were blinded to PGD and PH status.
Subject characteristics were compared using chi-square and t-test based on distributions. We evaluated the impact of pre-transplant PTX3 level on the association of PGD with PH by performing stratified logistic regression analysis, with subgroups defined by pre-transplant PTX3 level. Subgroups for logistic regression were defined as high or low PTX3 plasma level, with a threshold of 7.22 ng/ml (75% percentile for PTX3 levels in the cohort) chosen based on the distribution of PTX3 levels in the overall cohort and to ensure adequate sample sizes in the subgroups. Formal testing for interaction (differences in the magnitude of the association of PH with PGD based on PTX3 level) was also performed using logistic regression, although the study was not powered for such testing. A p-value<0.05 was considered statistically significant and STATA 14 (STATA Corp., College Station, TX) was used for all analyses.
The cohort consisted of 162 subjects who underwent lung transplantation, of whom 18 (11%) developed PGD (Table 1). Patients with PGD had significantly higher mPAP (36.5mmHg vs. 26.6mmHg, p=0.008) and a higher prevalence of at least moderate PH (mPAP≥40 mmHg) (33% vs. 9%, p=0.003). There were no significant differences in the overall distribution of pre-transplant pulmonary diagnosis based on PGD status (p=0.2). There was no significant difference in the median pre-transplant plasma concentration of PTX3 when comparing PGD to non-PGD subjects (3.0 ng/ml vs. 2.7 ng/ml, p=0.9). The result was similar when excluding the 6 subjects with PAH (p=0.8). There were differences in PTX3 median level based on pre-transplant diagnosis: COPD – 1.99 ng/ml, ILD 3.02 ng/ml, and CF, 4.08 ng/ml (p=0.03).
Table 1:
Patient demographics according to grade 3 PGD status at day 2 or day 3 post-transplantation
| Recipient Characteristics | Non-PGD (n=144) | PGD (n=18) | p-value |
|---|---|---|---|
| Male Gender, n, (%) | 78 (54) | 8 (44) | 0.4 |
| Age, years, mean | 52 | 57 | 0.1 |
| Race, n (%) | 0.5 | ||
| Caucasian | 116 (81) | 13 (72) | |
| Black | 13 (9) | 4 (22) | |
| Other | 14 (10) | 1 (6) | |
| Pulmonary Diagnosis, n (%) | 0.2 | ||
| Chronic Obstructive Pulmonary Disease | 44 (31) | 4 (22) | |
| Pulmonary Fibrosis | 51 (35) | 10 (56) | |
| Cystic Fibrosis/Bronchiectasis | 30 (21 | 0 (0) | |
| Pulmonary Arterial Hypertension | 5 (4) | 1 (6) | |
| Other | 14 (9) | 3 (17) | |
| Cardiopulmonary Bypass, yes | 47 (33) | 8 (44) | 0.3 |
| Bilateral Transplant Procedure, n (%) | 108 (75) | 10 (59) | 0.2 |
| Pulmonary Arterial Pressure, mean mmHg | 26.6 | 36.5 | 0.008 |
| Mean Pulmonary Artery Pressure ≥ 40 mmHg, n (%) | 13 (9) | 6 (33) | 0.003 |
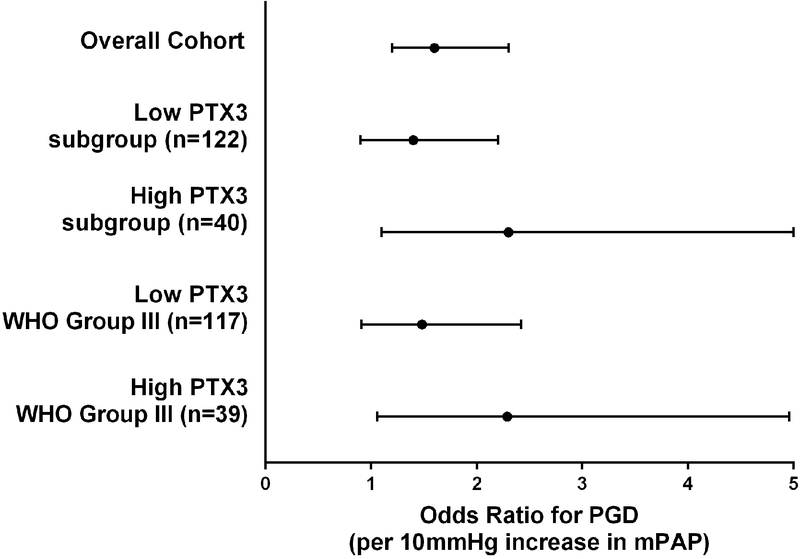
We evaluated the impact of pre-transplant PTX3 concentrations on the association of PH with PGD (Figure 1). In the overall cohort, mPAP was strongly associated with PGD (OR per 10mmHg=1.6, 95%CI 1.2–2.3, p=0.003). We then stratified the cohort into high and low PTX3 level strata (cutoff=7.22 ng/ml). Among patients with low PTX3 level prior to transplant (n=122), mPAP was not significantly associated with PGD (OR per 10mmHg=1.4, 95%CI 0.9–2.2, p=0.09). Among patients with high PTX3 level prior to transplant (n=40), there was a strong association between mPAP with PGD (OR per 10mmHg=2.3, 95%CI 1.1, 5.0), p=0.04). These findings were unchanged if the 6 patients with PAH are removed or, given the differences in PTX3 level by diagnosis, if underlying pulmonary diagnosis was added to the regression model. The p-value for interaction was 0.3.
Figure 1:
Odds ratio (OR) for PGD per 10 mmHg increase in mean pulmonary arterial pressure. The dot represents the point estimate and the error bars the 95% confidence interval. The dashed line represents an OR=1. The results in the overall cohort are presented first (n=161). Second, the subgroup of patients with PTX3 plasma level <7.22 ng/ml (n=122) is presented. Third, the subgroup of patients with PTX3 plasma level ≥7.22 ng/ml (n=40) is presented. Fourth, the subgroup of WHO pulmonary hypertension Group III patients with PTX3 plasma level <7.22 ng/ml (n=117) is presented. Finally, the subgroup of WHO pulmonary hypertension Group III patients with PTX3 plasma level ≥7.22 ng/ml (n=39) is presented.
In this cohort of patients with advanced lung disease undergoing lung transplantation, we identified that PH in the setting of higher pre-transplant PTX3 plasma level had a particularly elevated risk of PGD compared to the overall population of patients.
Higher basal levels of PTX3 seemed to identify patients with PH at the highest risk of PGD after lung transplantation. Higher PTX3 levels have been associated with increased RV mass and RV end diastolic pressure (5), and may therefore identify patients with more clinically significant PH who are more at risk for PGD. We have previously shown that, irrespective of underlying lung disease, moderate (mPAP≥40 mmHg, <55 mmHg) and severe PH (mPAP≥55 mmHg) were independently associated with a 7% and 18% absolute increased risk of PGD, respectively, when compared to patients without PH (1). Significant efforts have been devoted to the development of clinically useful predictive algorithms for PGD based on data available prior to organ allocation, including pulmonary pressures. Incorporating basal protein biomarkers, such as PTX plasma concentrations, into future clinical predictive models will be the focus of future investigations.
In conclusion, in this preliminary, exploratory study, pre-transplant PTX3 plasma level may impact the relationship of PH with PGD, with higher PTX3 plasma concentrations significantly magnifying the association of PH with PGD. PTX3 is an intriguing target for PH interventions and for future incorporation into improved PGD risk predictive models.
Funding/Support:
This study was supported by the National Institutes of Health [Grants: T32 HL007891, K24 HL103844, R01 HL087115, R01 HL081619, R01 HL096845, K23: K23 HL121406] and the Actelion Entelligence Young Investigator Grant.
References:
- 1.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, Bhorade SM, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah AS, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Ware LB, Palmer SM, Christie JD, Lung Transplant Outcomes G. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond JM, Akimova T, Kazi A, Shah RJ, Cantu E, Feng R, Levine MH, Kawut SM, Meyer NJ, Lee JC, Hancock WW, Aplenc R, Ware LB, Palmer SM, Bhorade S, Lama VN, Weinacker A, Orens J, Wille K, Crespo M, Lederer DJ, Arcasoy S, Demissie E, Christie JD, Lung Transplant Outcomes G. Genetic variation in the prostaglandin e2 pathway is associated with primary graft dysfunction. Am J Respir Crit Care Med 2014;189:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diamond JM, Lederer DJ, Kawut SM, Lee J, Ahya VN, Bellamy S, Palmer SM, Lama VN, Bhorade S, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah PD, Weinacker A, Weill D, Kohl BA, Deutschman CC, Arcasoy S, Shah AS, Belperio JA, Wilkes D, Reynolds JM, Ware LB, Christie JD. Elevated plasma long pentraxin-3 levels and primary graft dysfunction after lung transplantation for idiopathic pulmonary fibrosis. Am J Transplant 2011;11:2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura Y, Ono T, Kuwana M, Inoue K, Takei M, Yamamoto T, Kawakami T, Fujita J, Kataoka M, Kimura K, Sano M, Daida H, Satoh T, Fukuda K. Human pentraxin 3 (ptx3) as a novel biomarker for the diagnosis of pulmonary arterial hypertension. PloS one 2012;7:e45834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leary PJ, Jenny NS, Barr RG, Bluemke DA, Harhay MO, Heckbert SR, Kronmal RA, Lima JA, Mikacenic C, Tracy RP, Kawut SM. Pentraxin-3 and the right ventricle: The multi-ethnic study of atherosclerosis-right ventricle study. Pulmonary circulation 2014;4:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]