Abstract
Heterocyclic compounds are inevitable in a numerous part of life sciences. These molecules perform various noteworthy functions in nature, medication and innovation. Nitrogen-containing heterocycles exceptionally azoles family are the matter of interest in synthesis attributable to the way that they happen pervasively in pharmacologically dynamic natural products, multipurpose arranged useful materials also profoundly powerful pharmaceuticals and agrochemicals. Benzimidazole moiety is the key building block for several heterocyclic scaffolds that play central role in the biologically functioning of essential molecules. They are considered as promising class of bioactive scaffolds encompassing diverse varieties of activities like antiprotozoal, antihelminthic, antimalarial, antiviral, anti-inflammatory, antimicrobial, anti-mycobacterial and antiparasitic. Therefore in the present review we tried to compile the various pharmacological activities of different derivatives of heterocyclic benzimidazole moiety.
Keywords: Benzimidazole derivatives, Antiprotozoal activity, Anti-inflammatory activity, Antimalarial activity, Antimycobacterial activity, Antiviral activity, Anticancer activity
Introduction
Among heterocyclic pharmacophores, the benzimidazole ring system is quite common. These substructures are often called ‘privileged’ due to their wide recurrence in bioactive compounds [1]. Benzimidazole moiety is a fusion of benzene and imidazole ring system at the 4 and 5 positions of imidazole ring. They have properties of both acids and bases. The NH group here is highly acidic and also feebly basic. Another feature of it is that they comprise the ability to form salts. The benzimidazole moiety is useful for the development of novel medicinal compounds in pharmaceutical field. Benzimidazole is also a vital pharmacophore, a privileged sub-structure in medicinal chemistry which contributes as a key part for different natural activities [2].
Pharmacological significance of benzimidazole derivatives
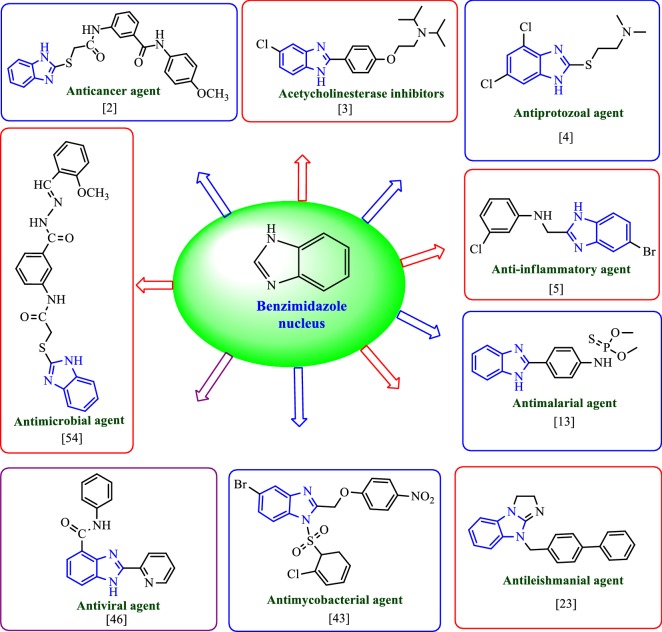
Literature survey reveals that the various derivatives of benzimidazole have been synthesized for their pharmacological activities such as antimicrobial [3], anticancer [2], acetylcholinesterase [4], antiprotozoal [5], anti-inflammatory [6], analgesic [7], antihistaminic [8], antimalarial [9], antitubercular [10], anti-HIV [11] and antiviral [12]. Some of the already synthesized compounds from the above mentioned field have found very strong application in medicine praxis. The activity against bacteria, fungi and helminthes resulted their mode of action, which resulted in the blockage of microtubule in various nematode, trematode and cystode [13]. Benzimidazole-based drugs exhibit a wide range of different biological activities as a result of changing the groups on the core structure. Some marketed drugs containing benzimidazole nucleus are shown in Fig. 1.
Fig. 1.
Some benzimidazoles containing medicinal preparation
Acetylcholinesterase (AChE) is a core chemical engaged with the ending of nerve signs via the hydrolysis of acetylcholine. It is an objective of medication advancement to battle the neuromuscular issue, for example, glaucoma, myasthenia gravis and Alzheimer’s disease (AD). AChE has been focused in the cure of AD, a dynamic neurodegenerative disease portrayed by neurofibrillary tangles, β-amyloid plaques and loss of focal cholinergic ability. A lack in cholinergic neurotransmission is viewed as one of the real reasons for reminiscence weaknesses in the patients with AD. One of the compelling methodologies for improving the cholinergic transmission is to utilize the inhibitors of acetylcholinesterase [4]. Parasitic ailments are as yet overall issues that deeply affect general wellbeing. Contaminations brought about by protozoa, for example, Trypanosoma cruzi, Plasmodium falciparum, Entamoeba histolytica, Leishmania Mexicana, Trichomonas vaginalis, Giardia intestinalis and helminth, for example, Taenia solium or Trichinella spiralis are overall spread ailments that influence predominantly immature nations, where tropical or template temperatures exist, yet in addition poor uncontaminated and cleanliness conditions are normal [14].
Irritation is a confined reaction of body tissues to destructive incentives or injures bringing about the arrangement of protein-rich exudates. It is a defensive reaction of the nonspecific resistant framework that expels the essential driver of cell damage; eradicate necrotic cells and tissues harmed from the incendiary procedure and commence tissue repair. The essential indications of aggravation are redness, heat, torment, swelling and loss of capacity. Reason for aggravation is physical as well chemical means, immunological responses and contamination by pathogenic life form. Aggravation can be assigned as acute and chronic. Acute irritation is described by the exudation of liquid and plasma proteins (oedema) and the development of leukocytes, particularly neutrophils. Chronic irritation is otherwise called constant aggravation, in which tissue destruction and recovering are continuing all the while, for example, tuberculosis, rheumatoid joint inflammation, constant lung infections and atherosclerosis [6].
Mosquitoes are one of the deadliest creepy crawlies in earth which generate biting irritation and also transmit lethal infections, for example, intestinal sickness, yellow fever, filariasis, chikungunya, encephalitis and dengue. Mosquitoes in the class Aedes are liable for the transmission of chikungunya, dengue, yellow fever and other pathogenic arbo-infections. Likewise, the prime vector for lymphatic filariasis is Culex quinquefasciatus, as well called southern house mosquito. Cx. quinquefasciatus ordinarily stay around human lodging and on maturing like to nibble people than different warm blooded creatures. Intestinal sickness is a mosquito-borne infectious ailment which is mostly transmitted by a contaminated female Anopheles mosquito [15].
Tuberculosis (TB), which is caused prevalently by Mycobacterium tuberculosis (Mtb), is the main source of death from a reparable irresistible ailment, and has been recognized by the World Health Organization (WHO) as one of the three need illnesses for medication innovative work [16]. Viral hemorrhagic fever is a genuine sickness portrayed by broad vascular harm and draining diathesis, fever and various organ inclusions. Various infections can cause this disorder, each with its very own creature repository, method of transmission, mortality rate, and clinical result in people [17].
Worldwide infectious disease figures have attained an alarming level following the proliferation of Gram-positive and Gram-negative multi-drug-resistant species. Patient non-compliance and the occurrence of multidrug-resistant pathogens often interfere innovative infection therapies that depend on a sustained multidrug course. Rational drug design has been shown to be very beneficial in this respect, since the biochemical basis of intrinsic and acquired resistance mechanisms is largely known [3].
One of the most commonly known gastrointestinal malignancies is colorectal tumor (CRC). Alterations in lifestyle, elevated-fat diet, physiological disillusionment and smoking are associated to pathogenesis of CRC. Approximately 25% of CRC cases were identified with early analysis metastases and at some stage of life nearly 50% of CRC patients would suffer from metastasis. The therapy results for these patients are largely unsatisfactory as normal regimens consider the possibility of homogeneous tumor mass distribution [2].
Rational designed based on literature survey of benzimidazole derivatives is shown in Fig. 2.
Fig. 2.
Rational designed based on literature survey of benzimidazole derivatives
Reported pharmacological activities of benzimidazole derivatives
Acetyl cholinesterase inhibitory
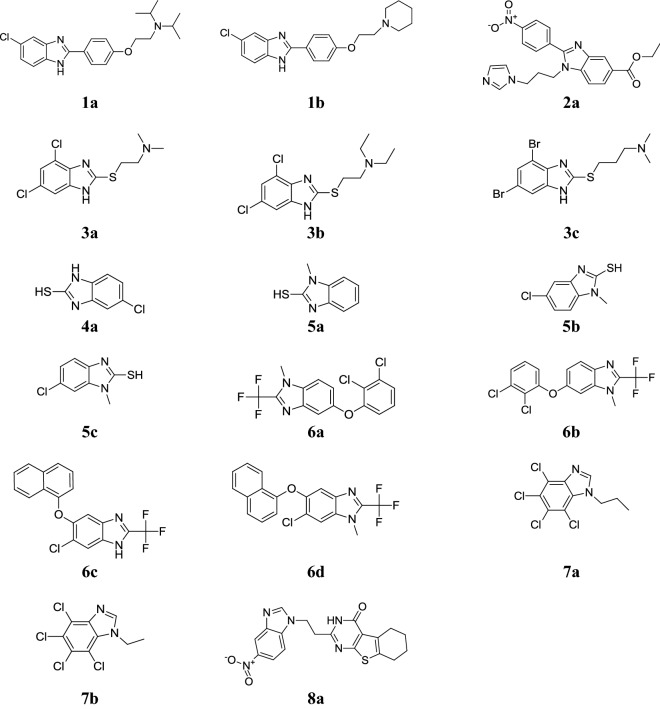
Alpan et al. designed a class of N-{2-[4-(1H-benzimidazole-2-yl)phenoxy]ethyl} substituted amines and evaluated for its butyrylcholinesterase and acetylcholinesterase inhibitor activity. Among the synthesized derivatives, compounds 1a and 1b were found to be the most active against eeAChE and hAChE using tacrine as standard drug (Table 1, Fig. 3) [4].
Table 1.
In vitro inhibition of AChE/BuChE of compounds (1a and 1b)
| Comp. | IC50 ± SEM (µM) | ||
|---|---|---|---|
| eeAChE | hAChE | BchE | |
| 1a | 0.58 ± 0.06 | 3.68 ± 0.39 | 7.44 ± 1.51 |
| 1b | 0.61 ± 0.07 | 0.13 ± 0.03 | > 100 |
| Tacrine | 0.075 ± 0.02 | 0.52 ± 0.09 | 0.0098 ± 0.0002 |
Fig. 3.
Molecular structures of compounds (1a–1b, 2a, 3a–3c, 4a, 5a–5c, 6a–6d, 7a–7b, 8a)
Yoon et al. synthesized a class of benzimidazoles and screened for its acetylcholinesterase and butyrylcholinesterase inhibitor activity. Compound 2a (Fig. 3) showed promising inhibitory activity with IC50 = 5.12 µM for BChE and IC50 = 8.63 µM for AChE using rivastigmine and donepezil (22.00, 7.95 µM for BChE and 50.20, 0.03 µM for AChE) as standard [18].
Antiprotozoal activity
Andrzejewska et al. synthesized two series of S-substituted 4,6-dihalogeno-2-mercapto-1H-benzimidazoles and assessed for their in vitro antiprotozoal potential towards G. intestinalis and T. vaginalis using albendazole and metronidazole as standard. Among them, compounds 3a, 3b and 3c were found to be most potent and comparable to standard drugs (Table 2, Fig. 3) [5].
Table 2.
Antiprotozoal activity of benzimidazole compounds (3a–3c)
| Comp. | IC50 µg/mL | |
|---|---|---|
| Giardia intestinalis | Trichomonas vaginalis | |
| 3a | 0.006 | 0.021 |
| 3b | 0.006 | 0.013 |
| 3c | 0.008 | 0.004 |
| Albendazole | 0.010 | 0.422 |
| Metronidazole | 0.210 | 0.037 |
Diaz-Chiguer et al. prepared a new series of benzimidazole derivatives and evaluated in vitro (via the % of lysis of bloodstream) and in vivo for its trypanocidal activity against of Trypanosoma cruzi (NINOA and INC5). In this series, compound 4a showed significant in vitro and in vivo [INC5: 68.4 (% lysis); NINOA: 46.4 (% lysis)] trypanocidal activity (Table 3, Fig. 3) [19].
Table 3.
In vitro trypanocidal activity of synthesized compound 4a
| Comp. | Trypanosoma cruzi | ||||
|---|---|---|---|---|---|
| LC50 (mM) | CC50 (mM) | Selectivity index (SI) | |||
| INC5 | NINOA | INC5 | NINOA | ||
| 4a | 0.32 | 0.014 | 43.2 | 135 | 3085.71 |
| Nifurtimox | 0.69 | 0.78 | 25.4 | 36.81 | 32.56 |
| Benznidazole | 0.31 | 0.60 | 23.6 | 76.13 | 39.3 |
Hernandez-Covarrubias et al. reported a class of benzimidazoles and evaluated for its antiprotozoal activity against G. duodenalis. All the tested compounds were found to be more active than standard metronidazole but the better activity observed with SH group compounds 5a–5c (Fig. 3) (IC50 = 18–45 µM) which exhibited considerable activity as compared to metronidazole (IC50 = 1.22 µM) [20].
Hernandez-Luis et al. synthesized a series of 2-(trifluoromethyl)-1H-benzimidazole molecules and assessed in vitro for its antiparasitic activity towards various protozoan parasites: G. intestinalis (GI), T. vaginalis (TV) E. histolytica (EH) and L. mexicana (LM) using albendazole (ABZ), mebendazole (MBZ), pentamidine as standard drugs and in vivo towards Trichinella spiralis (TS) using albendazole (ABZ), triclabendazole (TBZ) and pentamidine as standard drugs. In this class, compounds 6a, 6b and 6c exhibited good antiparasitic activity and in addition, compound 6a and 6c showed good activity against T. spiralis at adult phase and 6d possessed the good antiprotozoal potential against the muscle larvae stage (Tables 4 and 5, Fig. 3) [14].
Table 4.
In vitro antiprotozoal and anthelmintic screening results
| Comp. | Microbial strains (IC50 µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| GI | EH | TV | LM | TS (% reduction, 0.18 µM) | TS (% reduction, 0.37 µM) | TS (% reduction, 1.80 µM) | |
| 6a | 0.030 | 0.009 | 0.016 | 24.00 | 54 ± 2 | 62 ± 2 | 80 ± 3 |
| 6b | 0.063 | 0.019 | 0.110 | 4.10 | 44 ± 2 | 48 ± 3 | 67 ± 2 |
| 6c | 0.005 | 0.019 | 0.086 | 13.78 | 43 ± 3 | 50 ± 2 | 65 ± 3 |
| ABZ | 0.037 | 56.6 | 1.592 | a | 58.6 ± 2 | 61.9 ± 3 | 67 ± 6 |
| MTZ | 1.228 | 0.350 | 0.216 | b | b | b | b |
| Pentamidine | b | b | b | 2.421 | b | b | b |
aNo effect
bNot determined
Table 5.
Percentage of adult and muscle larvae load reduction in T. spiralis
| Comp. | Adult phase | Muscle larvae stage | |
|---|---|---|---|
| 50 mg/kg | 75 mg/kg | 75 mg/kg | |
| 6a | a | 58 | 46 |
| 6c | 69 | 80 | 40 |
| 6d | b | 36 | 64 |
| ABZ | 62 | 73 | 63 |
| MTZ | 41 | 7 | 25 |
| Alpha | 28 | a | 24 |
| Control | 0 | 0 | 0 |
aNot determined
bNo reduction
Kopanska et al. reported a series of 1H-benzimidazole analogues and assessed for its in vitro antiprotozoal activity against Acanthamoeba castellanii and compared with chlorhexidine as reference. The screening results indicated that compounds 7a and 7b were found most efficient in reducing the figure of trophozoites and cysts (Table 6, Fig. 3) [21].
Table 6.
Reduction in viability of A. castellanii trophozoites and cysts
| Comp. | Concentrations [µmol/L] | % of survivors | Percentage content of particular stages | |||
|---|---|---|---|---|---|---|
| Trophozoites | Cysts | Total | Trophozoites | Cysts | ||
| 7a | 5.5 | 23.3 ± 2.0 | 15.0 ± 2.3 | 22.5 ± 2.0 | 93.4 ± 8.0 | 6.6 ± 1.0 |
| 11.1 | 41.2 ± 2.8 | 76.0 ± 9.7 | 44.5 ± 3.5 | 83.6 ± 5.7 | 16.4 ± 2.1 | |
| 7b | 5.2 | 26.5 ± 2.3 | 19.0 ± 3.4 | 25.8 ± 2.4 | 92.7 ± 7.9 | 7.3 ± 1.3 |
| 7.9 | 22.0 ± 1.8 | 121.0 ± 12.6 | 31.6 ± 2.9 | 62.5 ± 5.2 | 37.5 ± 3.9 | |
| Chlorohexidine | 4.4 | 23.4 ± 0.7 | 11.0 ± 1.6 | 22.3 ± 0.8 | 95.3 ± 2.9 | 4.7 ± 0.7 |
| 11.0 | 24.2 ± 1.1 | 31.0 ± 4.8 | 24.8 ± 2.6 | 88.4 ± 3.9 | 11.6 ± 1.8 | |
Mavrova et al. synthesized novel derivatives of thieno[2,3-d]pyrimidin-4(3H)-ones and screened for their in vitro antiparasitic activity against Trichinella spiralis using albendazole (as standard drug). Among them, compound 8a showed good antiparasitic activity. The significance results of the active compound shown in Table 7 and Fig. 3 [22].
Table 7.
Antihelmintic activity of compound 8a against Trichinella spiralis
| Comp. | Efficacy (%) after 24 h | Efficacy (%) after 48 h |
|---|---|---|
| 8a | 95.05 | 95.05 |
| Albendazole | 10.6 | 14.8 |
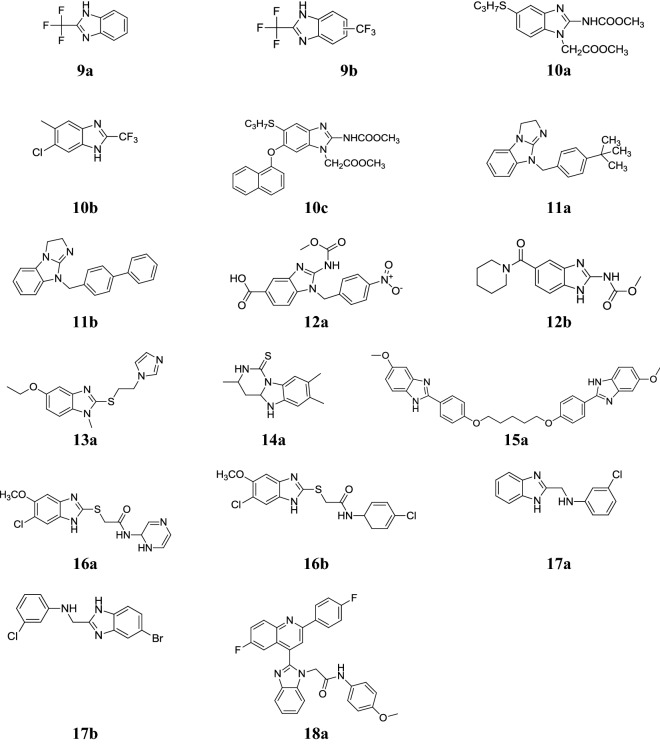
Navarrete-Vazquez et al. synthesized a sequence of 2-(trifluoromethyl)-1H-benzimidazoles along with various bioisosteric substituents at 5- and 6-position (–Cl, –F, –CF3, –CN) and examined for its in vitro antiprotozoal activity towards the protozoa T. vaginalis and G. intestinalis using metronidazole and albendazole as reference. In this series, compound 9a showed most promising activity than metronidazole against G. intestinalis and compound 9b found more active against T. vaginalis than the reference drugs. The compound 9b as well displayed modest antimalarial activity against D6 and W2 strains of Plasmodium falciparum (Table 8, Fig. 4) [23].
Table 8.
IC50 (μM) of synthesized compounds (9a and 9b)
| Comp. | G. intestinalis | T. vaginalis | P. falciparum | |
|---|---|---|---|---|
| D6 | W2 | |||
| 9a | 0.107 ± 0.017 | 3.314 ± 0.130 | > 20 | > 20 |
| 9b | 0.672 ± 0.020 | 0.232 ± 0.021 | 5.98 ± 0.25 | 6.12 ± 0.32 |
| Metronidazole | 1.226 ± 0.125 | 0.236 ± 0.016 | – | – |
| Albendazole | 0.038 ± 0.003 | 3.390 ± 0.125 | > 20 | >20 |
Fig. 4.
Molecular structures of compounds (9a–9b, 10a–10c, 11a–11b, 12a–12b, 13a, 14a, 15a, 16a–16b, 17a–17b, 18a)
Marquez-Navarro et al. developed new derivatives of benzimidazole moiety and examined for their in vivo antiprotozoal activity toward Hymenolepis nana adult and in vitro toward Toxocara canis larvae. In vitro screening results indicated that compound 10a showed significant activity toward T. canis whereas compounds 10b and 10c showed the good in vivo results against H. nana and compared to standard albendazole (Table 9, Fig. 4) [24].
Table 9.
Antihelmintic screening results
| Comp. | C log P | T. canis J2 larvae in vitro relative mobility (%) | H. nana in vivo adult reduction (%) | ||
|---|---|---|---|---|---|
| 0.18 µM | 1.8 µM | 18 µM | 50 mg/kg | ||
| 10a | 3.23 | 40 | 40 | 30 | – |
| 10b | – | – | – | – | 97 |
| 10c | – | – | – | – | 96 |
| Albendazole | 3.01 | 80 | 40 | 40 | 83 |
Oh et al. synthesized a novel class of 2,3-dihydroimidazo[1,2-a]benzimidazole and screened for its anti-leishmanial and anti-trypanosomal activities towards Leishmania donovani and Trypanosoma cruzi using miltefosine, benznidazole and amphotericin B as standard. Compounds 11a and 11b showed promising antiprotozoal activity (Tables 10 and 11, Fig. 4) [25].
Table 10.
In vitro anti-leishmanial screening results
| Comp. | Leishmania donovani | |||
|---|---|---|---|---|
| Amastigote form | Promastigote form | |||
| EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | |
| 11a | 3.05 | > 50 | > 16.4 | 1.25 |
| 11b | 5.29 | 39.7 | 7.5 | 1.48 |
| Miltefosine | 4.83 | 18.9 | 3.91 | 11.1 |
| Amphotericin B | 0.25 | 7.57 | 30.2 | 0.22 |
CC50 cytotoxicity, EC50 half maximal effective concentration, SI selective index (EC50/CC50)
Table 11.
In vitro anti-trypanosomal screening results
| Comp. | Trypanosoma cruzi | ||
|---|---|---|---|
| EC50 (μM) | CC50 (μM) | SI | |
| 11a | 1.10 | 36.5 | 33.2 |
| 11b | 2.10 | 18.8 | 8.95 |
| Benznidazole | 20.7 | > 50 | > 2.42 |
Palomares-Alonso et al. developed new substituted benzimidazoles and assessed for their cysticidal activity against Taenia crassiceps cysts (ORF and WFU strain) using albendazole sulfoxide as control drug. Among them, compounds 12a and 12b displayed superior cysticidal activity (Table 12, Fig. 4) [26].
Table 12.
Cysticidal activity against T. crassiceps (ORF and WFU strains)
| Comp. | Cysts mortality (%) | |||
|---|---|---|---|---|
| ORF strain | WFU strain | |||
| 0.28 µM | 1.70 µM | 0.28 µM | 1.70 µM | |
| 12a | 41 ± 4.6 | 68 ± 7 | 22.6 ± 2.3 | 26 ± 4 |
| 12b | 37 ± 6.1 | 62 ± 8 | 6.3 ± 2.3 | 16.7 ± 3 |
| Albendazole sulfoxide | 46 ± 5 | 88 ± 7 | 25 ± 2.3 | 35 ± 2.3 |
Perez-Villanueva et al. synthesized a new class of 2-{[2-(1H-imidazol-1-yl)ethyl]-sulfanyl}-1H-benzimidazole derivatives and assessed for its in vitro antiprotozoal activity against protozoa G. intestinalis, T. vaginalis and E. histolytica using metronidazole and albendazole as standard drugs. Among them, compound 13a showed highest activity against G. intestinalis (Table 13, Fig. 4) [27].
Table 13.
Antiprotozoal screening results
| Comp. | Microbial strains IC50 (µM) | ||
|---|---|---|---|
| T. vaginalis | G. intestinalis | E. histolytica | |
| 13a | 0.0761 ± 0.0094 | 0.0083 ± 0.0023 | 0.0298 ± 0.0047 |
| Meteronidazole | 0.2360 ± 0.0160 | 1.2260 ± 0.1250 | 0.3798 ± 0.1461 |
| Albendazole | 1.5905 ± 0.0113 | 0.0370 ± 0.0030 | 56.5334 ± 18.8445 |
Sondhi et al. synthesized pyrimido[1,6-a]benzimidazoles and assessed for their in vitro antiamoebic activity by microdilution method against E. histolytica. In this series, compound 14a (Fig. 4) showed best IC50 value 1.82 µM as compared to metronidazole which showed IC50 value 1.22 µM [28].
Torres-Gomez et al. reported some benzimidazole pentamidine compounds and assessed for their in vitro antiprotozoal activity against L. Mexicana, E. histolytica, Giardia lamblia, T. vaginalis and Plasmodium berghei using pentamidine and metronidazole (as reference drugs). Among the reported compounds, compound 15a showed good activity against G. lamblia, E. histolytica, L. mexicana and T. vaginalis and comparable to standard pentamidine (Table 14, Fig. 4) [29].
Table 14.
Antiprotozoal screening results
| Comp. | Microbial strains (IC50 µ/M) | ||||
|---|---|---|---|---|---|
| T. vaginalis | G. lamblia | E. histolytica | L. mexicana | P. berghei | |
| 15a | 0.164 | 0.435 | 0.109 | 34.641 | 0.712 |
| Pentamidine | 3.815 | 4.079 | 11.801 | a | 9.568 |
| Meteronidazole | 0.286 | 1.286 | 0.771 | – | – |
– Not tested
aCell damage, due to cytophatic effect caused by pentamidine
Velazquez-Lopez et al. reported some new benzimidazole derivatives and evaluated for their in vitro antiprotozoal activity against T. cruzi epimastigotes INC-5 and NINOA using reference drug (nifurtimox). Among the synthesized compounds, compound 16a showed potent activity towards the T. cruzi epimastigote INC-5 strain while compound 16b found active against the NINOA strain and comparable to nifurtimox (Table 15, Fig. 4) [30].
Table 15.
In vitro susceptibility of bloodstream epimastigote
| Comp. | IC50 INC-5 (μM) | IC50 NINOA (μM) | CC50 (μM) |
|---|---|---|---|
| 16a | 28.672 ± 0.602 | 98.799 ± 1.990 | 134.580 ± 1.995 |
| 16b | 186.230 ± 4.103 | 56.967 ± 0.961 | 90.436 ± 1.426 |
| Nifurtimox | 50.750 ± 0.839 | 89.804 ± 1.138 | 131.503 ± 0.490 |
Anti-inflammatory activity
Achar et al. prepared a class of 2-methylaminobenzimidazole compounds and screened in vivo for its analgesic (acetic acid induced writhing in mice) and anti-inflammatory activities (carrageenan induced paw oedema in rats). Among them, compounds 17a and 17b were displayed considerable analgesic and anti-inflammatory activities in comparison to reference nimesulide (Tables 16, 17 and 18, Fig. 4) [6].
Table 16.
Analgesic screening results
| Comp. | Mean values (X ± SE) | (%) Protection |
|---|---|---|
| Control | 300 ± 1.55 | – |
| 17a | 5.6 ± 1.85 | 81.33 |
| 17b | 3.3 ± 1.66 | 89.00 |
| Nimesulide | – | 100 |
Table 17.
Anti-inflammatory screening results
| Comp. | Paw oedema thickness (mm) | |||
|---|---|---|---|---|
| 30 m (X ± SE) | Oedema Inhibition (%) |
60 m (X ± SE) | Oedema Inhibition (%) |
|
| Control | 1.3 ± 0.05 | – | 1.5 ± 0.03 | – |
| 17a | 1.1 ± 0.03 | 15.3 | 1.1 ± 0.00 | 26.6 |
| 17b | 1.2 ± 0.03 | 7.6 | 1.1 ± 0.06 | 26.6 |
| Nimesulide | 1.1 ± 0.05 | 15.3 | 1.1 ± 0.00 | 26.6 |
Table 18.
Anti-inflammatory screening results
| Comp. | Paw oedema thickness (mm) | |||
|---|---|---|---|---|
| 120 m (X ± SE) | Oedema Inhibition (%) |
180 m (X ± SE) | Oedema Inhibition (%) |
|
| Control | 1.7 ± 0.03 | – | 1.8 ± 0.03 | – |
| 17a | 1.1 ± 0.03 | 41.1 | 1.0 ± 0.03 | 44.4 |
| 17b | 1.4 ± 0.03 | 17.6 | 1.5 ± 0.05 | 16.6 |
| Nimesulide | 1.0 ± 0.00 | 41.1 | 1.1 ± 0.00 | 44.4 |
El-Feky et al. designed novel fluorinated quinoline incorporated benzimidazoles and evaluated for their in vivo anti-inflammatory activity by carrageenin induced edema bioassay method in rats using celecoxib. Among them, compound 18a demonstrated the highest anti-inflammatory activity and exhibited best binding profiles into the COX-2 binding site as compared to celecoxib. The significance result of the active compound is shown in Table 19, Fig. 4 [31].
Table 19.
Anti-inflammatory screening results
| Comp | Anti-inflammatory activity |
|---|---|
| Protection at 50 mg/kg dose (%) | |
| 18a | 55 |
| Celecoxib | 50 |
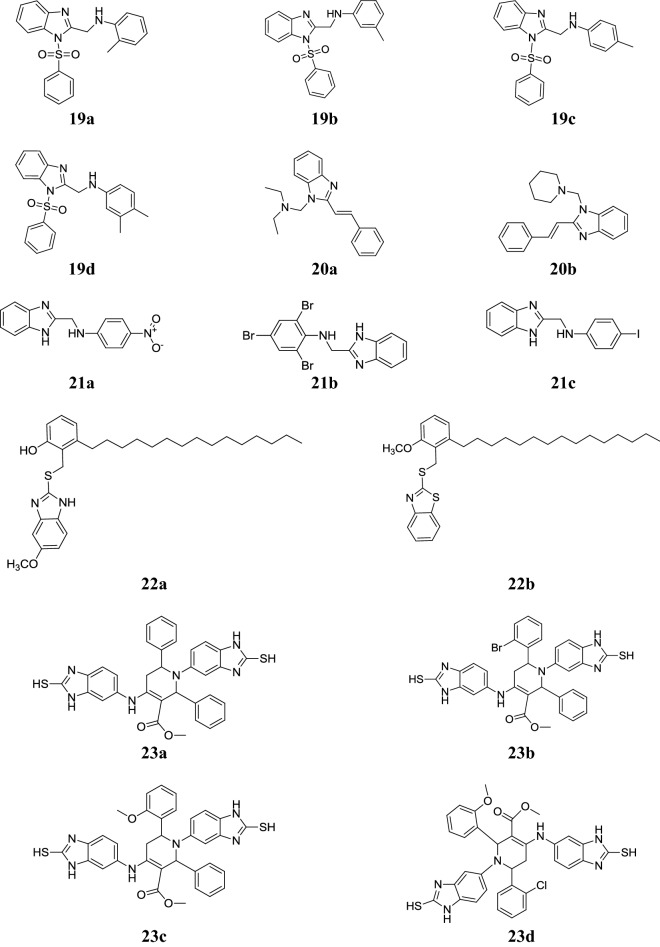
Gaba et al. reported phenylsulfonyl substituted benzimidazoles and evaluated in vivo for their anti-inflammatory activity (carrageenan-induced paw edema in rats) and analgesic activity (acetic acid-induced writhing test in mice), respectively. Among them, compounds 19a, 19b, 19c and 19d showed significant reduction in edema and compared to standard drug indomethacin and protection in the number of writhes produced by acetic acid, and comparable to the reference drug acetyl salicylic acid (Tables 20 and 21, Fig. 5) [7].
Table 20.
Anti-inflammatory screening results
| Comp. | Edema at 3 h (%, mean ± SEM) | Reduction in edema (%) |
|---|---|---|
| 19a | 68.66 ± 72.99 | 31.34 |
| 19b | 67.16 ± 73.06 | 32.84 |
| 19c | 65.67 ± 73.78 | 34.33 |
| 19d | 62.69 ± 73.27 | 37.31 |
| Control | 100.00 ± 73.59 | 0.00 |
| Indomethacin | 52.23 ± 74.27 | 47.76 |
Table 21.
Analgesic screening results
| Comp. | No. of writhes in 15 min (%, Mean ± SEM) | Protection (%) |
|---|---|---|
| 19a | 32.33 ± 73.62 | 54.03 |
| 19b | 33.17 ± 73.39 | 52.84 |
| 19c | 32.67 ± 73.57 | 53.55 |
| 19d | 29.83 ± 72.45 | 57.58 |
| Control | 70.33 ± 73.01 | 0.00 |
| Acetyl salicylic acid | 25.67 ± 71.45 6 | 3.51 |
Fig. 5.
Molecular structures of compounds (19a–19d, 20a–20b, 21a–21c, 22a–22b, 23a–23d)
Jesudason et al. reported a class of N-Mannich bases of benzimidazole compounds and screened for its analgesic activity by the acetic acid induced writhing method using Wistar albino mice and anti-inflammatory activity by the formalin-induced paw edema method on Wistar albino rats by plethysmography. In this series, compound 20a exhibited similar results to paracetamol and compound 20b showed more potent than diclofenac (Tables 22 and 23, Fig. 5) [32].
Table 22.
Analgesic screening results and LD50
| Comp. | Dose (mg/kg) | % Protection | LD50 (mg/kg) |
|---|---|---|---|
| 20a | 20 | 32.2 | 175 |
| 40 | 47.49 | ||
| Paracetamol | 100 | 47.76 | – |
Table 23.
Anti-inflammatory screening results
| Comp. | Dose (mg/kg) | % Reduction of edema | |||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| 20b | 40 | 48 | 56 | 59 | 62 |
| Diclofenac | 50 | 48 | 65 | 64 | 65 |
Mariappan et al. developed some 2-substituted benzimidazole molecules and screened for their in vivo anti-inflammatory and analgesic activities using pentazocine as standard. Among the synthesized derivatives, compounds 21a, 21b, 21c showed significant analgesic and anti-inflammatory activity (Tables 24 and 25, Fig. 5) [33].
Table 24.
Analgesic activities of benzimidazole compounds 21a-c via Tail-flick method
| Comp. | (Mean ± SEM) tail withdrawing time in second | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| Control (0.5% CMC) | 1.56 ± 0.16 | 2.16 ± 0.16 | 2.33 ± 0.21 | 2.66 ± 0.21 | 2.82 ± 0.72 |
| Pentazocine | 2.16 ± 0.16 | 8.5 ± 0.34 | 11.33 ± 0.21 | 10.16 ± 0.30 | 10.83 ± 0.30 |
| 21a | 2.0 ± 0.25 | 3.0 ± 0.25 | 4.16 ± 0.33 | 10.5 ± 0.22 | 9.83 ± 0.33 |
| 21b | 2.0 ± 0.25 | 4.33 ± 0.21 | 3.73 ± 0.30 | 8.63 ± 0.21 | 10.03 ± 0.30 |
| 21c | 2.0 ± 0.25 | 6.51 ± 0.21 | 7.83 ± 0.30 | 9.73 ± 0.21 | 9.25 ± 0.30 |
Table 25.
Anti-inflammatory activities of benzimidazole compounds 21a-c by carrageenan-induced rat paws edema method
| Comp. | (Mean ± SEM) tail withdrawing time in second | Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | 4 h | |
| Control (0.5% CMC) | 0.14 ± 0.01 | 0.23 ± 0.01 | 0.24 ± 0.02 | 0.25 ± 0.01 | 0.25 ± 0.01 | – |
| Pentazocine | 0.14 ± 0.01 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.09 ± 0.01 | 64 |
| 21a | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.10 ± 0.01 | 60 |
| 21b | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.10 ± 0.01 | 60 |
| 21c | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.11 | 0.09 ± 0.02 | 64 |
Paramashivappa et al. synthesized a class of substituted benzimidazoles and assessed for its human cyclooxygenase-2 (COX-2) and cyclooxygenase-1 (COX-1) enzyme inhibition activity in human whole blood assay using rofecoxib as reference. In this series, compound 22a and 22b were found as most active agents (Table 26, Fig. 5) [34].
Table 26.
Inhibitory effect on COX-2 and COX-1 activity in human whole blood assay
| Comp. | COX-2 IC50 µM | COX-1 IC50 µM | COX-1/COX-2 |
|---|---|---|---|
| 22a | 1 | 384 | 384 |
| 22b | 1.06 | > 500 | > 470 |
| Rofecoxib | 0.057 | 11.4 | 200 |
Ravindernath et al. designed new benzo[d]imidazolyl tetrahydropyridine carboxylates and evaluated for their anti-inflammatory activity by the Carrageenan-induced paw edema test in rats using diclofenac sodium as a reference drug for comparison. All synthesized compounds (23a–23d) displayed appreciable activity. The significance results of the active compounds are shown in Table 27, Fig. 5 [35].
Table 27.
Anti-inflammatory screening results
| Comp. | Time | |||
|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | |
| 23a | 0.78 ± 0.022 | 1.45 ± 0.057 | 0.5 ± 0.027 | 0.08 ± 0.003 |
| 23b | 0.55 ± 0.0389 | 1.583 ± 0.045 | 0.616 ± 0.0315 | 0.3 ± 0.023 |
| 23c | 0.64 ± 0.011 | 1.4 ± 0.038 | 0.31 ± 0.024 | 0.31 ± 0.024 |
| 23d | 0.82 ± 0.030 | 1.76 ± 0.07 | 0.58 ± 0.03 | 0.1 ± 0.002 |
| Control | 0.90 ± 0.04 | 1.60 ± 0.018 | 2.38 ± 0.02 | 3.25 ± 0.03 |
| Diclofenac sodium | 0.95 ± 0.03 | 1.72 ± 0.03 | 0.60 ± 0.03 | 0.60 ± 0.02 |
Sondhi et al. synthesized pyrimido[1,6-a]benzimidazoles and tested in vitro for their anti-inflammatory and analgesic activities using carrageenin induced paw oedema model. Among the synthesized compounds, compound 24a (Fig. 6) displayed superior anti-inflammatory (46%) and mild analgesic activity (50%) using ibuprofen as standard (51% and 75%) [28].
Fig. 6.
Molecular structures of compounds (24a, 25a–25c, 26a, 27a, 28a, 29a, 30a–30b, 31a–31g, 32a–32b, 33a and 34a)
Sondhi et al. developed a class of benzimidazole acridine derivatives and tested for its anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activities using ibuprofen as standard. Among the series, compound 25a displayed considerable activity against kinase while compounds 25b and 25c displayed significant anti-inflammatory and analgesic activities (Table 28, Fig. 6) [36].
Table 28.
Anti-inflammatory, analgesic and kinase inhibition activities
| Comp. | Anti-inflammatory activity (%) | Analgesic activity (%) | Kinase IC50 (µM) | ||
|---|---|---|---|---|---|
| CDK-1 | CDK-5 | GSK-3 | |||
| 25a | – | – | 7.4 | 4.6 | 42 |
| 25b | 31.4 | 60 | – | – | – |
| 25c | 35.8 | 50 | – | – | – |
| Ibuprofen | 38.8 | 50 | – | – | – |
Vicini et al. synthesized benzimidazole tetrazolyl- and carboxyl-derivatives and screened for their anti-inflammatory and antipyretic activities in rat paw oedema and rat Escherichia coli derived LPS-induced pyrexia along with antinociceptive property examined in writhing and hot plate tests in mice. Among them, compound 26a (1H-benzimidazol-2-yl) acetic acid showed central analgesic activity. The significance results of the active compounds are shown in Table 29, Fig. 6 [37].
Table 29.
Analgesic effect of compound 26a against acetic acid induced writhing in mice
| Comp. | ID50 (mg/kg os) | Maximal inhibition % mean ± SEM |
|---|---|---|
| 26a | > 200 | 42 ± 15 |
| Acetaminophen | 208 | 90 ± 17 |
Wang et al. prepared a class of benzimidazole compounds and assessed for its in vitro H1 antihistamine activity. Among them, compound 27a found to display excellent activity to reduce mast cell degranulation, moderate anti-PAF activity and decreased potency on hERG as compared to standard astermizole and desloratadine (Table 30, Fig. 6) [8].
Table 30.
Antihistamine, receptor binding and anti-PAF activities
| Comp. | Anti H1 activity ileum IC50 (µmol/L) | H1 receptor binding IC50 (µmol/L) | PAF-induced platelet Aggregation IC50 (µmol/L) |
|---|---|---|---|
| 27a | 0.00794 | 0.000881 | 78 |
| Desloratadine | 0.0721 | 0.00588 | 130 |
| Astermizole | 0.0453 | 0.004 | ND |
Yang et al. designed new benzimidazoles and then assessed for their in vitro phosphodiesterase 10A (PDE10A) inhibitor activity. From the newly developed compounds, compound 28a (Fig. 6) showed good IC50 = 3.73 ± 0.60 nM along with selectivity (> 1000-fold) for PDE10A [38].
Antimalarial activity
Bandyopadhyay et al. synthesized new thiophosphorylated and phosphorylated benzimidazole derivatives and examined for their antimalarial activity toward Aedes albopictus and Culex quinquefasciatus for mosquito larvicidal properties at different concentration. Compound 29a (Fig. 6) found most active toward Ae. albopictus (LC50—6.42 and 5.25 mg/L at 24 and 48 h) and Cx. Quinquefasciatus (LC50—7.01 and 3.88 mg/L) using temephos as positive control (2.85 ± 2.64, 2.8 ± 3.04 toward Ae. Albopictus and for Cx. Quinquefasciatus 3.04 ± 2.31, 3.55 ± 2.45) [15].
Camacho et al. synthesized a class of N′-substituted-2-(5-nitrofuran or 5-nitrothiophen-2-yl)-3H-benzo[d]imidazole-5-carbohydrazides and investigated in vitro for its efficiency to inhibit β-hematin formation (IβHS), hemoglobin hydrolysis and then in vivo in rodent Plasmodium berghei for its antimalarial efficacy. Compounds 30a and 30b showed good antimalarial activity via inhibition of β-hematin formation and as proficient as chloroquine (Table 31, Fig. 6) [9].
Table 31.
Antimalarial activity of benzimidazole derivatives (30a and 30b)
| Comp. | IβHS | IC50 (µM) | IGP | % P | SD |
|---|---|---|---|---|---|
| 30a | 95.43 ± 0.58 | 8.43 | 0 | 4.02 ± 0.45 | 17 ± 1.26 |
| 30b | 75.76 ± 0.99 | 11.10 | 14.08 ± 0.88 | 1.8 ± 0.49 | 18.8 ± 2.05 |
| Leupeptin | – | – | 91.62 ± 0.69 | – | – |
| Pepstatin | – | – | 95.45 ± 0.66 | – | – |
| Chloroquine | 94.19 ± 0.36 | – | 24.12 ± 1.16 | 1.3 ± 0.3 | > 30 |
| Saline Solution | – | – | – | 21.8 ± 2.31 | 11.66 ± 1.66 |
Divatia et al. synthesized novel thiosemicarbazones containing benzimidazole nucleus and evaluated for their in vitro antimalarial activity towards P. falciparum by minimum inhibitory concentration using chloroquine and quinine as standards. Among them, compounds 31a, 31b, 31c, 31d, 31e, 31f and 31g showed excellent antimalarial activity. From structure activity relationship study it was observed that compounds having electron withdrawing groups (EWG) (chloro, fluoro and iodo) showed promising activity (Table 32, Fig. 6) [39].
Table 32.
Antimalarial activity of benzimidazole derivatives 30a and 31 g
| Comp. | Minimum inhibitory concentration (IC50 µg/mL) |
|---|---|
| 31a | 0.023 |
| 31b | 0.003 |
| 31c | 0.012 |
| 31d | 0.025 |
| 31e | 0.005 |
| 31f | 0.26 |
| 31 g | 0.15 |
| Quinine | 0.268 |
| Chloroquine | 0.020 |
Toro et al. reported ferrocenyl and cyrhetrenyl benzimidazoles and evaluated for their in vitro antimalarial activity against chloroquine susceptible-strain (3D7) and the chloroquine resistant-strain (W2) of Plasmodium falciparum. A better activity was observed for the compounds 32a–32b (Fig. 6) (IC50 = 10.4–26.5 μM) than its ferrocenyl analog 1-Fe-(H, NO2) (IC50 = 23.9–48.0 μM) [40].
Anti-mycobacterial/antitubercular activity
Camacho et al. synthesized some novel N′-substituted-2-(5-nitrofuran or 5-nitrothiophen-2-yl)-3H-benzo[d]imidazole-5-carbohydrazide compounds and investigated for their antitubercular potency against multidrug resistant MDR-MTB and MTB H37Rv strains. Compounds 33a (Fig. 6) exhibited good anti-mycobacterial activity (MIC = 12.5 µg/mL) against sensitive M. tuberculosis H37Rv and MDR-MTB (MIC = 6.25 µg/mL) strains and compared to isoniazid (MIC = 0.063 µg/mL) and rifampin (MIC = 32 µg/mL) [9].
Gong et al. reported a new series of substituted benzimidazole derivatives and investigated for their antitubercular potency against M. tuberculosis in a replicating state (R-Mtb), a physiologically-induced non-replicating state (NR-Mtb). Among the synthesized derivatives, compound 34a (Fig. 6) (NR-Mtb: MIC90 = 0.20 µg/mL; R-Mtb: MIC90 < 0.049 µg/mL) [16].
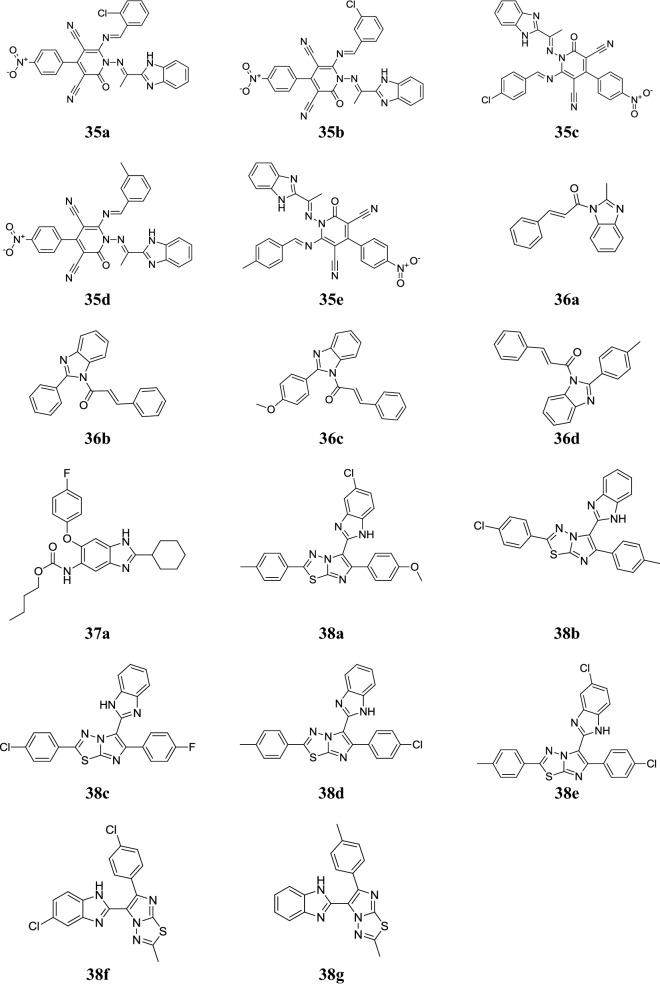
Desai et al. reported a class of benzimidazole containing 2-pyridones compounds and evaluated for its antimycobacterial potential towards M. tuberculosis H37Rv strain in Middlebrook 7H12 medium by microplate alamar blue assay (MABA) using isoniazid as a reference drug. In this series, compounds 35a, 35b, 35c, 35d and 35e (Fig. 7) exhibited good activity with MIC values (2.76–20.4 µM) as compared to isoniazid with MIC value (0.24 µM) [10].
Fig. 7.
Molecular structures of compounds (35a–35e, 36a–36d, 37a, 38a–38g)
Kalalbandi et al. developed a novel class of 1-[(2E)-3-phenylprop-2-enoyl]-1H-benzimidazoles and assessed for its antitubercular activity towards M. tuberculosis H37Rv by microplate alamar blue assay. Among them, compounds 36a, 36b, 36c and 36d (Fig. 7) (MIC = 3.12, 6.25, 3.12 and 1.6 µg/mL, respectively) showed excellent in vitro activity against H37Rv strain as compared to pyrazinamide, streptomycin and rifampicin having MIC = 3.12, 6.25 and 0.12 µg/mL, respectively [41].
Park et al. synthesized some new 2,5,6-trisubstituted benzimidazoles and assessed for their antitubercular potential against drug sensitive Mtb H37Rv strain using microplate alamar blue assay. Compound 37a (Fig. 7) displayed the best potency with the MIC value (0.63 µg/mL) against Mtb H37Rv [42].
Ramprasad et al. synthesized some imidazo[2,1-b] [1, 3, 4] thiadiazole-benzimidazole compounds and evaluated for their in vitro antituberculosis potency against M. tuberculosis H37Rv strain by agar dilution method using standard drugs ethambutol, isoniazid and pyrazinamide for comparison which showed the values in the range of 3.125–50.0 µg/mL. Among the synthesized compounds, compounds 38a, 38b, 38c, 38d, 38e, 38f and 38g (Fig. 7) showed potent anti-tubercular activity with MIC value (3.125 µg/mL) and comparable to standard ethambutol (MIC = 3.13 µg/mL) [43].
Ranjith et al. developed a class of positional isomers having benzimidazole moiety and evaluated for its antitubercular potency against M. smegmatis (MS), M. tuberculosis H37Rv (MTB), M. fortuitum (MF) and MDR-TB strains using isoniazid and rifampicin as standards. Among the synthesized derivatives, compounds 39a, 39b and 39c displayed significant activity against M. tuberculosis H37Rv (Table 33, Fig. 8) [44].
Table 33.
Antitubercular screening results
| Comp. | Screening results, MIC (µg/mL) | |||
|---|---|---|---|---|
| MTB | MS | MF | MDR-TB | |
| 39a | 0.625 | 10 | 10 | 6.25 |
| 39b | 0.625 | 1.25 | 10 | 6.25 |
| 39c | 0.625 | 1.25 | 10 | 6.25 |
| Isoniazid | 0.7 | 50 | 12.5 | 12.5 |
| Rifampicin | 0.5 | 1.5 | 1.5 | 25 |
Fig. 8.
Molecular structures of compounds (39a–39c, 40a–40e, 41a, 42a–42b, 43a–43c, 44a–44b)
Shingalapur et al. synthesized some novel 5-(nitro/bromo)-styryl-2-benzimidazole compounds and evaluated for their in vitro anti-tubercular activity towards M. tuberculosis H37 Rv by alamar blue assay using streptomycin (100% inhibition) as reference. Among them, compounds 40a, 40b, 40c, 40d and 40e showed significant antitubercular activity (Table 34, Fig. 8) [45].
Table 34.
Antitubercular activity {MIC (µg/mL)}
| Comp. | M. tuberculosis H37 Rv |
|---|---|
| 40a | > 7.25 (45) |
| 40b | > 7.25 (83) |
| 40c | > 7.25 (54) |
| 40d | > 7.25 (63) |
| 40e | > 7.25 (76) |
Yoon et al. prepared some new benzimidazole derivatives and evaluated for their antimycobacterial potency against M. tuberculosis H37Rv strain using BacTiter-Glo™ microbial cell viability (BTG) assay using six standard drugs (rifampicin, cycloserine, pyrimethamine, isoniazid, amikacin and ethambutol). In this series, compound 41a was found to be the highly potent agent as compared to standard drugs (Table 35, Fig. 8) [46].
Table 35.
Antimycobacterial activity of benzimidazole derivative 41a
| Comp. | M. tuberculosis H37Rv | |||||
|---|---|---|---|---|---|---|
| Alamar blue | BTG | |||||
| IC50 (µM) | IC90 (µM) | MIC (µM) | IC50 (µM) | IC90 (µM) | MIC (µM) | |
| 41a | 16.14 | 44.46 | 100 | 11.52 | 16.53 | 50 |
| Amikacin | 0.12 | 0.14 | 0.16 | 0.07 | 0.12 | 0.16 |
| Cycloserine | 24.76 | 28.01 | 100 | 23.55 | 26.38 | 100 |
| Ethambutol | 3.45 | > 200 | NA | 1.50 | 1.64 | 6.25 |
| Isoniazid | 0.19 | > 5 | NA | 0.13 | 0.20 | 0.31 |
| Pyrimethamine | 25.09 | 28.00 | 100 | 24.27 | 46.37 | 100 |
| Rifampicin | 0.02 | 0.02 | 0.16 | 0.02 | 0.03 | 0.04 |
Antiviral activity
Cheng et al. synthesized some novel benzimidazoles and demonstrated for their antiviral activity against Coxsackie virus B3 in VERO cells. Among the synthesized derivatives, compounds 42a and 42b (Fig. 8) showed potent selective activity with IC50 values (1.43 and 0.54 µg/mL) as compared to ribavirin (RVB) with IC50 value and eminent selective index (411.7 µg/mL and > 2.42) [47].
Fonseca et al. synthesized benzimidazole compounds incorporated into a hydrophenanthrene and naphthalene skeleton and screened for their in vitro antiviral activity against several RNA and DNA viruses. Among them, compounds 43a, 43b and 43c (Fig. 8) displayed good activity against VZV and CMV replication and comparable to that of acyclovir and ganciclovir (Table 36) [48].
Table 36.
Antiviral screening results of the synthesized compounds (43a-c)
| Comp. | Antiviral potency IC50 (µg/mL) | |
|---|---|---|
| CMV | VZV | |
| 43a | > 0.2 | 0.2–0.5 |
| 43b | 1.1–3.2 | 0.6–2.8 |
| 43c | 1.0–1.2 | 0.8–1.4 |
| Acyclovir | – | 0.3–3.0 |
| Ganciclovir | 0.9–1.5 | – |
Hwu et al. developed some new benzimidazole derivatives bearing coumarin ring and evaluated for their antiviral activity against the hepatitis C virus. Among the synthesized derivatives, compounds 44a and 44b (Fig. 8) were found to be most active and showed EC50 values (3.4 µM and 4.1 µM) [49].
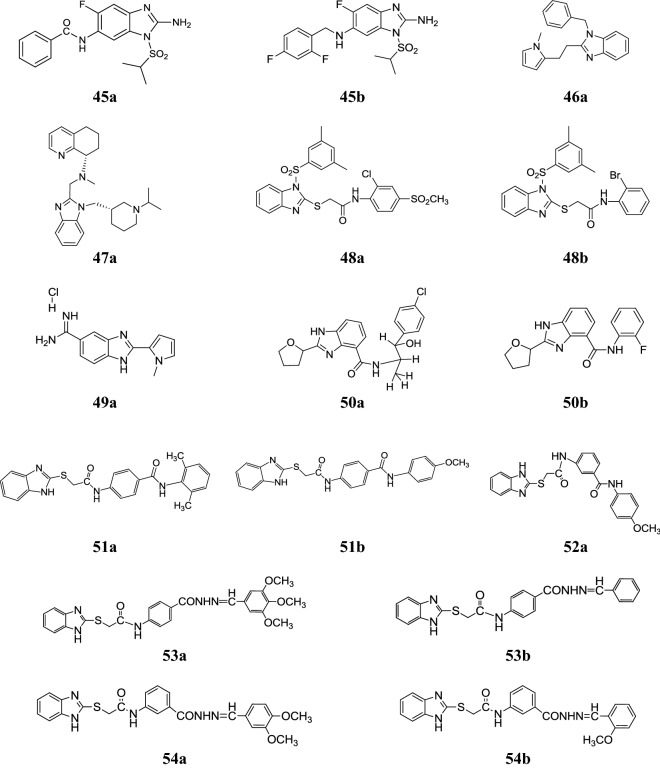
Li et al. synthesized a class of novel benzimidazoles and assessed for their hepatitis B virus inhibition activity. Among them, compounds 45a and 45b showed outstanding anti-HBV potency and comparable to lamivudine and adefovir (Table 37, Fig. 9) [50].
Table 37.
Antiviral activity results of the synthesized compounds (45a–45b)
| Comp. | IC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|
| 45a | 0.70 | 192 | 274 |
| 45b | 0.70 | 86 | 123 |
| Lamivudine | 0.38 | > 1000 | > 2632 |
| Adefovir | 1.7 | 57 | 34 |
Fig. 9.
Molecular structures of compounds (45a–45b, 46a, 47a, 48a–48b, 49a, 50a–50b, 51a–51b, 52a, 53a–53b, 54a–54b)
Luo et al. developed few novel benzimidazole compounds and evaluated for their anti-hepatitis B virus (HBV) activity and cytotoxicity in HepG 2.2.15 cells. In this study, compound 46a showed significant antiviral activity using lamivudine as reference (Table 38, Fig. 9) [51].
Table 38.
Antiviral activity results of the synthesized compounds 46a
| Comp. | IC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|
| 46a | < 0.41 | 33.3 | 81.2 |
| Lamivudine | 5 | 0.16 | 3.13 |
Miller et al. designed a series of N-substituted benzimidazoles as CXCR4 antagonists. In this series, compound 47a (Fig. 9) exhibited promising antiviral activity having IC50 of 2 nM, a 1000-fold cytotoxicity window and a twofold protein shift. A modification in side chain and stereochemical optimization led to significantly enhancement in potency and protein shift to afford compounds with low nanomolar anti-HIV activity [52].
Monforte et al. synthesized some novel N1-aryl-2-arylthioacetamido-benzimidazoles and screened for their human immunodeficiency virus type-1 (HIV-1) inhibitor activity. In this series, compounds 48a and 48b were found as the most active compounds with no toxicity (Table 39, Fig. 9) [11].
Table 39.
Anti-RT and anti-HIV-1 activities, cytotoxicity and selectivity index in MT-4 cells
| Comp. | IC50 (µM) | EC50 (µM) | CC50 (µM) | SI |
|---|---|---|---|---|
| 48a | 0.12 ± 0.035 | 0.04 ± 0.01 | > 221.59 | > 5540 |
| 48b | 0.18 ± 0.018 | 0.06 ± 0.02 | ≥ 235.64 | ≥ 3927 |
| Nevirapine | 2.55 ± 0.93 | 0.19 ± 0.06 | > 15.02 | > 79 |
| Efavirenz | 0.032 ± 0.009 | 0.006 ± 0.0001 | > 1056 | > 6.34 |
Starcevic et al. synthesized 2-substituted-5-amidino-benzimidazoles and assessed for their in vitro inhibitory activity against GMK cell line and HeLa cell line by MTT assay. From this series, compound 49a showed prominent activity against all four types of viruses with no cytotoxicity (Table 40, Fig. 9) [12].
Table 40.
Antiviral activity EC50 (µM)
| Comp. | HeLa | GMK | ||
|---|---|---|---|---|
| Adenovirus 5 | Herpesvirus 1 | Coxsackievirus B5 | Echovirus 7 | |
| 49a | 5.9 | 30 | 3.5 | 5 |
Zhang et al. reported some new benzimidazole derivatives and screened for their anti-Coxsackie virus B3 (CVB3) activity in VERO cells. In this series, compounds 50a and 50b (Fig. 9) exhibited better inhibitory activity with IC50 values (5.30 and 1.06 µg/mL) together with good selective indexes (12.1 and 7.5) than those of ribavirin (RBV) with IC50 value 353.33 [53].
Anticancer activity
In this study, Tahlan et al. developed a new class of benzimidazole benzamide compounds and demonstrated for its anticancer activity against cancer cell line (HCT116) by SRB method and compared to standard drugs (5-fluorouracil). From the synthesized derivatives, compound 51a and 51b (Fig. 9) showed the significant anticancer activity (Table 41) [3].
Table 41.
Anticancer activity results of synthesized compounds (51a and 51b)
| Comp. | Cancer cell line (IC50 = μM) |
|---|---|
| HCT116 | |
| 51a | 5.85 |
| 51b | 4.53 |
| 5-Fluorouracil | 9.99 |
Designed and synthesized a novel series of benzimidazole derivatives by Tahlan et al. and evaluated for its anticancer potency towards cancer cell line (HCT116) by SRB assay. In this series, compound 52a (Fig. 9) was found to be most promising anticancer compound. The significant result of the most active compound is shown in Table 42 [2].
Table 42.
Anticancer activity results of synthesized compound (52a)
| Comp. | Cancer cell line (IC50 = μM) |
|---|---|
| HCT116 | |
| 52a | 4.12 |
| 5-Fluorouracil | 7.69 |
Antimicrobial activity
Novel class of benzimidazole Schiff base derivatives has been synthesized by Tahlan et al. and evaluated for their antimicrobial activity against Gram positive and Gram negative bacterial and fungal species by tube dilution method. In this series, compounds 53a and 53b (Fig. 9) displayed potent antifungal activity against A. niger and C. albicans. The significant result of the active compounds is shown in Table 43 [54].
Table 43.
Antimicrobial results of compounds (53a–53b)
| Comp. | Microbial strains (MIC = µM/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Bacterial strains | Fungal strains | ||||||
| S. aureus | E. coli | B. subtilis | P. aeruginosa | S. enterica | C. albicans | A. niger | |
| 53a | 9.62 | 9.62 | 2.41 | 2.41 | 4.81 | 2.41 | 1.20 |
| 53b | 5.82 | 2.91 | 5.82 | 5.82 | 5.82 | 1.46 | 2.91 |
| Cefadroxil | 1.72 | 1.72 | 1.72 | 1.72 | 1.72 | – | – |
| Fluconazole | – | – | – | – | – | 2.04 | 2.04 |
Tahlan et al. synthesized a class of benzimidazole Schiff base derivatives and screened for its antimicrobial activity toward selected microbial species. From the series compounds 54a and 54b (Fig. 9) exhibited promising antimicrobial activity towards bacterial and fungal species. The significant result of the active compounds is shown in Table 44 [55].
Table 44.
Antimicrobial results of compounds (54a–54b)
| Comp. | Microbial strains (MIC = µM/mL) | ||||||
|---|---|---|---|---|---|---|---|
| Bacterial species | Fungal species | ||||||
| B. subtilis | P. aeruginosa | E. coli | S. typhi | K. pneumoniae | C. albicans | A. niger | |
| 54a | 1.28 | 1.28 | 1.28 | 2.55 | 5.11 | 5.11 | 2.55 |
| 54b | 0.68 | 0.68 | 2.72 | 2.72 | 5.44 | 5.44 | 2.72 |
| Cefadroxil | 1.73 | 3.46 | 3.46 | 0.86 | 3.46 | – | – |
| Fluconazole | – | – | – | – | – | 4.08 | 4.08 |
Conclusion
The present review based on reported heterocyclic benzimidazole derivatives which displayed the significant biological potentials in medicinal chemistry. Benzimidazole moiety is the key building block for several heterocyclic scaffolds that play central role in the biologically functioning of essential molecules and are surprisingly effective with their restraint movement and favorable selectiveness. The present review article is based on various reported pharmacological activities of heterocyclic 1H-benzimidazole derivatives. The review article shows the pharmacological activities of the reported synthesized benzimidazole derivatives in medicinal field. We hope this paper may be helpful in the development of new derivatives of benzimidazole based on medicinal chemistry and as well as designing of new drug molecule in future.
Acknowledgements
The authors are thankful to Head, Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, for providing necessary facilities to carry out this research work.
Abbreviations
- AChE
acetylcholinesterase
- AD
Alzheimer’s disease
- TB
tuberculosis
- M. tuberculosis
Mycobacterium tuberculosis
- GI
Giardia intestinalis
- TV
Trichomonas vaginalis
- EH
Entamoeba histolytica
- LM
Leishmania mexicana
- ABZ
albendazole
- SI
selectivity index
- TS
Trichinella spiralis
- TBZ
triclabendazole
- MBZ
mebendazole
- EWG
electron withdrawing groups
- VZV
varicella-zoster virus
- CMV
cytomegalovirus
- WHO
World Health Organization
- CRC
colorectal tumour
- CSC
cancer stem cell
- CDK
cyclin-dependent kinase
Authors’ contributions
Authors BN, ST and SK—designed the review article of benzimidazole derivatives on pharmacological significance. All authors read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sumit Tahlan, Email: sumittahlan1989@gmail.com.
Sanjiv Kumar, Email: sanjiv.pharmchem@gmail.com.
Balasubramanian Narasimhan, Email: naru2000us@yahoo.com.
References
- 1.Alaqeel SI. Synthetic approaches to benzimidazoles from o-phenylenediamine: a literature review. J Saudi Chem Soc. 2017;21:229–237. doi: 10.1016/j.jscs.2016.08.001. [DOI] [Google Scholar]
- 2.Tahlan S, Ramasamy K, Lim SM, Shah SAA, Mani V, Narasimhan B. Design, synthesis and therapeutic potential of 3-(2-(1H-benzo[d]imidazol-2-ylthio) acetamido)-N-(substituted phenyl)benzamide analogues. Chem Cent J. 2019;12(139):1–12. doi: 10.1186/s13065-018-0513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahlan S, Ramasamy K, Lim SM, Shah SAA, Mani V, Narasimhan B. (2-(1H-Benzo[d]imidazol-2-ylthio)acetamido)-N-(substituted phenyl) benzamides: design, synthesis and biological evaluation. BMC Chem. 2019;3(12):1–16. doi: 10.1186/s13065-019-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpan AS, Parlar S, Carlino L, Tarikogullari AH, Alptuzun V, Gunes HS. Synthesis biological activity and molecular modeling studies on 1H-benzimidazole derivatives as acetylcholinesterase inhibitors. Bioorg Med Chem. 2013;21:4928–4937. doi: 10.1016/j.bmc.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 5.Andrzejewskaa M, Yepez-Mulia L, Tapia A, Cedillo-Rivera R, Laudy AE, Starosciak BJ, Kazimierczuk Z. Synthesis and antiprotozoal and antibacterial activities of S-substituted 4,6-dibromo- and 4,6-dichloro-2-mercaptobenzimidazoles. Eur J Pharm Sci. 2004;21:323–329. doi: 10.1016/j.ejps.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Achar KCS, Hosamani KM, Seetharamareddy HR. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur J Med Chem. 2010;45:2048–2054. doi: 10.1016/j.ejmech.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Gaba M, Gaba P, Uppal D, Dhingra N, Bahiad MS, Silakari O, Mohan C. Benzimidazole derivatives: search for GI-friendly anti-inflammatory analgesic agents. Acta Pharm Sin B. 2015;5(4):337–342. doi: 10.1016/j.apsb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang XJ, Xi MY, Fu JH, Zhang FR, Cheng GF, Yin DL, You QD. Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents. Chin Chem Lett. 2012;23:707–710. doi: 10.1016/j.cclet.2012.04.020. [DOI] [Google Scholar]
- 9.Camacho J, Barazarte A, Gamboa N, Rodrigues J, Rojas R, Vaisberg A, Gilman R, Charris J. Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial, cytotoxic and antitubercular agents. Bioorg Med Chem. 2011;19:2023–2029. doi: 10.1016/j.bmc.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 10.Desai NC, Shihory NR, Kotadiya GM, Desai P. Synthesis, antibacterial and antitubercular activities of benzimidazole bearing substituted 2-pyridone motifs. Eur J Med Chem. 2014;82:480–489. doi: 10.1016/j.ejmech.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Monforte AM, Ferro S, Luca LD, Surdo GL, Morreale F, Pannecouque C, Balzarini J, Chimirri A. Design and synthesis of N1-aryl-benzimidazoles 2-substituted as novel HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem. 2014;22:1459–1467. doi: 10.1016/j.bmc.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 12.Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K, Karminski-Zamolaa G. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem. 2007;15:4419–4426. doi: 10.1016/j.bmc.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Salahuddin Shaharyar M, Mazumder A. Benzimidazoles: a biologically active compounds. Arab J Chem. 2017;10:S157–S173. doi: 10.1016/j.arabjc.2012.07.017. [DOI] [Google Scholar]
- 14.Hernandez-Luis F, Hernandez-Campos A, Castillo R, Navarrete-Vazquez G, Soria-Arteche O, Hernandez-Hernandez M, Yepez-Mulia L. Synthesis and biological activity of 2-(trifluoromethyl)-1H-benzimidazole derivatives against some protozoa and Trichinella spiralis. Eur J Med Chem. 2010;45:3135–3141. doi: 10.1016/j.ejmech.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay P, Sathe M, Tikar SN, Yadav R, Sharma P, Kumar A, Kaushik MP. Synthesis of some novel phosphorylated and thiophosphorylated benzimidazoles and benzothiazoles and their evaluation for larvicidal potential to Aedes albopictus and Culex quinquefasciatus. Bioorg Med Chem Lett. 2014;24:2934–2939. doi: 10.1016/j.bmcl.2014.04.082. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Karakaya SS, Guo X, Zheng P, Gold B, Ma Y, Little D, Roberts J, Warrier T, Jiang X, Pingle M, Nathan CF, Liu G. Benzimidazole-based compounds kill Mycobacterium tuberculosis. Eur J Med Chem. 2014;75:336–353. doi: 10.1016/j.ejmech.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Dai D, Burgeson JR, Gharaibeh DN, Moore AL, Larson RA, Cerruti NR, Amberg SM, Bolken TC, Hruby DE. Discovery and optimization of potent broad-spectrum arenavirus inhibitors derived from benzimidazole. Bioorg Med Chem Lett. 2013;23:744–749. doi: 10.1016/j.bmcl.2012.11.095. [DOI] [PubMed] [Google Scholar]
- 18.Yoon YK, Ali MA, Wei AC, Choon TS, Khaw KY, Murugaiyah V, Osman H, Masand VH. Synthesis characterization and molecular docking analysis of novel benzimidazole derivatives as cholinesterase inhibitors. Bioorg Chem. 2013;49:33–39. doi: 10.1016/j.bioorg.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 19.DIaz-Chiguer DL, Marquez-Navarro A, Nogueda-Torres B, Leon-Avila GL, Perez-Villanueva J, Hernandez-Campos A, Castillo R, Ambrosio JR, Nieto-Meneses R, Yepez-Mulia L, Hernandez-Luis F. In vitro and in vivo trypanocidal activity of some benzimidazole derivatives against two strains of Trypanosoma cruzi. Acta Trop. 2012;122:108–112. doi: 10.1016/j.actatropica.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Covarrubias C, Vilchis-Reyes MA, Yepez-Mulia L, Sanchez-Diaz R, Navarrete-Vazquez G, Hernandez-Campos A, Castillo R, Hernandez-Luis F. Exploring the interplay of physicochemical properties, membrane permeability and giardicidal activity of some benzimidazole derivatives. Eur J Med Chem. 2012;52:193–204. doi: 10.1016/j.ejmech.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Kopanska K, Najda A, Zebrowska J, Chomicz L, Piekarczyk J, Myjakd P, Bretnera M. Synthesis and activity of 1H-benzimidazole and 1H-benzotriazole derivatives as inhibitors of Acanthamoeba castellanii. Bioorg Med Chem. 2004;12:2617–2624. doi: 10.1016/j.bmc.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Mavrova AT, Vuchev D, Anichina K, Vassilev N. Synthesis, antitrichinnellosis and antiprotozoal activity of some novel thieno[2,3-d]pyrimidin-4(3H)-ones containing benzimidazole ring. Eur J Med Chem. 2010;45:5856–5861. doi: 10.1016/j.ejmech.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Navarrete-Vazquez G, Rojano-Vilchis MM, Yepez-Mulia L, Melendez V, Gerena L, Hernandez-Campos A, Castillo R, Hernandez-Luis F. Synthesis and antiprotozoal activity of some 2-(trifluoromethyl)-1H-benzimidazole bioisosteres. Eur J Med Chem. 2006;41:135–141. doi: 10.1016/j.ejmech.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Marquez-Navarro A, Nogueda-Torres B, Hernandez-Campos A, Soria-Arteche O, Castillo R, Rodriguez-Morales S, Yepez-Mulia L, Hernandez-Luis F. Anthelmintic activity of benzimidazole derivatives against Toxocara canis second-stage larvae and Hymenolepis nana adults. Acta Trop. 2009;109:232–235. doi: 10.1016/j.actatropica.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Oh S, Kim S, Kong S, Yang G, Lee N, Han D, Goo J, Siqueira-Neto JL, Freitas-Junior LH, Song R. Synthesis and biological evaluation of 2,3-dihydroimidazo[1,2-a] benzimidazole derivatives against Leishmania donovani and Trypanosoma cruzi. Eur J Med Chem. 2014;84:395–403. doi: 10.1016/j.ejmech.2014.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Palomares-Alonso F, Jung-Cook H, Perez-Villanueva J, Piliado JC, Rodriguez-Morales S, Palencia-Hernandez G, Lopez-Balbiaux N, Hernandez-Campos A, Castillo R, Hernandez-Luis F. Synthesis and in vitro cysticidal activity of new benzimidazole derivatives. Eur J Med Chem. 2009;44:1794–1800. doi: 10.1016/j.ejmech.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Villanueva J, Hernandez-Campos A, Yepez-Mulia L, Mendez-Cuesta C, Mendez-Lucio O, Hernandez-Luis F, Castillo R. Synthesis and antiprotozoal activity of novel 2-{[2-(1H-imidazol-1-yl)ethyl]sulfanyl}-1H-benzimidazole derivatives. Bioorg Med Chem Lett. 2013;23:4221–4224. doi: 10.1016/j.bmcl.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Sondhi SM, Rajvanshi S, Johar M, Bharti N, Azam A, Singh AK. Anti-inflammatory, analgesic and antiamoebic activity evaluation of pyrimido[1,6-a] benzimidazole derivatives synthesized by the reaction of ketoisothiocyanates with mono and diamines. Eur J Med Chem. 2002;37:835–843. doi: 10.1016/S0223-5234(02)01403-4. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Gomez H, Hernandez-Nunez E, Leon-Rivera I, Guerrero-Alvarez J, Cedillo-Rivera R, Moo-Puc R, Argotte-Ramos R, Rodriguez-Gutierrez MC, Chan-Bacab MJ, Navarrete-Vazquez G. Design, synthesis and in vitro antiprotozoal activity of benzimidazolepentamidine hybrids. Bioorg Med Chem Lett. 2008;18:3147–3151. doi: 10.1016/j.bmcl.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Velazquez-Lopez JM, Hernandez-Campos A, Yepez-Mulia L, Tellez-Valencia A, Flores-Carillo P, Nieto-Meneses R, Castillo R. Synthesis and trypanocidal activity of novel benzimidazole derivatives. Bioorg Med Chem Lett. 2016;26(17):4377–4381. doi: 10.1016/j.bmcl.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 31.El-Feky SA, Thabet HK, Ubeid MT. Synthesis, molecular modeling and anti-inflammatory screening of novel fluorinated quinoline incorporated benzimidazole derivatives using the Pfitzinger reaction. J Fluorine Chem. 2014;161:87–94. doi: 10.1016/j.jfluchem.2014.02.012. [DOI] [Google Scholar]
- 32.Jesudason EP, Sridhar SK, Malar EJP, Shanmugapandiyan P, Inayathullah M, Arul V, Selvaraj D, Jayakumar R. Synthesis, pharmacological screening, quantum chemical and in vitro permeability studies of N-Mannich bases of benzimidazoles through bovine cornea. Eur J Med Chem. 2009;44:2307–2312. doi: 10.1016/j.ejmech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 33.Mariappan G, Hazarika R, Alam F, Karki R, Patangia U, Nath S. Synthesis and biological evaluation of 2-substituted benzimidazole derivatives. Arab J Chem. 2015;8:715–719. doi: 10.1016/j.arabjc.2011.11.008. [DOI] [Google Scholar]
- 34.Paramashivappa R, Kumar PP, Rao PVS, Rao AS. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett. 2003;13:657–660. doi: 10.1016/S0960-894X(02)01006-5. [DOI] [PubMed] [Google Scholar]
- 35.Ravindernath A, Reddy MS. Synthesis and evaluation of anti-inflammatory, antioxidant and antimicrobial activities of densely functionalized novel benzo[d] imidazolyl tetrahydropyridine carboxylates. Arab J Chem. 2017;10:S1172–S1179. doi: 10.1016/j.arabjc.2013.02.011. [DOI] [Google Scholar]
- 36.Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L. Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg Med Chem. 2006;14:3758–3765. doi: 10.1016/j.bmc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 37.Vicini P, Incerti M, Amoretti L, Ballabeni V, Tognolini M, Barocelli E. Synthesis and pharmacological properties of benzisothiazole/benzimidazole derivatives with acidic groups. Farmaco. 2002;57:363–367. doi: 10.1016/S0014-827X(02)01219-3. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Murigi FN, Wang Z, Li J, Jin H, Tu Z. Synthesis and in vitro characterization of cinnoline and benzimidazole analogues as phosphodiesterase 10A inhibitors. Bioorg Med Chem Lett. 2015;25:919–924. doi: 10.1016/j.bmcl.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Divatia SM, Rajani DP, Rajani SD, Patel HD. Novel thiosemicarbazone derivatives containing benzimidazole moiety: green synthesis and anti-malarial activity. Arab J Chem. 2014 doi: 10.1016/j.arabjc.2014.09.007. [DOI] [Google Scholar]
- 40.Toro P, Klahn AH, Pradines B, Lahoz F, Pascual A, Biot C, Arancibia R. Organometallic benzimidazoles: synthesis, characterization and antimalarial activity. Inorg Chem Commun. 2013;35:126–129. doi: 10.1016/j.inoche.2013.06.019. [DOI] [Google Scholar]
- 41.Kalalbandi VKA, Seetharamappa J, Katrahalli U, Bhat KG. Synthesis, crystal studies, anti-tuberculosis and cytotoxic studies of 1-[(2E)-3-phenylprop-2-enoyl]-1H-benzimidazole derivatives. Eur J Med Chem. 2014;79:194–202. doi: 10.1016/j.ejmech.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Park B, Awasthi D, Chowdhury SR, Melief EH, Kumar K, Knudson SE, Slayden RA, Ojima I. Design, synthesis and evaluation of novel 2,5,6-trisubstituted benzimidazoles targeting FtsZ as antitubercular agents. Bioorg Med Chem. 2014;22:2602–2612. doi: 10.1016/j.bmc.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramprasad J, Nayak N, Dalimba U, Yogeeswari P, Sriram D, Peethambar SK, Achur R, Kumar HSS. Synthesis and biological evaluation of new imidazo[2,1-b][1,3,4] thiadiazole-benzimidazole derivatives. Eur J Med Chem. 2015;95:49–63. doi: 10.1016/j.ejmech.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Ranjith PK, Rajeesh P, Haridas KR, Susanta NK, Row TNG, Rishikesan R, Kumari NS. Design and synthesis of positional isomers of 5 and 6-bromo-1-[(phenyl)sulfonyl]-2-[(4-nitrophenoxy)methyl]-1H benzimidazoles as possible antimicrobial and antitubercular agents. Bioorg Med Chem Lett. 2013;23:5228–5234. doi: 10.1016/j.bmcl.2013.06.072. [DOI] [PubMed] [Google Scholar]
- 45.Shingalapur RV, Hosamani KM, Keri RS. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur J Med Chem. 2009;44:4244–4248. doi: 10.1016/j.ejmech.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Yoon YK, Ali MA, Wei AC, Choon TS, Ismail R. Synthesis and evaluation of antimycobacterial activity of new benzimidazole aminoesters. Eur J Med Chem. 2015;93:614–624. doi: 10.1016/j.ejmech.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Cheng J, Xie J, Luo X. Synthesis and antiviral activity against Coxsackie virus B3 of some novel benzimidazole derivatives. Bioorg Med Chem Lett. 2005;15:267–269. doi: 10.1016/j.bmcl.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 48.Fonseca T, Gigante B, Marques MM, Gilchrist TL, Clercq ED. Synthesis and antiviral evaluation of benzimidazoles, quinoxalines and indoles from dehydroabietic acid. Bioorg Med Chem. 2004;12:103–112. doi: 10.1016/j.bmc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 49.Hwu JR, Singha R, Hong SC, Chang YH, Das AR, Vliegen I, Clercq ED, Neyts J. Synthesis of new benzimidazole–coumarin conjugates as anti-hepatitis C virus agents. Antivir Res. 2008;77:157–162. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Li YF, Wang GF, Luo Y, Huang WG, Tang W, Feng CL, Shi LP, Ren YD, Zuo JP, Lu W. Identification of 1-isopropylsulfonyl-2-amine benzimidazoles as a new class of inhibitors of hepatitis B virus. Eur J Med Chem. 2007;42:1358–1364. doi: 10.1016/j.ejmech.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 51.Luo Y, Yao JP, Yang L, Feng CL, Tang W, Wang GF, Zuo JP, Lu W. Design and synthesis of novel benzimidazole derivatives as inhibitors of hepatitis B virus. Bioorg Med Chem. 2010;18:5048–5055. doi: 10.1016/j.bmc.2010.05.076. [DOI] [PubMed] [Google Scholar]
- 52.Miller JF, Turner EM, Gudmundsson KS, Jenkinson S, Spaltenstein A, Thomson M, Wheelan P. Novel N-substituted benzimidazole CXCR56 antagonists as potential anti-HIV agents. Bioorg Med Chem Lett. 2010;20:2125–2128. doi: 10.1016/j.bmcl.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 53.Zhang ZL, Sun ZJ, Xue F, Luo XJ, Xiu NY, Teng L, Peng ZG. Design, synthesis and biological activity of some novel benzimidazole derivatives against Coxsackie virus B3. Chin Chem Lett. 2009;20:921–923. doi: 10.1016/j.cclet.2009.03.035. [DOI] [Google Scholar]
- 54.Tahlan S, Narasimhan B, Lim SM, Ramasamy K, Mani V, Shah SAA. Mercaptobenzimidazole Schiff bases: design, synthesis, antimicrobial studies and anticancer activity on HCT-116 cell line. Mini-Rev Med Chem. 2018 doi: 10.2174/1389557518666181009151008. [DOI] [PubMed] [Google Scholar]
- 55.Tahlan S, Narasimhan B, Lim SM, Ramasamy K, Mani V, Shah SAA. Design, synthesis, SAR study, antimicrobial and anticancer evaluation of novel -mercaptobenzimidazole azomethine derivatives. Mini-Rev Med Chem. 2018;1:1. doi: 10.2174/1389557518666180903151849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable