Abstract
Background
Glycemic control is vital in the care of type 2 diabetes mellitus (T2DM) and is significantly associated with the incidence of clinical complications. This Bayesian network analysis was conducted with an aim of evaluating the efficacy of scaling and root planning (SRP) and SRP + adjuvant treatments in improving glycemic control in chronic periodontitis (CP) and T2DM patients, and to guide clinical practice.
Methods
We searched the Pubmed, Embase, Cochrane Library and Web of Science databases up to 4 May 2018 for randomized controlled trials (RCTs). This was at least three months of the duration of study that involved patients with periodontitis and T2DM without other systemic diseases given SRP. Patients in the control group did not receive treatment or SRP combination with adjuvant therapy. Outcomes were given as HbA1c% and levels fasting plasma glucose (FPG). Random-effects meta-analysis and Bayesian network meta-analysis were conducted to pool RCT data. Cochrane’s risk of bias tool was used to assess the risk of bias.
Results
Fourteen RCTs were included. Most were unclear or with high risk of bias. Compared to patients who did not receive treatment, patients who received periodontal treatments showed improved HbA1c% level, including SRP (the mean difference (MD) -0.399 95% CrI 0.088 to 0.79), SRP + antibiotic (MD 0.62, 95% CrI 0.18 to 1.11), SRP + photodynamic therapy (aPDT) + doxycycline (Doxy) (MD 1.082 95% CrI 0.13 to 2.077) and SRP + laser (MD 0.66 95% CrI 0.1037, 1.33). Among the different treatments, SRP + aPDT + Doxy ranked best. Regarding fasting plasma glucose (FPG), SRP did not show advantage over no treatment (MD 4.91 95% CI − 1.95 to 11.78) and SRP with adjuvant treatments were not better than SRP alone (MD -0.28 95% CI -8.66, 8.11).
Conclusion
The results of this meta-analysis seem to support that periodontal treatment with aPDT + Doxy possesses the best efficacy in lowering HbA1c% of non-smoking CP without severe T2DM complications. However, longer-term well-executed, multi-center trails are required to corroborate the results.
Electronic supplementary material
The online version of this article (10.1186/s12903-019-0829-y) contains supplementary material, which is available to authorized users.
Keywords: Type 2 diabetes mellitus, Periodontitis, Non-surgical periodontal therapy, Scaling and root planing, Adjuvant therapy
Background
Periodontitis is a chronic inflammatory disease caused by pathogens in the surrounding periodontal tissues, that results in the periodontal pocket formation, clinical attachment loss and alveolar bone resorption and ultimately leads to tooth loss [1, 2]. Epidemiological evidence has shown that periodontitis affects over 50% of the adult worldwide, indicating a dose-response relationship with oral health relates to the quality of life [3, 4]. It is well-known that periodontitis is highly associated with T2DM, and now periodontitis is regarded as the sixth complicated form of T2DM [5]. Compared to non-diabetics, patients with diabetes present worse clinical manifestation of periodontitis [6]. Besides, moderate to severe periodontitis increases the risk of T2DM and leads to poor glycemic control in diabetics [7, 8].
Glycemic control is vital in the care of T2DM and is significantly associated with the incidence of clinical complications. One percent reduction in HbA1c% is associated with 14% reductions in risk of myocardial infarction, 21% for deaths related to diabetes and 37% for microvascular complications [9]. Studies indicate that scaling and root planing (SRP) or SRP plus adjuvant treatment could improve glycemic control in patients with T2DM and CP, but no effective conclusion has been reached regarding the best treatment. Besides, there is conflicting evidence regarding glycemic control of various adjuvant treatments in SRP. For instance, SRP followed by locally delivered Atorvastatin (ATV) did not show a reduction of HbA1c% compared to SRP [10], while additional benefits were found after adjuvant therapy with aPDT [11]. Previous meta-analyses usually compared periodontal treatment with no treatment [12]. Few of meta-analyses have focused on adjuvant therapy, but adjuvant therapy is limited to a single type, such as aPDT [13] and systemic antibiotics [14, 15]. Therefore, a more comprehensive study is needed to clarify whether SRP or SRP with adjuvant treatments could improve glycemic control in patients diagnosed with T2DM, and potentially find the best treatment to provide evidence for clinical practice.
This Bayesian network analysis aimed to address the following focused question based on the Population, Intervention, Comparison, Outcomes, Study Design (PICOS) schema: “In chronic periodontitis with T2DM, does periodontal treatment with/without adjuvant treatment compared to no treatment or periodontal treatment with adjuvant treatment compared to periodontal treatment alone, result in better glycemic control in randomized controlled clinical trials?”
Methods
This review was not registered as a priori protocol but followed the PRISMA and the PRISMA extension for Systematic reviews and Meta-Analyses network meta-analysis, respectively.
Eligibility criteria
The studies were considered eligible for inclusion if they met the following criteria based on PICO schema:
Participants: Adult patients (aged ≥30 years) with no gender, age and career predilection diagnosed as periodontitis and T2DM
Intervention: Comparing SRP with no treatment, or comparing SRP with SRP plus adjuvant treatment, or comparing SRP plus adjuvant therapy with different adjuvant therapies
Outcoming: HbA1c% and fasting plasma glucose (FPG) (mean change in parameters from baseline to follow-up visits)
Study design: Randomized clinical trial (RCT)
Follow up: At least three months
Language: English
Exclusion criteria
Split-mouth randomized controlled clinical trial
Pregnancy and lactation
Current smoking and smoking within the past 5 years
Studies including subjects who had systemic conditions (except T2DM) and major complications of T2DM
Periodontal treatment and antibiotic use within the previous three months
Periodontal support therapy within three months
Sample sizes of each group less than 10
Studies that did not report the value of HbA1c and FPG
Information sources and literature search
We searched the Pubmed, Embase, Cochrane Library and Web of Science Databases from inception to 4 May 2018. The following MeSH terms/free terms and their combinations were searched to find potentially eligible studies (Additional file 1). Also, the reference lists of relevant articles and relevant systematic reviews were manually searched to find other potentially eligible studies.
Study selection
Two independent reviewers (R.Y. Cao and Q.L. Li) screen identified eligible studies based on their titles/abstracts and full texts. Any disagreements were resolved by discussion or through an adjudication by a third review (M.F. Yao).
Data extraction and data items
Two independent investigators (R.Y. Cao and Q.L. Li) extracted and recorded relevant data from eligible studies by pre-designed data-extraction forms: study characteristics (first author, publication year, country), patient characteristics (age, sex,inclusion criteria of T2DM and periodontitis, T2DM duration, T2DM treatment), intervention and control treatment protocols, sample size, outcome details (detection method of HbA1c%, ΔHbA1c%, ΔFPG), follow-up period, adverse events. Inconsistencies were settled through discussions until an agreement was reached.
Risk of bias in individual trails
The risk of bias of included studies was assessed independently by two reviewers (R.Y. Cao and Q.L. Li) with Cochrane Collaboration tool. The items for Cochrane tool included random sequence generation; allocation concealment; blinding of participants and personnel assessment; incomplete outcome data; selective outcome reporting and other bias. We did not assess the blinding of the outcome because our outcomes (HbA1c% and FPG) were objective indicators. Inconsistencies were settled through discussions until an agreement was reached.
Outcomes and data synthesis
The treatment outcomes were the absolute difference (AD) in HbA1c% and FPG at three to four months after periodontal treatment. When the standard deviations (SD) for the outcomes were not available, it was calculated by assuming that the correlation coefficient was 0.5 as previously described [16, 17].
The data used was a follow-up of 3–4 months. First, a random-effects pairwise meta-analysis was performed with the Stata 14.2 (Stata Corporation, College Station, TX, USA). The mean difference (MD) and 95% confidence intervals (CIs) were used to compare continuous variables as previously described [12]. Second, we did a Bayesian network analysis by using Markov chain Monte Carlo methods via GeMTC package 0.8 implemented in R 3.2.2. We also assessed study design information and patient characteristics to evaluate the transitivity assumption for reliable data pooling with sufficient similarity between the included trials [18, 19]. Both non-informative uniform and normal prior distributions were used throughout the network meta-analysis [20]. Markov chain Monte Carlo methods with four chains of 300,000 iterations after a burn-in phase of 180,000 iterations were used to gain MD and 95% credible intervals (CrIs). We used CrIs beyond the null value to assess significance. We also performed a sensitivity analysis by setting a uniform prior standard deviation (“std.dev”, “dunif”, 0, 2). Model selection (fixed vs random effects) was based on the assessment of the deviance information criterion (DIC) [21]. We then reported the results of the random-effects model. Brooks-Gelman-Rubin diagnostic plot was used to assess model convergence of network analysis [19]. We also ranked the different treatments in terms of lowering the HbA1c% level using the same methods [20].
The goodness of fit of the model assessed the consistency assumption of this network analysis and it was tested by calculating the posterior mean residual deviance (Dbar). When the Dbar was similar to the number of data points in the study, the model was taken into account for fitting the data well [22]. Moreover, we also examined the pooled effects from traditional pairwise meta-analysis and network meta-analysis to further verify the consistency of the network. Inconsistency was assessed by comparing direct evidence with indirect evidence from the entire network on each node (node-splitting analysis) with p < 0.05 [23]. Heterogeneity was assessed by I2 calculation. To verify the robustness of our analyses, we performed a sensitivity analysis by excluding studies with HbA1c% > 9 or < 7 as the baseline. We also conducted comparison-adjusted funnel plots to assess publication bias. All statistical analyses were performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK), R 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 14.2 (Stata Corporation, College Station, TX, USA).
Quality of network meta-evidence
We assessed the quality of evidence for direct estimates based on the GRADE criteria [24] using five items (risk of bias, imprecision, indirectness, inconsistency and publication bias). We used an approach that proposed by Puhan et al. and Brignardello-Petersen et al. to assess the quality of evidence for indirect and network estimates.
Results
Study selection
The literature search identified 586 possibly eligible articles, 86 articles were fully accessed after excluding duplicates and unsuitable studies by title/abstract, while 72 of them were not considered eligible for inclusion (Additional file 2). A total of 14 trials with 629 patients were included in this network meta-analysis. Figure 1 shows phases of the screening process.
Fig. 1.
Flow chart of articles search and screening process
Characteristics of included studies
Tables 1 and 2 presented the main characteristics of the 14 included studies. All studies were RCTs, and were followed up for 3–12 months. All the patients had T2DM and chronic periodontitis, but the diagnostic criteria of T2DM were not reported in most studies. The T2DM duration was from 4 to 11.8 years. T2DM treatments mainly included diet and insulin supplementation or oral hypoglycemic agents. The baseline of HbA1c% varied widely, from 6.2 to 10.4. Periodontal treatments were performed by SRP, within 24 h - 2 weeks. Most studies gave oral hygiene instruction to subjects. All studies reported the outcomes of HbA1c%, few reported on FPG. Four studies did not report adverse events, and three studies [30, 32, 33] showed side effects, such as taste perception, dry mouth, headache, diarrhoea. Ten studies reported the method of HbA1c% determination. The network meta-analysis included 7 treatments (Fig. 2), including SRP (n = 280), no treatment (n = 76), SRP + antibiotic (doxycycline (Doxy), metronidazole + amoxicillin, Doxy) (n = 130), subantimicrobial dose doxycycline (SDD) (n = 17), locally-delivered drugs (atorvastatin gel, simvastatin gel, chlorhexidine gel) (n = 66), laser (diode laser, aPDT) (n = 45) and SRP + Doxy + aPDT (n = 15). The plots indicated that most of the direct evidence between different adjuvantive treatments was lacking.
Table 1.
Characteristics of studies included in the network meta-analysis
| First author, year | Country | Inclusion criteria of T2DM | T2DM duration (year) | T2DM treatment | Age (years) | Female/male | Intervention | Control |
|---|---|---|---|---|---|---|---|---|
| Singh (2008) [25] | India | T2DM | na | na | > 30 | na |
A: SRP + Doxy (100 mg bid for day 1 and then 100 mg/day for 13 days) (n = 15) B: SRP (n = 15) |
No treatment (n = 15) |
| Gilowski (2012) [26] | Poland |
T2DM diagnosed for > 6 months |
T: 4.0 (1.5–13.0) C: 5.0 (3.0–18.0) |
diet regimen, insulin, and or oral hypoglycemic drugs |
T: 57.6 ± 8.0 C: 56.0 ± 9.0 |
T: 10/ 7 C: 8/ 9 |
SRP + SDD (20 mg bid for 3 months) (n = 17) |
SRP + placebo (n = 17) |
| Moeintaghavi (2012) [27] | Iran | Diagnosis of T2DM with glycated HbA1c values over 7% | na | oral hypoglycemic drugs (no insulin) |
Female: 48.1 ± 3 Male: 52.48 ± 3 |
T: 13/ 9 C: 7/ 11 |
SRP (n = 22) |
No treatment (n = 18) |
| Gaikwad (2013) [28] | India | T2DM | na |
Received antidiabetic therapy |
na | 16/ 34 | SRP + Doxy (100 mg/d for15 days) (n = 25) | SRP (n = 25) |
| Pradeep (2013) [29] | India | Well-controlled type 2 DM (American Diabetic Association in 2011 and glycated hemoglobin levels) | na | na | 30 - 50 | 18/ 20 |
SRP + SMV gel (1.2%) (n = 17) |
SRP + placebo (n = 18) |
| Santos (2013) [30] | Brazil | T2DM diagnosed > 5 years |
T: 6.3 ± 0.8 C: 6.8 ± 1.1 |
Diet: 1/ 0 Diet + insulin: 1/ 4 Diet + oral hypoglycemic agents: 14/ 14 Diet + oral hypoglycemic agents + insulin: 3/ 1 |
T: 50.3 ± 9.5 C: 53.9 ± 10.8 |
T: 15/ 4 C: 13/ 6 |
SRP + CHX gel (1%) + CHX solution (0.12%) rinse (bid/day for 60 days) (n = 19) |
SRP + placebo gel + placebo solutions rinse (n = 18) |
| Telgi (2013) [31] | India | T2DM | na | oral hypoglycemic agents | 35 - 45 | na |
A: SRP + 0.12% CHX mouthwash (once daily) and brush (twice daily) (n = 20) B: 0.12% CHX mouthwash (once daily) and brush (twice daily) (n = 20) |
brush (twice daily) (n = 20) |
| Macedo (2014) [11] | Brazil | T2DM diagnosed for >5 years and HbA1c >7 % | > 5 | na |
T: 49.4 ± 6.8 C: 48.1 ± 9 |
T: 9/ 6 C: 10/ 5 |
SRP + aPDT (10 mg/ml PC) + Doxy (100 mg bid for day 1 and then 100 mg/day for 13 days) (n = 15) | SRP + Doxy (100 mg bid for day 1 and then 100 mg/day for 13 days) (n = 15) |
| Miranda (2014) [32] | Brazil | T2DM diagnosed for ≥ 5 years, HbA1c ≥ 6.5% ≤ 11% |
T: 8.0 ± 3.2 C: 7.4 ± 3.6 |
Diet and insulin supplementation or oral hypoglycemic agents |
T: 54.0 ± 8.2 C: 53.7 ± 8.0 |
T: 17/ 12 C: 9/ 18 |
SRP + MTZ + AMX (400/500 mg tid for 14 days) (n = 29) |
SRP + placebos (n = 27) |
| Tsalikis (2014) [33] | Greece | T2DM diagnosed for ≥ 1 year |
T: 11.8 ± 5.9 C: 10.2 ± 5.7 |
na |
T: 62.9 ± 10 C: 57.94 ±8.22 |
T: 13/ 18 C: 15/ 20 |
SRP+ Doxy (100 mg bid for day 1 and then 100 mg/day for 20 days) (n = 31) |
SRP + placebos (n = 35) |
| Wu (2015) [34] | China | T2DM diagnosed for > 1 year with no medication changes in the last 3 months |
T: 4.00 ± 1.76 C: 4.22 ± 1.57 |
na |
T: 54.09 ±6.57 C: 55.52 ±5.22 |
T: 12/11 C: 10/13 |
SRP + OHI (n = 23) | OHI (n = 23) |
| Koçak (2016) [35] | Turkey |
TDM2 (5.7 % ≤ glycated hemoglobin (HbA1c) ≤8.5 %) with no alteration in the diabetes treatment in the last year prior to the study |
Na | na |
T: 51.7 ± 5.2 C: 53.1 ± 5.1 |
T: 15/15 C: 15/15 |
SRP + DL (n = 30) | SRP (n = 30) |
| Kumari (2016) [10] | India | Well-controlled T2DM (American Diabetic Association in 2012 and glycated hemoglobin Levels) | Na | na | 40-50 | 37/38 |
SRP + ATV gel (1.2%) (n = 30) |
SRP + placebo gel (n = 30) |
| Ramos (2016) [36] | Brazil |
Type 2 DM diagnosed for > 5 years and HbA1c >7% |
>5 | na |
T: 48.9 ± 9.5 C: 49.3 ± 7.4 |
T: 8/7 C: 8/7 |
SRP + aPDT (10 mg/ml PC) (n = 15) |
SRP + Doxy (100 mg bid for day 1 and then 100 mg/day for 13 days) (n = 15) |
SRP scaling and root planing, Doxy doxycycline, SDD subantimicrobial dose doxycycline, SMV simvastatin, CHX chlorhexidine, aPDT antimicrobial photodynamic therapy, AMX amoxicillin, MTZ metronidazole, ATV atorvastatin, DL diode laser, PC phenothiazine chloride solution, na not available
Table 2.
Outcomes of studies included in the network meta-analysis
| First author, year | Inclusion criteria of chronic periodontitis | Method of HbA1c% determination | Initial HbA1c (%) (T vs. C) |
Outcome (ΔHbA1c%) (T vs. C) |
Outcome (ΔFPG) (T vs. C) |
Follow up (m) |
Adverse events (T vs. C) |
|---|---|---|---|---|---|---|---|
| Singh (2008) [25] | Moderate to advanced periodontitis; at least 30% teeth with PPD ≥ 4 mm | Liquid chromatography method |
TA: 8.3 ± 0.7 TB: 7.9 ± 0.7 C: 8.08 ± 0.7 |
TA: 0.8 ± 0.66 TB: 0.6 ± 0.66 C: -0.02 ± 0.72 |
TA: 4.16 ± 11.55 TB: 4 ± 14.45 |
3 | No |
| Gilowski (2012) [26] | Periodontitis; at least four nonadjacent sites with PPD ≥ 4 mm | Turbidimetric inhibition immunoassay |
T: 6.7 (6.3–7.0) C: 6.2 (6.0–7.8) |
T: 0 ± 0.9 C: 0.1 ± 1.41 |
3 | No | |
| Moeintaghavi (2012) [27] | Mild to moderate periodontitis (American Academy of Periodontology) | Cobas Integra 700 apparatus (Roche Diagnostics, Germany) |
T: 8.15 ± 1.18 C: 8.72 ± 2.22 |
T: 0.74 ± 1.18 C: -0.25 ± 2.05 |
T: 17.5 ± 48.91 C: -9.78 ± 38.02 |
3 | na |
| Gaikwad (2013) [28] | Chronic generalized periodontitis | na |
T: 8.38 ± 0.89 C: 8.06 ± 1.10 |
T: 1.38 ± 0.83 C: 0.95 ± 1.05 |
na | 4 | No |
| Pradeep (2013) [29] | Chronic periodontitis; PPD ≥ 5 mm or CAL ≥ 4 mm and vertical bone loss ≥ 3mm on intraoral periapical radiographs | na |
T: 6.66 ± 0.11 C: 6.71 ± 0.13 |
3m: T: 0.02 ± 0.12 C: 0.03 ± 0.13 6m: T: 0.01 ± 0.12 C:0.03 ± 0.13 9m: T: 0.03 ± 0.11 C: 0.05 ± 0.13 |
na | 9 | No |
| Santos (2013) [30] | Generalized chronic periodontitis (Armitage 1999); at least 30% of sites with PPD and CAL ≥ 4 mm | high-performance liquid chromatography |
T: 10 ± 2.41 C: 10.4 ± 2.9 |
3m: T: 0.78 ± 3.5 C: 0.68 ± 2.5 6m: T: 0.19 ± 2.9 C: 0.74 ± 4.1 12m: T: 0.35 ± 3.4 C: 1.4 ± 3.0 |
3m: T: 5.4 ± 65.09 C: 1.4 ± 77.31 6m: T: 5.5 ± 57.34 C: 15.4 ± 80.06 12m: T: 7.2 ± 72.37 C: 20.6 ± 79.15 |
12 |
T: 7, C: 12 reported taste perception change/ dry mouth/ staining |
| Telgi (2013) [31] | mild to moderate periodontitis; PD: 4 – 5 mm | na |
TA: 7.68 ± 0.63 TB: 7.56 ± 0.59 C: 7.74 ± 0.59 |
TA: 0.58 ± 0.27 TB: 0.25 ± 0.14 C: 0.004 ± 0.12 |
TA: 2.88 ± 1.07 TB: 1.29 ± 0.53 C: 0.42 ± 0.71 |
3 | na |
| Macedo (2014) [11] | Chronic periodontitis, at least one site with PPD ≥ 5 mm on each quadrant, two teeth with CAL ≥ 6 mm | automated immunoturbidimetric method |
T: 8.6 ± 1.1 C: 8.0 ± 0.93 |
T: 0.87 ± 0.9 C: 0.41 ± 0.84 |
na | 3 | No |
| Miranda (2014) [32] | the sites with PPD and CAL ≥ 4 mm, a minimum of six teeth with at least one site with PPD and CAL ≥ 5 mm, BOP | high-performance liquid chromatography |
T: 8.53 ± 1.56 C: 8.99 ± 1.63 |
3m: T: -0.07 ± 1.83 C: 0.05 ± 1.67 6m: T: 0.04 ± 1.94 C: -0.08 ± 1.66 12m: T: -0.24 ± 2.54 C: 0.59 ± 1.81 |
3m: T: 5.46 ±38.30 C: 8.71 ± 40.41 6m: T: 6.19 ±34.01 C: 5.95 ± 45.50 12m:T:1.59± 36.53 C: 6.9 ± 47.09 |
12 |
Diarrhea: T: 7, C:3 Headache: T: 4, C: 1 Metallic taste: T: 4, C: 2 Nausea/Vomiting: T: 5, C: 2 |
| Tsalikis (2014) [33] | Moderate or advanced periodontitis; six pockets > 5mm, CAL > 3mm with radiographic bone loss | A1CNow + Multitest HbA1c system |
T: 6.70 ± 0.61 C: 6.89 ± 0.60 |
3m: T: 0.08 ± 0.58 C: -0.07 ± 0.88 6m: T: 0.22 ± 0.67 C: 0.09 ± 0.77 |
na | 6 | C: 1 reported Dizziness and difficulty to swallow |
| Wu (2015) [34] | mean CAL > 1mm (American Academy of Periodontology criteria) | immunoturbidimetry |
T: 7.41 ± 0.20 C: 7.39 ± 0.16 |
3m: T: 0.01 ± 0.19 C: 0.01 ± 0.16 6m: T: 0.32 ± 0.17 C: 0.03 ± 0.17 |
na | 6 | na |
| Koçak (2016) [35] | ≥ 8 sites with PPD ≥5mm | na |
T: 6.9 ± 0.7 C: 6.5 ± 0.6 |
T: 0.41 ± 0.19 C: 0.22 ± 0.25 |
na | 3 | na |
| Kumari (2016) [10] | Chronic periodontitis; PPD ≥ 5 mm or CAL ≥ 4 mm, and vertical bone loss ≥ 3 mm on intraoral periapical radiographs | na |
T: 6.72 ± 0.13 C: 6.75 ± 0.12 |
3m: T: 0.04 ± 0.14 C: 0.03 ± 0.13 6m: T: 0.06 ± 0.13 C: 0.04 ± 0.13 9m: T: 0.07 ± 0.13 C: 0.06 ± 0.13 |
na | 9 | No |
| Ramos (2016) [36] | At least one site with PPD ≥ 5 mm on each quadrant and two teeth with CAL ≥ 6 mm | High pressure liquid chromatography |
T: 10.6 ± 1.99 C: 9.69 ± 1.68 |
T: 0.99 ± 1.00 C: 0.76 ± 0.73 |
na | 3 | No |
CAL clinical attachment loss, PPD probing pocket depth, BOP bleeding on probe, na not avail
Fig. 2.
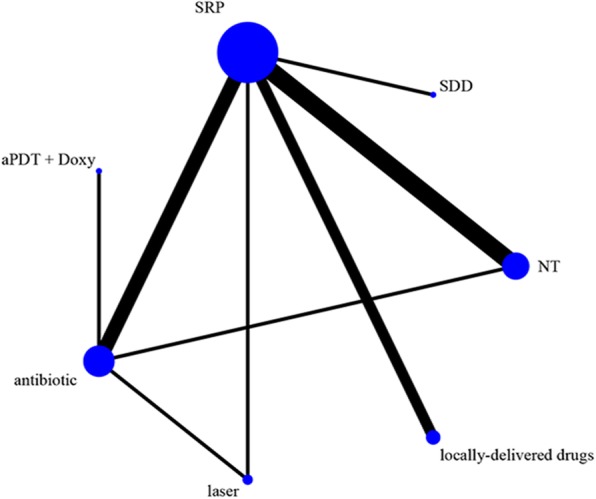
Network of the interventional comparisons for the Bayesian network analysis. The size of the nodes is proportional to the number of subjects (sample size) randomized to receive the therapy. The width of the lines is proportional to the number of trials comparing each pair of treatments. aPDT, antimicrobial photodynamic therapy; Doxy, doxycycline; antibiotics (Doxy, metronidazole + amoxicillin); local, locally-delivered drugs (atorvastatin gel, chlorhexidine gel, simvastatin gel); laser (diode laser, aPDT); SDD, subantimicrobial dose doxycycline; SRP, scaling and root planing, NT, no treatment
Risk of bias in included studies
Figure 3 presented reviewers’ judgments on the risk of bias in the referred RCTs. Most included studies were found to have methodological issues, with the most problematic domains being the allocation concealment (unclear or high risk in 35.7% of studies). Though the included studies were RCTs, 35.7% studies did not report the details of the randomized method. One study had an low risk of selective outcome reporting bias. In addition, two studies also might have had other bias, because we could not judge if there were baseline imbalances between groups (Additional file 3).
Fig. 3.

Judgements about each risk of bias item for each included study
Efficacy of different treatments for the reduction of HbA1c% in subjects with CP and T2DM
In traditional meta-analysis (Table 3), SRP + laser showed additional benefits compared to SRP alone (0.19 [0.08, 0.30]). There was statistically significant reduction in HbA1c% after treated with SRP + antibiotic (0.82 [0.33, 1.31]) compared to no treatment. In addition, there was no other significant difference was observed among the rest of the comparisons. The overall heterogeneity was high (Global I2 = 83.2), but mainly between SRP and no treatment (I2 = 94.2), while the heterogeneity was lower in other subgroups (I2 = 0).
Table 3.
Comparison of outcomes between traditional meta-analysis and Bayesian network meta-analysis
| treatment comparison | traditional meta-analysis | network meta-analysis |
|---|---|---|
| SRP + Antibiotic vs. SRP | 0.21 (-0.03, 0.45) | 0.20 (-0.17, 0.56) |
| SRP + Laser vs. SRP | 0.19 (0.08, 0.30) | 0.25 (-0.24, 0.78) |
| SRP + locally-delivered drug vs. SRP | 0.00 (-0.05, 0.05) | 0.0026 (-0.41, 0.43) |
| SRP + SDD vs. SRP | -0.10 (-0.90, 0.70) | -0.10 (-1.1, 0.89) |
| SRP + aPDT + Doxy vs. SRP + Antibiotic | 0.46 (-0.16, 1.08) | 0.46 (-0.40, 1.31) |
| SRP + Laser vs. SRP + Antibiotic | 0.23 (-0.40, 0.86) | 0.053 (-0.48, 0.61) |
| SRP vs. No treatment | 0.45 (0.00, 0.89) | 0.40 (0.088, 0.80) |
| SRP + Antibiotic vs. No treatment | 0.82 (0.33, 1.31) | 0.61 (0.16, 1.11) |
SRP scaling and root planing, SDD subantimicrobial dose doxycycline, aPDT antimicrobial photodynamic therapy, Doxy doxycycline
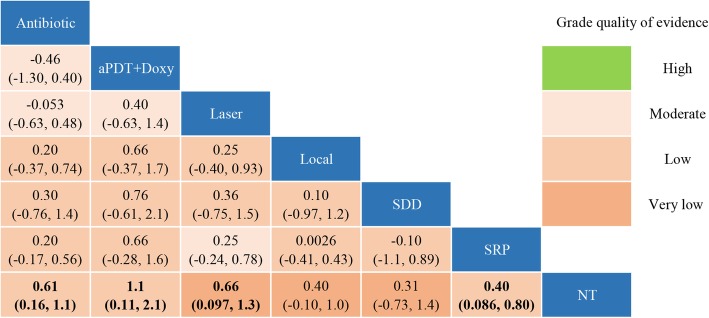
In network meta-analysis, sensitivity analysis indicated that both non-informative uniform and normal prior distributions were properly used (Additional file 4). SRP + antibiotic (0.61 [0.16, 1.1]), SRP + aPDT + Doxy (1.1 [0.11, 2.1]), SRP + laser (0.66 [0.097, 1.3]) and SRP alone (0.40 [0.086, 0.80]) was significantly better than no treatment in improving HbA1c%, separately (Fig. 4). No other significant difference was observed among the rest of the comparisons. The probabilities the most effective treatment methods of decreasing HbA1c% was SRP + aPDT + Doxy (71.2%), followed by SRP + laser (13.6%), SRP + SDD (8.6%), SRP + antibiotic (3.8%), SRP (0.2%) and no treatment (0.02%) (Fig. 5). The Global I2 was 86.18, except that between SRP and no treatment or SRP + laser were 95.63 and 23.56, respectively, the other subgroups were 0 (Table 4).
Fig. 4.
Multiple-treatment comparisons and the quality of evidence for ΔHbAlc%. aPDT, antimicrobial photodynamic therapy; Doxy, doxycycline; antibiotics (Doxy, metronidazole + amoxicillin); local, locally-delivered drugs (atorvastatin gel, chlorhexidine gel, simvastatin gel); laser (diode laser, aPDT); SDD, subantimicrobial dose doxycycline; SRP, scaling and root planing, NT, no treatment
Fig. 5.
The rank of different treatments. aPDT, antimicrobial photodynamic therapy; Doxy, doxycycline; antibiotics (Doxy, metronidazole + amoxicillin); local, locally-delivered drugs (atorvastatin gel, chlorhexidine gel, simvastatin gel); laser (diode laser, aPDT); SDD, subantimicrobial dose doxycycline; SRP, scaling and root planing, NT, no treatment
Table 4.
Analysis of heterogeneity
| t1 | t2 | i2.pair | i2.cons | incons.p |
|---|---|---|---|---|
| HbA1c% | ||||
| Per-comparison I-squared | ||||
| SRP + Antibiotic | SRP + aPDT + Doxy | NA | NA | NA |
| SRP + Antibiotic | SRP + Laser | NA | 0.00 | 0.62 |
| SRP + Antibiotic | NT | NA | 0.00 | 0.587 |
| SRP + Antibiotic | SRP | 0.00 | 0.00 | 0.98 |
| SRP + SDD | SRP | NA | NA | NA |
| SRP + Laser | SRP | NA | 23.56 | 0.77 |
| SRP + Locally-delivered drugs | SRP | 0.00 | 0.00 | NA |
| NT | SRP | 95.43 | 95.63 | NA |
| Global I-squared | 88.11 | 86.18 | ||
SRP scaling and root planing, SDD subantimicrobial dose doxycycline, t1 treatment 1, t2 treatment 2, i2.pair i-square of pair-wise meta-analysis, i2.cons i-square of network meta-analysis, incons.p inconsistency p-values for pairwise and network meta-analysis, NA not applicable
Efficacy of different treatments for the reduction of FPG in subjects with CP and T2DM
We only did a pairwise meta-analysis, because there were only 5 available studies. SRP was not better than no treatment in FPG reduction (4.91 [− 1.95, 11.78], I2 = 46.6%) (Additional file 5). Moreover, no significant difference was observed between SRP + adjuvant and SRP (− 0.28 [− 8.66, 8.11], I2 = 0.0%).
Model fit and evaluation of consistency
The model fitted the data well for the Dbar approximated data points in both outcomes (Additional file 6), and the effects of most comparisons between pairwise and network meta-analysis were similar in the relevant CI or CrI (Table 3). In addition, the node-splitting analysis also showed that there was no inconsistency among direct, indirect and network outcomes with P > 0.05 (Table 5).
Table 5.
The results of node-spitting analysis
| comparison | p-value | MD (95%CrI) |
|---|---|---|
| HbA1c% | ||
| SRP + Antibiotic vs. SRP + Laser | ||
| direct | 0.61455 | 0.23 (-0.66, 1.1) |
| indirect | -0.059 (-0.83, 0.72) | |
| network | 0.043 (-0.49, 0.62) | |
| SRP + Antibiotic vs. No treatment | ||
| direct | 0.5605667 | -0.82 (-1.7, 0.036) |
| indirect | -0.53 (-1.2, 0.090) | |
| network | -0.62 (-1.1, -0.18) | |
| SRP + Antibiotic vs. SRP | ||
| direct | 0.6489667 | -0.22 (-0.65, 0.22) |
| indirect | 0.040 (-1.1, 1.2) | |
| network | -0.22 (-0.58, 0.15) | |
| SRP + Laser vs. SRP | ||
| direct | 0.5950500 | -0.19 (-0.85, 0.47) |
| indirect | -0.48 (-1.5, 0.50) | |
| network | -0.26 (-0.79, 0.23) | |
SRP scaling and root planing
Sensitivity analysis and publication bias
To explore whether the baseline level affects the final effects, we excluded studies with relative higher HbA1c% (> 9%) or relative lower HbA1c% (< 7%). The results were shown in Additional file 7. When we excluded the studies with HbA1c% > 9%, no major changes were found except for SRP + laser versus no treatment, from 0.66 [0.097, 1.3] to 0.59 [− 0.15, 1.4]. Nevertheless, if we excluded studies with HbA1c% < 7%, no significant difference was found any treatment comparisons. The result of the comparison-adjusted funnel plot of HbA1c% reduction revealed there may be publication bias owing asymmetry (Fig. 6).
Fig. 6.
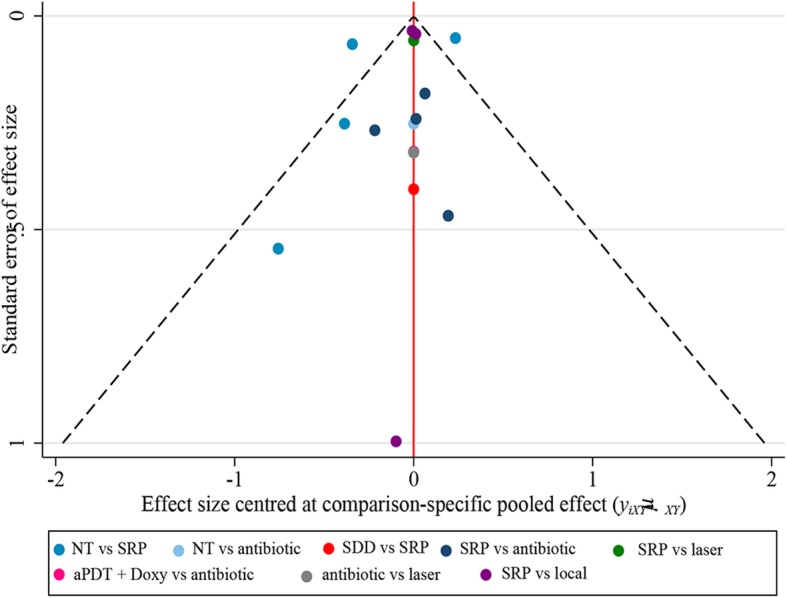
Publication bias assessment for ΔHbAlc%. aPDT, antimicrobial photodynamic therapy; Doxy, doxycycline; antibiotics (Doxy, metronidazole + amoxicillin); local, locally-delivered drugs (atorvastatin gel, chlorhexidine gel, simvastatin gel); laser (diode laser, aPDT); SDD, subantimicrobial dose doxycycline; SRP, scaling and root planing, NT, no treatment
Quality of network meta-evidence
We used Grade approach to assess the quality of evidence and the results were presented in Fig. 4. Most of the comparisons were found to be of low quality evidence, four comparisons (SRP + antibiotic vs. SRP + aPDT + Doxy, SRP + laser vs. SRP/SRP + antibiotic/aPDT + Doxy) were considered to have moderate quality of evidence and three comparisons (SRP + laser/ local-delivery drugs) were considered as low-quality of evidence. These indicate that we have limited confidence in these recommendations, and future research may change them.
Discussion
This network meta-analysis of 14 RCTs including 629 patients that compared the HbA1c% reduction of different treatments indicated that all the treatments might improve HcA1c% than no treatment except for SDD or local-delivery drugs adjunctive to SRP. Furthermore, among the treatments, SRP + aPDT + Doxy was the most effective. No evidence of effectiveness was found in the lowering of FPG.
Evidence-based data of the periodontal treatment effect on glycemic control in T2DM are limited. Although some studies have compared SRP with no treatment or SRP + adjuvant treatment, there was still a lack of sufficient data comparing the different adjuvant treatments. Our study included all periodontal treatments that met our inclusion criteria and made a more overall evaluation. The results from network meta-analysis and traditional meta-analysis were largely consistent. Even so, laser might improve the efficacy of SRP in HbA1c% reduction in our pairwise meta-analysis (0.19 [0.08, 0.30]), but not in our network meta-analysis (0.25 [− 0.24, 0.78]). This inconsistency may be attributed to chance alone, as only one study with small sample size provided direct evidence. The inconsistency was also found when comparing SRP with no treatment. SRP could reduce periodontal pathogens, and subsequently inhibit cytokines associated with inflammatory markers, leading to decreased glucose [37]. Our network meta-analysis supported the efficacy of SRP (0.40 [0.088, 0.80]), and was consistent with previous meta-analyses [5, 38]. Although our pairwise meta-analysis showed that SRP was not better than no treatment (0.45 [0.00, 0.89]), the lower bound of the 95% CI was 0. Heterogeneity between SRP and no treatment is extremely high in both pairwise and network meta-analysis, this may due to the fact that the baseline of HbA1c% is not balanced in the 3 included studies and they may have serious methodological issues. For example, they did not report how to perform allocation concealment. More well-executed and multi-center trails are still required to explore the effect of SRP.
We also found an interesting phenomenon that SRP + SDD / local-delivery drugs did not showed any advantages of improving HbA1c% than no treatment. It seems illogical since SRP alone was better than no treatment. There was only one study with small sample sizes in SRP + SDD; further trials are needed to ascertain the role of SDD in improving HbA1c%. The uncomparable baseline of HbA1c% may be attributed to the inefficacy of SRP + local-delivery drugs: HbA1c% > 10 for Santos et al. [30]; HbA1c% < 7 for Pradeep et al. [29], Kumari et al. Sensitivity analyses indicated that the baseline of HbA1c% may influence the efficacy of the periodontal treatments. After we excluded the studies with HbA1c% > 9, the major results did not change greatly. Nonetheless, when the studies with HbA1c% < 7 were excluded, all treatments except SRP + antibiotic could not significantly decrease HbA1c% compared to no treatment. Longitudinal studies involving subjects with different HbA1c% is therefore warranted.
Antibiotics are commonly applied in periodontal treatment. They could significantly decrease the levels of TNF and IL-6 in serum [39]. TNF and IL-6 could impair intracellular insulin signalling, potentially leading to insulin resistance [40]. Contrary to our expectations, we found it couldn’t provide additional benefits compared to SRP alone (0.20 [− 0.17, 0.56]). This was in conformance with findings of Wang et al.(− 0.238 [− 0.616, 0.140]) [11] and Lira Junior et al.(− 0.11[− 0.35,0.13]) [14].
Laser, especially aPDT has been attracting huge interest in adjunct to SRP due to its better antibacterial ability with lower technically sensitive. In our network meta-analysis, we included three RCTs that compared laser to other treatments (SRP + aPDT vs. SRP, SRP + diode laser vs. SRP and SRP + aPDT vs. SRP + Doxy). We found SRP + laser was better compared to no treatment (0.66 [0.097, 1.3]) but not SRP (0.25 [− 0.24, 0.78]). Similarly, a meta-analysis of 2 RCTs was conducted by Abduljabbar et al. showed that aPDT didn’t provide additional benefits compared to SRP alone (0.035 [− 0.47, 0.54]) [13]. However, one of its included RCT [11] was used in treating their patients with doxycycline. In our opinion, it might intensify the effect of aPDT, so we considered it as an independent group (aPDT + Doxy) rather than the laser group. In our network meta-analysis, aPDT + Doxy adjunctive to SRP was ranked best among the comparisons, although there was only one trial of 30 patients was included. It is difficult to draw a solid conclusion with such a small amount of RCTs. Thus, we look forward to more well-executed and multi-center trails.
Regarding FPG, SRP was not better than no treatment (4.91 [− 1.95, 11.78]), and adjuvant treatments did not show any advantage than SRP alone (− 0.28 [− 8.66, 8.11]). The results are consistent with Corbella et al. [17]. However, Sgolastra et al. [41]and Teshome et al. [42] support the effectiveness of periodontal treatment in lowering FPG. Since few studies have reported the results of FPG, it is difficult to draw a conclusion. Therefore, more RCTs were expected to clear out the real effect of periodontal treatments.
This network meta-analysis has several limitations. First, only included English studies were included and their quality is the principal limitation, a low risk of bias was found in six of the 14 included studies. Second, the sample sizes were relatively small, from 30 to 66 subjects. The follow-up duration of the trials was short, resulting in uncertain outcomes. With the foregoing limitations, well-executed and multi-center trails comparing the different adjuvant treatments, and extending the follow-up duration up to 12 or 24 months should be conducted. Finally, this meta-analysis excluded split-mouth RCT, patients with severe T2DM complications and smorkers to meet the transitivity, indicating that the conclusions of this meta-analysis apply to non-smoking CP patients without severe T2DM complications. In addition, though we have adopted strict eligibility criteria, we also found high heterogeneity in comparing SRP with no treatment. We also found a certain publication bias.
Conclusion
The results of this meta-analysis seem to support that periodontal treatment with aPDT + Doxy possesses the best efficacy in lowering HbA1c% of non-smoking CP without severe T2DM complications. However, the quality of evidence is low or very low, and therefore further studies are needed to confirm the results.
Additional files
Search strategy used in PubMed/MEDLINE. (DOCX 14 kb)
List of excluded duplicate studies. (DOCX 32 kb)
Sensitivity analysis for informative uniform distribution. (DOCX 24 kb)
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. (DOCX 342 kb)
Forest plot of changes in FPG. (DOCX 172 kb)
Evaluation of model fit. (DOCX 14 kb)
Sensitivity analysis. (DOCX 16 kb)
Data extraction table of this network meta-analysis. (DOCX 16 kb)
Acknowledgements
We want to acknowledge Dr. Jianying Zhang (Central South University) for her help during this manuscript development.
Abbreviations
- CP
Chronic periodontitis
- T2DM
Type 2 diabetes mellitus
- SRP
Scaling and root planning
- Doxy
Doxycycline
- SDD
Subantimicrobial dose doxycycline
- SMV
Simvastatin
- CHX
Chlorhexidine
- aPDT
Antimicrobial photodynamic therapy
- AMX
Amoxicillin
- MTZ
Metronidazole
- ATV
Atorvastatin
- DL
Diode laser
- PC
Phenothiazine chloride solution
Authors’ contributions
RYC, QLL and MFY were responsible for study selection, quality assessment, data extraction and data synthesis. RYC drafted the manuscript. HBZ, QQW and YC participated in the research design and revision of the manuscript. All authors read and approved the final manuscript.
Funding
There was no funding for this review.
Availability of data and materials
All data generated or analysed during this study are included in this published article and Additional file 8.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Lu Y, Zhang L, Liu Y, Zhou Y, Chen Y, Yu H. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells. Arch Med Sci. 2015;11(3):638–646. doi: 10.5114/aoms.2015.52370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buset SL, Walter C, Friedmann A, Weiger R, Borgnakke WS, Zitzmann NU. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J Clin Periodontol. 2016;43(4):333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti MS, Eickholz P, Loos BG, Papapanou P, van der Velden U, Armitage G, Bouchard P, Deinzer R, Dietrich T, Hughes F, et al. Principles in prevention of periodontal diseases: consensus report of group 1 of the 11th European workshop on periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol. 2015;42(Suppl 16):S5–11. doi: 10.1111/jcpe.12368. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno H, Ekuni D, Maruyama T, Kataoka K, Yoneda T, Fukuhara D, Sugiura Y, Tomofuji T, Wada J, Morita M. The effects of non-surgical periodontal treatment on glycemic control, oxidative stress balance and quality of life in patients with type 2 diabetes: a randomized clinical trial. PLoS One. 2017;12(11):e0188171. doi: 10.1371/journal.pone.0188171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Mao Y, Dai P, Li X, Gu W, Wang H, Wu G, Ma J, Huang S. Mitochondrial dysfunction is involved in the aggravation of periodontitis by diabetes. J Clin Periodontol. 2017;44(5):463–471. doi: 10.1111/jcpe.12711. [DOI] [PubMed] [Google Scholar]
- 7.Winning L, Patterson CC, Neville CE, Kee F, Linden GJ. Periodontitis and incident type 2 diabetes: a prospective cohort study. J Clin Periodontol. 2017;44(3):266–274. doi: 10.1111/jcpe.12691. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Zhang W, Liu X, Zhang W, Li Y. Interrelationship between diabetes and periodontitis: role of hyperlipidemia. Arch Oral Biol. 2015;60(4):667–674. doi: 10.1016/j.archoralbio.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari M, Martande SS, Pradeep AR, Naik SB. Efficacy of Subgingivally delivered 1.2% atorvastatin in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. J Periodontol. 2016;87(11):1278–1285. doi: 10.1902/jop.2016.130227. [DOI] [PubMed] [Google Scholar]
- 11.Macedo Gde O, Novaes AB, Jr, Souza SL, Taba M, Jr, Palioto DB, Grisi MF. Additional effects of aPDT on nonsurgical periodontal treatment with doxycycline in type II diabetes: a randomized, controlled clinical trial. Lasers Med Sci. 2014;29(3):881–886. doi: 10.1007/s10103-013-1285-6. [DOI] [PubMed] [Google Scholar]
- 12.Simpson TC, Weldon JC, Worthington HV, Needleman I, Wild SH, Moles DR, Stevenson B, Furness S, Iheozor-Ejiofor Z. Treatment of periodontal disease for glycaemic control in people with diabetes mellitus. Cochrane Database Syst Rev. 2015;(11):CD004714. [DOI] [PMC free article] [PubMed]
- 13.Abduljabbar T, Vohra F, Javed F, Akram Z. Antimicrobial photodynamic therapy adjuvant to non-surgical periodontal therapy in patients with diabetes mellitus: a meta-analysis. Photodiagn Photodyn Ther. 2017;17:138–146. doi: 10.1016/j.pdpdt.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Lira Junior R, Santos CMM, Oliveira BH, Fischer RG, Santos APP. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: a systematic review. J Dent. 2017;66:1–7. doi: 10.1016/j.jdent.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang TF, Jen IA, Chou C, Lei YP. Effects of periodontal therapy on metabolic control in patients with type 2 diabetes mellitus and periodontal disease: a meta-analysis. Medicine. 2014;93(28):e292. doi: 10.1097/MD.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Han X, Guo X, Luo X, Wang D. The effect of periodontal treatment on hemoglobin a1c levels of diabetic patients: a systematic review and meta-analysis. PLoS One. 2014;9(9):e108412. doi: 10.1371/journal.pone.0108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbella S, Francetti L, Taschieri S, De Siena F, Fabbro MD. Effect of periodontal treatment on glycemic control of patients with diabetes: a systematic review and meta-analysis. J Diabetes Investig. 2013;4(5):502–509. doi: 10.1111/jdi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Wang X, Yang K, Zhang J, Luo J, Gao P, Ma Y, Jiao L, Ling F. Endovascular treatment for symptomatic intracranial artery stenosis: protocol for a systematic review and network meta-analysis. BMJ Open. 2018;8(7):e022359. doi: 10.1136/bmjopen-2018-022359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yogendrakumar V, Lun R, Hutton B, Fergusson DA, Dowlatshahi D. Comparing pharmacological venous thromboembolism prophylaxis to intermittent pneumatic compression in acute intracerebral haemorrhage: protocol for a systematic review and network meta-analysis. BMJ Open. 2018;8(11):e024405. doi: 10.1136/bmjopen-2018-024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- 21.Liew TM, Lee CS. Comparative efficacy and acceptability of interventions for major depression in older persons: protocol for Bayesian network meta-analysis. BMJ Open. 2018;8(1):e019819. doi: 10.1136/bmjopen-2017-019819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelhalter DJ, Best NG, Carlin BR, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002;64:583–616. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- 23.Zhang Y, Kang S, Fang W, Wu X, Liang W. Network meta-analysis on prophylactic regimens against recurrent hepatitis B virus infection after liver transplantation. Hepatobiliary Surg Nutr. 2013;2(6):297–303. doi: 10.3978/j.issn.2304-3881.2013.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Singh S, Kumar V, Kumar S, Subbappa A. The effect of periodontal therapy on the improvement of glycemic control in patients with type 2 diabetes mellitus: a randomized controlled clinical trial. Int J Diabetes Dev Ctries. 2008;28(2):38–44. doi: 10.4103/0973-3930.43097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilowski L, Kondzielnik P, Wiench R, Plocica I, Strojek K, Krzeminski TF. Efficacy of short-term adjunctive subantimicrobial dose doxycycline in diabetic patients - randomized study. Oral Dis. 2012;18(8):763–770. doi: 10.1111/j.1601-0825.2012.01943.x. [DOI] [PubMed] [Google Scholar]
- 27.Moeintaghavi A, Arab HR, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: a randomized controlled clinical trial. Aust Dent J. 2012;57:31–37. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 28.Gaikwad SP, Gurav AN, Shete AR, Desarda HM. Effect of scaling and root planing combined with systemic doxycycline therapy on glycemic control in diabetes mellitus subjects with chronic generalized periodontitis: a clinical study. J Periodontal Implant Sci. 2013;43:79–86. doi: 10.5051/jpis.2013.43.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradeep AR, Rao NS, Bajaj P, Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol. 2013;84:24–31. doi: 10.1902/jop.2012.110721. [DOI] [PubMed] [Google Scholar]
- 30.Santos VR, Lima JA, Miranda TS, Gonçalves TE, Figueiredo LC, Faveri M, Duarte PM. Full-mouth disinfection as a therapeutic protocol for type-2 diabetic subjects with chronic periodontitis: twelve-month clinical outcomes: a randomized controlled clinical trial. J Clin Periodontol. 2013;40:155–162. doi: 10.1111/jcpe.12040. [DOI] [PubMed] [Google Scholar]
- 31.Telgi RL, Tandon V, Tangade PS, Tirth A, Kumar S, Yadav V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci. 2013;43(4):177–182. doi: 10.5051/jpis.2013.43.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda TS, Feres M, Perez-Chaparro PJ, Faveri M, Figueiredo LC, Tamashiro NS, Bastos MF, Duarte PM. Metronidazole and amoxicillin as adjuncts to scaling and root planing for the treatment of type 2 diabetic subjects with periodontitis: 1-year outcomes of a randomized placebo-controlled clinical trial. J Clin Periodontol. 2014;41:890–899. doi: 10.1111/jcpe.12282. [DOI] [PubMed] [Google Scholar]
- 33.Tsalikis L, Sakellari D, Dagalis P, Boura P, Konstantinidis A. Effects of doxycycline on clinical, microbiological and immunological parameters in well-controlled diabetes type-2 patients with periodontal disease: a randomized, controlled clinical trial. J Clin Periodontol. 2014;41:972–980. doi: 10.1111/jcpe.12287. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Chen L, Wei B, Luo K, Yan F. Effect of non-surgical periodontal treatment on visfatin concentrations in serum and gingival crevicular fluid of patients with chronic periodontitis and type 2 diabetes mellitus. J Periodontol. 2015;86:795–800. doi: 10.1902/jop.2015.140476. [DOI] [PubMed] [Google Scholar]
- 35.Kocak E, Saglam M, Kayis SA, Dundar N, Kebapcilar L, Loos BG, Hakki SS. Nonsurgical periodontal therapy with/without diode laser modulates metabolic control of type 2 diabetics with periodontitis: a randomized clinical trial. Lasers Med Sci. 2016;31(2):343–353. doi: 10.1007/s10103-016-1868-0. [DOI] [PubMed] [Google Scholar]
- 36.Ramos UD, Ayub LG, Reino DM, Grisi MF, Taba M, Souza SL, Palioto DB, Novaes AB. Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J Clin Periodontol. 2016;43:147–155. doi: 10.1111/jcpe.12498. [DOI] [PubMed] [Google Scholar]
- 37.Perez CM, Munoz F, Andriankaja OM, Ritchie CS, Martinez S, Vergara J, Vivaldi J, Lopez L, Campos M, Joshipura KJ. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J Clin Periodontol. 2017;44(2):142–149. doi: 10.1111/jcpe.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liew AK, Punnanithinont N, Lee YC, Yang J. Effect of non-surgical periodontal treatment on HbA1c: a meta-analysis of randomized controlled trials. Aust Dent J. 2013;58(3):350–357. doi: 10.1111/adj.12091. [DOI] [PubMed] [Google Scholar]
- 39.Fredeking TM, Zavala-Castro JE, Gonzalez-Martinez P, Moguel-Rodriguez W, Sanchez EC, Foster MJ, Diaz-Quijano FA. Dengue patients treated with doxycycline showed lower mortality associated to a reduction in IL-6 and TNF levels. Recent Pat Antiinfect Drug Discov. 2015;10(1):51–58. doi: 10.2174/1574891X10666150410153839. [DOI] [PubMed] [Google Scholar]
- 40.Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, Taylor R. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgolastra F, Severino M, Pietropaoli D, Gatto R, Monaco A. Effectiveness of periodontal treatment to improve metabolic control in patients with chronic periodontitis and type 2 diabetes: a meta-analysis of randomized clinical trials. J Periodontol. 2013;84(7):958–973. doi: 10.1902/jop.2012.120377. [DOI] [PubMed] [Google Scholar]
- 42.Teshome A, Yitayeh A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: systematic review and meta-analysis. BMC Oral Health. 2016;17(1):31. doi: 10.1186/s12903-016-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy used in PubMed/MEDLINE. (DOCX 14 kb)
List of excluded duplicate studies. (DOCX 32 kb)
Sensitivity analysis for informative uniform distribution. (DOCX 24 kb)
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. (DOCX 342 kb)
Forest plot of changes in FPG. (DOCX 172 kb)
Evaluation of model fit. (DOCX 14 kb)
Sensitivity analysis. (DOCX 16 kb)
Data extraction table of this network meta-analysis. (DOCX 16 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and Additional file 8.