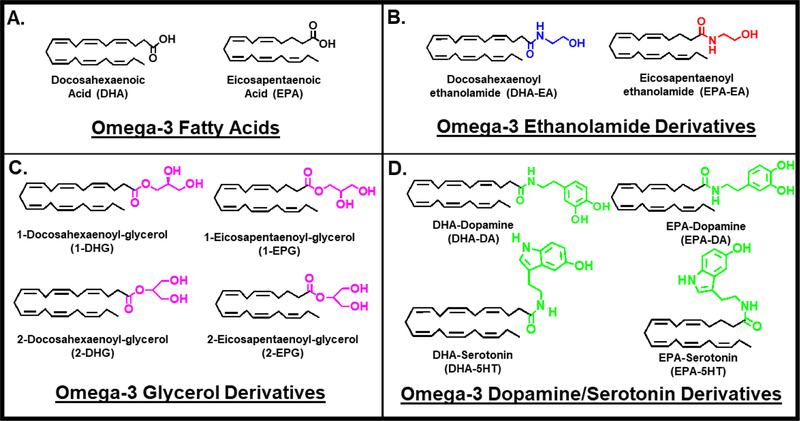
Abstract
Cannabinoid receptor activation is involved in homeostatic regulation of the body. These receptors are activated by cannabinoids, that include the active constituents of Cannabis sativa as well as endocannabinoids (eCBs). The eCBs are endogenously synthesized from the omega-6 and omega-3 polyunsaturated fatty acids (PUFAs). The consumption of omega-3 fatty acids shifts the balance towards a higher proportion of omega-3 eCBs, whose physiological functions warrants further investigation. Herein, we review the discovery of omega-3 fatty acid derived eCBs that are generated from long chain omega-3 PUFAs - docosahexaenoyl ethanolamide (DHA-EA or synaptamide), docosahexanoyl-glycerol (DHG), eicosapentaenoyl ethanolamide (EPA-EA), eicosapentanoylglycerol (EPG). Furthermore, we outline the lesser known omega-3 eCB-like molecules that arise from the conjugation of the omega-3 fatty acids with neurotransmitters serotonin and dopamine - DHA-serotonin (DHA-5HT), EPA-serotonin (EPA-5HT), DHA-dopamine (DHA-DA) and EPA-dopamine (EPA-DA). Additionally, we describe the role of these omega-3 eCBs and their derivatives in different disease states such as pain, inflammation and cancer. Moreover, we detail the formation and potential physiological roles of the oxidative metabolites that arise from the metabolism of omega-3 eCBs by eicosanoid synthesizing enzymes - cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 epoxygenase (CYP450). In summary, we outline the novel findings regarding a growing class of signaling molecules, omega-3 eCBs, that can control the physiological and pathophysiological processes in the body.
Keywords: Endocannabinoids, omega-3 fatty acids, inflammation, cancer, metabolism
Introduction
Cannabis sativa is used for both medical and recreational use (1, 2). In the early 1950s, Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive molecule, was isolated from Cannabis sativa (3). This led to a strong interest in the synthesis of cannabinoid (CB) analogs to understand the structure-activity relationship with putative CB receptors (4–8). Eventually, two G-protein coupled receptors (GPCRs), cannabinoid receptor 1 and 2 (CB1 and CB2), were discovered (9, 10). While CB1 is highly expressed in the central nervous system (CNS), CB2 is present primarily in immune and peripheral cells. Since Δ9-THC and other CBs that bind to CB1 and CB2 are lipophilic, there was a search for endogenous lipids that interacted with these receptors.
In the 1990s, the first endogenous cannabinoids (eCBs) that were shown to activate CB1 were anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (11–13). AEA and 2-AG are synthesized from omega-6 polyunsaturated fatty acid (PUFA), arachidonic acid (AA), and are hydrolyzed by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. Later studies identified other eCB-metabolizing enzymes, such as FAAH 1 and 2 for AEA; MAGL, α,β-hydrolase domain-containing 6 and 12 (ABDH6 and ABDH12) for 2-AG. Both AEA and 2-AG are also metabolized by eicosanoid synthesizing enzymes such as lipoxygenases (LOX), cyclooxygenase 2 (COX-2) and cytochrome P450 epoxygenases (CYP450) to form new bioactive molecules (14, 15). Together, the interactions between the CB receptors, eCB ligands, as well as their biosynthetic and degradative enzymes, constitute the eCB system (ECS) and are involved in maintaining homeostasis in the biological system (16).
Broadly, the eCBs are members of a large group of structurally related fatty acid derived signaling molecules. It is important to note that eCBs are traditionally defined as ligands with significant affinity and agonism to CB1 and CB2 according to the NC-IUPHAR committee (17). This definition resulted in the discovery and synthesis of several other eCB and “eCB-like” analogues (18–21). Thus, many of these fatty acid derived molecules are eCB-like ligands that have weak affinity to CB receptors and cannot be considered as classical eCBs. Additionally, as the definition of the ECS system is expanding, it is currently known that CBs and other eCB-like compounds, such as N-arachidonylglycine (NA-Gly), can target more receptors, such as TRPV1, GPR18, GPR119 and GPR55 (22–26).
With the increasing focus towards the consumption of omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), there has been a growing interest in the discovery of omega-3 fatty acid derived eCB or eCB-like molecules with novel bioactivity (27). Herein, we review the discovery and physiological role of omega-3 eCBs that are derived from DHA and EPA (Figure 1A). We illustrate that the derivatization of carboxylic acid end of the omega-3 fatty acid with ethanolamide, glycerol or other groups, such as neurotransmitters, leads to substantial changes in their biological activity and receptor interactions. We also review the discovery, mechanism and activity of the following omega-3 eCBs- DHA-ethanolamide (DHA-EA), 2-docosahexaenoyl-glycerol (2-DHG) and eCB-like- DHA-serotonin (DHA-5HT), DHA-dopamine (DHA-DA) and the EPA analogs. Additionally, we also evaluate the formation and potential physiological roles of their oxidative metabolites, which arise from the metabolism of omega-3 eCBs by eicosanoid synthesizing enzymes.
Figure 1. Omega-3 endocannabinoids and derivatives.
Chemical Structures of (A) Omega-3 fatty acids, Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) and N-acyl amide derivatives that have been identified in literature. (B) Docosahexaenoyl ethanolamide (DHA-EA), Eicosapentaenoyl ethanolamide (EPA-EA), (C) 1-Docosahexaenoyl-glycerol (1-DHG), 1-Eicosapentaenoyl- glycerol (1-EPG), (D) DHA-Dopamine (DHA-DA), EPA-Dopamine (EPA-DA), DHA-Serotonin (DHA-5HT) and EPA-Serotonin (EPA-5HT).
1. Omega-3 fatty acid DHA ethanolamide (DHA-EA or DHEA)
In order to study the structure-activity relationship of eCB-like analogues to CB1, an omega-3 derivative of DHA, N-docosahexaenoylethanolamide (DHA-EA or DHEA), was synthesized. In comparison to AEA, DHA-EA was shown to be 8- to 22.5-fold less potent to CB1 receptor (Figure 1B & Table 1) (12, 28). Later, DHA-EA was found endogenously in brain and retina (29, 30). When piglets were supplemented with a DHA diet, a several fold increase in DHA-EA levels were measured (Table ST1) (30). Additionally, dietary consumption of fish oil led to higher levels of DHA-EA in rodent brain, jejunum, liver and adipose tissue (Table ST1) (31–34). DHA-EA was also measured under inflammatory conditions in rat cerebrospinal fluid and the spinal cord tissue (Table ST1) (35). Most recently, DHA-EA has been detected in human milk (Table ST1) (36). As DHA-EA is identified in the body, further investigation of its physiological and pharmacological roles are necessitated.
Table 1.
Cannabinoid Receptor Binding and Activation of DHA-EA, EPA-EA and oxygenated metabolites
| Compound | CB1 EC50 values (nM) | CB2 EC50 values (nM) | References |
|---|---|---|---|
| AEA | 0.7a | 1352a | (19) |
| 39.2 ± 5.7b | NDb | (28) | |
| 543 ± 83b | NDb | (12) | |
| 89 ± 10b (with PMSF) | NDb | (60) | |
| DHA-EA | 1044a | 305a | (19) |
| 9.8a | NDa | (20) | |
| 12200 ± 520b | NDb | (12) | |
| 324.1 ± 9.2b | NDb | (28) | |
| 19,20 EDP-EA | 108a | 280a | (19) |
| 10,17-diHDHA-EA | NDa | 3.9a | (20) |
| 15-HEDPEA | NDa | 1a | (20) |
| EPA-EA | 0.1a | 2.1a | (19) |
| 1470 ± 500b (with PMSF) | NDb | (60) | |
| 162.3 ± 13.6b | NDb | (28) | |
| 17,18 EEQ-EA | 18.5a | 1.4a | (19) |
Abbreviations: N-docosahexaenoylethanolamine (DHA-EA), eicosapentaenoyl ethanolamide (EPA-EA), epoxydocosapentaenoic acid-ethanolamide (EDP-EA), epoxyeicosatetraenoic acid-ethanolamide (EEQ-EA), dihydroxydocosahexaenoyl ethanolamide (diHDHA-EA), hydroxy-epoxy-docosapentaenoyl ethanolamide (HEDPEA) and not determined (ND).
Experiments were performed with cell cultures transfected with CB receptors
Experiments were performed with displacement of CB receptor agonist
Experiments were measured by inhibition of cAMP
1.1. Role of DHA-EA in the Central Nervous System (CNS)
Since DHA-EA is detected in the brain, its role in synaptogenesis (formation of synapses between neurons) were investigated (37). Previously, DHA-EA production in neurons correlated with enhanced glutamatergic synaptic activity (38, 39). This activity is required for the synaptogenesis process; hence, DHA-EA was named “synaptamide” (40). In dose-dependent studies, DHA-EA significantly induced synaptic activity at 0.1 μM and 1 μM. Additionally, DHA-EA treatment increased hippocampal neurite growth 10-fold more than that of DHA following three days of supplementation. Similar to AEA, DHA-EA is hydrolyzed to DHA by FAAH. Interestingly, in the presence of a FAAH inhibitor, the synaptogenic properties of DHA were potentiated and DHA-EA levels increased (39, 40). Collectively, results from these studies helped in establishing the dual significance of the combined amide moiety and omega-3 scaffold in promoting synaptogenesis (Table 2) (41).
Table 2.
Biological Effects of DHA-derived eCBs and Derivatives
| Effects of DHEA in Brain | References |
|---|---|
| 9.5-fold increase in piglet brain from DHA supplementation | (30) |
| Synaptic activity induced in hippocampal neurons | (39) |
| Neurite growth increased 10-fold greater than DHA | (39) |
| 50 to 100-fold more effective than DHA in differentiating NSCs | (44) |
| Phosphorylation of PKA substrates increased | (44) |
| Ethanol impairment reversed | (45) |
| Cellular cAMP production increased | (45) |
| GPCR activation increased | (45) |
| Reversed cAMP reduction caused by ethanol (25mM) | (45) |
| Promoted axon development | (46) |
| Negatively regulated sonic hedgehog signaling in cortical neurons | (46) |
| Increased cortical neuron growth | (46) |
| Inhibited sonic hedgehog induced signaling | (46) |
| Effects of DHEA in adipose, peritoneum and pain | |
| Reduced IL-6 and MCP-1 levels in LPS-stimulated adipocytes | (47) |
| Causes negative correlation in size of adipocytes | (33) |
| Reduced nitric oxide production and MCP-1 levels in peritoneal | |
| macrophages and RAW264.7 cells | (48) |
| Increases glucose up-take, CB1, CB2 and NAPE-PLD expression in C2C12 myoblasts | (55) |
| Reduced headache severity by High ω−3/Low ω−6 diet | (56) |
| Effects of DHEA in cancer | |
| Induced death of LNCaP and PC3 prostate cancer cells to androgen | (18) |
| Inhibits proliferation of MCF-7 breast cancer cells | (50) |
| Yield reduction of AKT-mTOR pathway | (50) |
| Effects of DHEA metabolized by lipooxygenase (LOX) | |
| Reduced migration of PMN chemotaxis by IL-8 | (20) |
| Activates CB2 receptors (10,17-diDHEA & 15-HEDPEA) | (20) |
| Decreased platelet-leukocyte aggregation by 30%−40% | (20) |
| Effects of Docosahexaenoyl Ethanolamide Epoxides (EDP-EA) | |
| Significantly reduced pro-inflammatory nitric oxide and IL-6 | (19) |
| Induced anti-inflammatory cytokine production in primary microglial cultures | (19) |
| Effects of Monoacylglyceride Docosahexaenoic Acid (1-DHG) | |
| Reduced vasoconstriction from human pulmonary tissues | (68) |
| Reduced arterial tension comparably to H-1152 by 50% | (68) |
| Reduced RhoA activity by lowering coupling to GTP | (68) |
| Reduced phosphorylation of Rho-kinase, MYPT-1 and MLC | (67, 99) |
| Reverse hypertension in human pulmonary arteries | (67, 99) |
| Reduced expression of VEGF | (67, 99) |
| Reduced inflammatory markers in cystic fibrosis induced by LPS | (74) |
| Effects of Docosahexaenoic Acid Dopamine (DHA-DA) | |
| Decelerated development of Parkinson’s disease symptoms | (85) |
| Elicits antioxidative properties under oxidative stress conditions | (85) |
| Suppressed nitric oxide production | (85) |
| Reduced pro-inflammatory markers, MCP-1, CCL-20 and IL-6 | (85) |
| Suppressed PGE2 by 75% | (85) |
| Reduced breast cancer cell viability | (87) |
| Decreases COX-2 transcript levels | (77) |
| Effects of Docosahexaenoic Acid Serotonin (DHA-5HT) | |
| Inhibited IL-6 by 75% in LPS-stimulated RAW262.7 cells | (89) |
| Inhibited PGE2 in LPS-stimulated RAW262.7 cells | (89) |
| Suppressed pro-inflammatory mediator transcript levels | (89) |
| Downregulated pro-inflammatory IL’s and co-stimulatory T cell molecules | (89) |
| Inhibited migration of RAW264.7 cells | (89) |
| Suppressed IL-17 in PBMC’s by 80% | (90) |
Given that eCBs elicit their activities through receptor activation, it was hypothesized that DHA-EA promoted synaptogenesis by acting as an endogenous ligand of GPR110. In the study, use of GPR110 KO mice abolished DHA-EA bioactivity by significantly reducing synaptic protein expression and synapse number in developing brains. DHA-EA also induced neurogenic differentiation and neurite outgrowth, which were also dependent upon GPR110 activation. Hence, the DHA-EA/GPR110 signaling is hypothesized to be an essential mechanism for active neurogenesis and synaptogenesis, proving vital for the development of brain function (42).
The role of DHA-EA in neural development may further be supported by the detection of DHA-EA in murine embryonic stem cells and neural stem cells (NSCs) (43, 44). In NSCs, DHA-EA treatment induced neuronal differentiation for seven days. The maximum effect for DHA-EA was measured at 10 nM alone and at 5 nM in the presence of a FAAH inhibition. At nanomolar concentrations, DHA-EA was 50 to 100-fold more effective, than DHA, at increasing expression of neuron-specific markers, microtubule-associated protein 2 (MAP2) and class III beta-tubulin (Tuj-1). Interestingly, DHA-EA also increased phosphorylation of protein kinase A (PKA) substrates, which have been demonstrated to mediate the CREB signaling pathway (44). For instance, ethanol reduced levels of cAMP and hindered the ability of NSCs to differentiate, yet, DHA-EA treatment reversed these ethanol-induced effects. Additionally, at concentrations between 1 and 10 nM, DHA-EA significantly increased the cellular cAMP production and upregulated expression of adenylyl cyclase’s, AC7 and AC8, which are important regulators of cAMP levels. Notably, in the presence of 25 mM ethanol, 1 to 5 nM of DHA-EA completely reversed cAMP reduction (45).
Furthermore, DHA-EA has shown to promote axon development by negatively regulating sonic hedgehog (Shh) signaling in cortical neurons (46). Shh signaling is activated when Shh binds to patched-1 receptor that leads to transactivation of a smoothened GPCR (SMO). In the presence of SMO, 1 μM DHA increased levels of DHA-EA and caused a 28% increase in cortical neuron growth. Additionally, at 100 nM, DHA-EA prevented SMO-dependent transcription of GLI family zinc finger and blocked signaling induced by Shh. Intriguingly, when SMO was overexpressed in cortical cells, these cells failed to significantly decrease axonal growth caused by DHA-EA. Yet, in the presence of adenylyl cyclase inhibition, DHA-EA axonal growth was decreased, highlighting the demand for cAMP to drive this process. Collectively, these studies showed that DHA-EA enhances synapto- and neuro-genesis several folds more than DHA (44–46).
1.2. Role of DHA-EA in Inflammation
DHA-EA has been shown to be anti-inflammatory in several different inflammation models. For instance, the production and anti-inflammatory properties of DHA-EA were first demonstrated in 3T3-L1 adipocytes (Table 2). After 24 hours, 10 to 50 μM of DHA supplementation increased the conversion of DHA to DHA-EA by 2- to 7-fold higher compared to controls. To investigate the properties of DHA-EA in adipocytes, a pro-inflammatory response was evoked using lipopolysaccharide (LPS), a component from gram-negative bacteria commonly used to study inflammatory processes. DHA-EA supplementation at 1 nM to 10 μM reduced production of pro-inflammatory cytokines, such as IL-6 and monocyte chemotactic protein-1 (MCP-1), in differentiated adipocytes. Notably, both markers were reduced by 50% in the presence of 10 nM DHA-EA, compared to DHA, which had no effect at this concentration (47). Furthermore, receptor inhibition studies demonstrated that the reduction of pro-inflammatory markers were partially mediated by PPARγ and CB2. These studies confirmed that DHA-EA in adipocytes exhibit anti-inflammatory properties that are mediated through both CB- and non-CB receptors mediated pathways (47).
The effects of DHA-EA were further investigated in macrophages. At 10 μM, DHA-EA significantly inhibited pro-inflammatory nitric oxide (NO) and MCP-1 production in LPS-stimulated RAW264.7 and peritoneal macrophages (Table 2) (48). To elucidate the potential role of toll-like receptor (TLR) signaling in the anti-inflammatory effects caused by DHA-EA, the involvement of the TLR3/TLR4 myeloid differentiation primary response 88 pathway was examined. Interestingly, DHA-EA treatment in LPS and poly-IC induced macrophages led to significant reduction in the level of NO. However, DHA-EA did not inhibit NF-κB or IFN-β activation that is associated with this TLR3/TLR4 pathway. Additionally, pharmacological inhibition studies determined that CB1, CB2, and PPARγ minimally contributed to NO reduction by DHA-EA. Further suggesting that reduction in NO production is mediated through an unknown or alternate pathway. Currently, it is hypothesized that DHA-EA may indirectly reduce the pro-inflammatory response by reducing AA-derived COX eicosanoids or it is converted further by eicosanoid synthesizing enzymes to form anti-inflammatory lipid metabolites (49).
1.3. Role of DHA-EA in Cancer
The bioactivity of omega-3 eCBs have also been demonstrated in cancer models. In this study, DHA-EA significantly induced the cell death of prostate cancer cells sensitive to androgen (LNCaP) and insensitive to androgen (PC3). Moreover, expression of CB1 and CB2 in PC3 and LNCaP were determined and radioligand binding studies confirmed DHA-EA as an agonist of both the CB receptors. Additionally, degradation of DHA-EA by FAAH was measured and the inhibition of this enzyme in LNCaP cells led to a potent increase in its anti-proliferative properties (18). In other cancer cell types, such as breast cancer cells, DHA-EA mediated anti-proliferation effects through the PPARγ activation pathway (50).
1.4. Role of DHA-EA in Exercise and Pain
In recent years, eCBs have been evaluated for their potential role in exercise and pain. Exercise-induced hypoalgesia (EIH) is characterized by elevations in pain tolerance which has been attributed to the involvement of opioid and non-opioid systems. Previously, activation of CBs receptors and EIH have shown to lower nociceptor activity, enhance exercise-induced analgesia and release endogenous opioid-like molecules to modulate and reduce pain (51–53). Thus, it has been suggested that EIH may be reduced via elevated levels of eCBs after exercising to reduce pain sensitivity. In fact, DHA-EA levels become significantly increased in human serum after exercise (54). Additionally, exogenous treatment of DHA-EA to muscle tissue increased glucose-uptake and expression of CB1, CB2, and NAPE-PLD, a key enzyme involved in the synthesis of AEA (Table 2) (55).
DHA-EA bioactivity was also demonstrated in humans undergoing a high omega-3 and low omega-6 fatty acid diet intervention. This diet was shown to reduce headache frequency, headache severity, and psychological distress. Adults undergoing a high omega-3 and low omega-6 fatty acid diet intervention had significantly higher levels of DHA-EA in circulation, but lower levels of 2-AG in blood plasma (Table ST1). Notably, DHA-EA also caused an increase of anti-nociceptive omega-3 metabolites, such as 18-HEPE and 17-HDHA. Individuals on a high omega-3 diet reduced medication use for pain by 37%, while these results were not paralleled in a high omega-6 diet. Collectively, this evidence demonstrates that omega-3 dietary interventions increase the production of DHA-EA. Likewise, when DHA-EA increases in circulation, a downstream increase in other eCBs and DHA metabolites that may aid in reducing pain activities (56).
1.5. Biosynthesis of DHA-EA
DHA-EA was first isolated from bovine retina. A potential precursor, N-docosahexanoyl-phosphatidylethanolamine (NDHPE), was identified (29). There are three hypothesized models for DHA-EA endogenous biosynthesis. Similar to AEA synthesis, one mechanism involves a phospholipase D2 enzyme that cleaves NDHPE from the phospholipid backbone to form free DHA-EA. Secondly, it is possible that α/β-hydrolase 4 can be used to create an intermediate susceptible to hydrolysis of the NDHPE such that free DHA-EA is released. Thirdly, direct condensation of DHA and ethanolamine to form DHA-EA can occur by FAAH, though this reversed hydrolysis would likely occur at significantly higher concentrations. Collectively, more studies are needed to elucidate the synthesis of DHA-EA and confirm which mode(s) of synthesis occurs predominantly in vivo (29, 57).
1.6. Metabolism of DHA-EA and Role of Metabolites
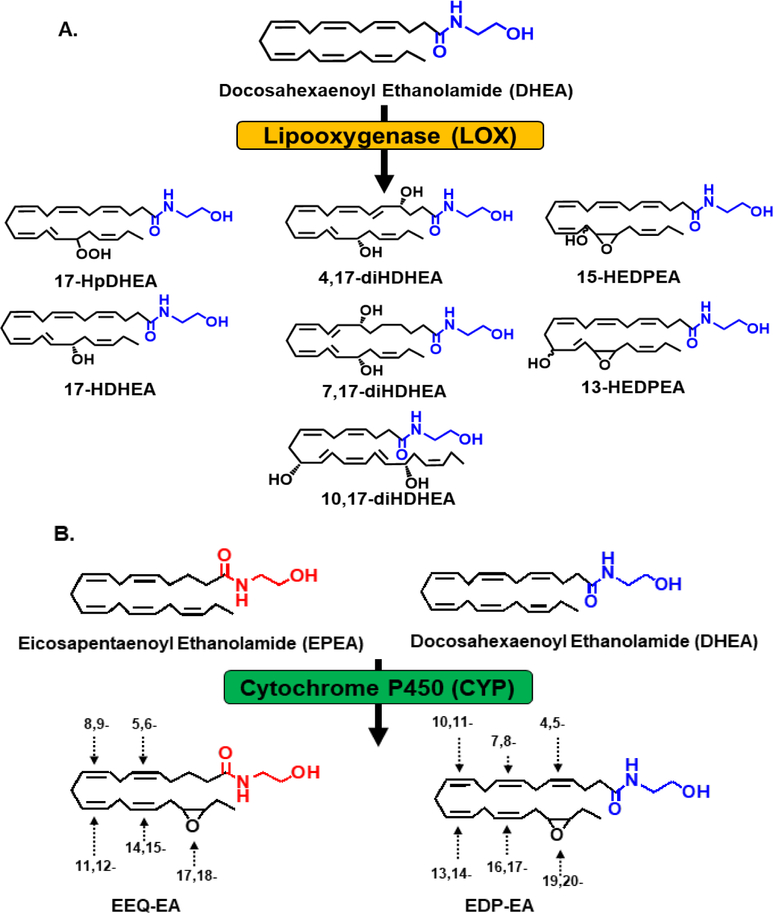
The eCBs are further metabolized by three eicosanoid synthesizing enzymes – COX, LOX and CYP450 – to form eCB metabolites. Studies have extensively investigated the generation of AEA and 2-AG oxy metabolites that have been produced through these enzymes (15). In recent years, DHA-EA was proposed to be a substrate for the LOX and CYP450 enzymes (Figure 2). The first reported metabolism of DHA-EA was through the LOX pathway. The primary DHA-EA metabolites were identified in mouse brain, human hemoglobin and polymorphonuclear (PMN) leukocytes (Figure 2A). During inflammation, PMN leukocytes migrate to the originating sites of inflammation, a process known as chemotaxis. In contrast, chemotaxis can be reduced in the presence of anti-inflammatory molecules, such as resolvins (58). Among the LOX products formed, 10,17-diHDHA-EA and 15-HEDPEA reduced the migration of PMN chemotaxis up to 10 nM and decreased platelet-leukocyte aggregation formation by ~30–40% in human whole blood at 10 and 100 pM (Table 2). In addition, 10,17-diDHA-EA and 15-HEDPEA demonstrated CB2 receptor agonist activity with EC50 values at 3.9 nM and 1 nM, respectively (Table 1) (20).
Figure 2. Oxidation of Omega-3 endocannabinoids (DHA-EA and EPA-EA) by the LOX and CYP450 pathway.
(A) For the LOX pathway, oxidation can occur once and metabolites formed can undergo additionally oxidation or reduction to form seven DHA-EA-LOX metabolites; 17-hydroperoxydocosahexaenoyl ethanolamide (17-HpDHA-EA), 17-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoylethanolamide (17-HDHA-EA), 10,17-dihydroxydocosahexaenoyl ethanolamide (10,17-diHDHA-EA), 7,17-dihydroxydocosahexaenoyl ethanolamide (7,17-diHDHA-EA), 4,17-dihydroxydocosahexaenoyl ethanolamide (4,17-diHDHA-EA), 13-hydroxy-16(17)-epoxy-docosapentaenyolethanolamide (13-HEDPEA) and 15-hydroxy-16(17)-epoxy-docosapentaenyolethanolamide (15-HEDPEA). (B) The CYP450 pathway oxidizes DHA-EA and EPA-EA into six epoxydocosapentaenoic acid-ethanolamide (EDP-EA) and five epoxyeicosatetraenoic acid-ethanolamide (EEQ-EA) regioisomers, respectively.
Recently, our group demonstrated that the CYP450 pathway plays a role in the metabolism of DHA-EA (Figure 2B). We have developed a targeted liquid chromatography tandem mass spectroscopy (LC-MS/MS) method to quantitatively measure and detect DHA-EA epoxide metabolites in rat tissues. Additionally, when rat brain homogenates were incubated with DHA-EA, six DHA-EA epoxide regioisomers called epoxydocosapentaenoic-ethanolamides (EDP-EAs) were produced. As proof of concept, one brain CYP450 isozyme, CYP2J2, was shown to be able to metabolize DHA-EA to form the corresponding epoxides. Furthermore, one regioisomer, 19,20-EDP-EA, was predominately produced by CYP450s in rat brain. This metabolite significantly reduced pro-inflammatory markers, IL-6 and NO, while inducing the expression of anti-inflammatory marker, IL-10, in microglial cells. Additionally, 19,20-EDP-EA meditated platelet aggregation, vasodilation, angiogenesis, while activating CB1 and CB2 receptors with EC50 values of 108 nM and 280 nM, respectively (Table 1 and 2) (19). These studies suggest that omega-3 eCB metabolites may serve as potential therapeutic targets in neuroinflammation and cerebrovascular diseases.
2. Omega-3 fatty acid EPA ethanolamide (EPA-EA or EPEA)
When AEA was discovered to bind and activate CB1, researchers synthesized AEA analogs to investigate which structural requirements were essential for binding to CB1. One of the compounds synthesized was eicosapentanoyl ethanolamide (EPA-EA or EPEA) (Figure 1B) (59). Competitive binding experiments showed that EPA-EA had a 4- to 16.5-fold weaker binding to CB1 compared to AEA (Table 1) (28, 59, 60).
Years later, EPA-EA was isolated from animal tissues, compared to AEA and DHA-EA, and levels were significantly lower or undetectable at basal levels (43). Additionally, when piglets were fed a fish oil diet, phospholipids containing esterified EPA significantly increased, while EPA-EA levels were undetectable in the brain, jejunum and liver (31). Yet, in adipose tissue, mice fed with omega-3 oil, levels of EPA-EA increased (33). Lastly, EPA-EA has also been detected in human milk, similar to DHA-EA (36) (Table ST1). Together, these findings further support that endogenous levels can be altered through dietary interventions and the levels of EPA-EA vary by tissue type.
2.1. Role of EPA-EA in Inflammation
Since EPA has shown to possess anti-inflammatory properties, the anti-inflammatory effects of EPA-EA was studied in adipocytes and myoblasts. In 3T3-L1 adipocytes, 10 to 50 μM of EPA supplementation led to a 3.5- to 10.9-fold increase of EPA-EA. When LPS-stimulated adipocytes were treated with EPA-EA, levels of pro-inflammatory cytokines, IL-6 and MCP-1, were reduced by 50% at 100 nM and 10 nM, respectively (Table 3). Moreover, LPS-stimulation increased EPA-EA levels several fold higher than DHA-EA, possibly due to the higher endogenous levels of esterified EPA than DHA (47, 61).
Table 3.
Biological effects of EPA-derived eCBs and Derivatives
| Effects of Eicosapentaenoyl Ethanolamide (EPEA) | References |
|---|---|
| Reduced IL-6 levels in LPS-stimulated 3T3-L1 adipocytes | (47) |
| Reduced MCP-1 production by 50% in LPS-stimulated 3T3-L1 cells | (47) |
| Inhibited MCF-7 cancer cell proliferation | (50) |
| Effects of Eicosapentaenoyl Ethanolamide Epoxides (EEQ-EA) | |
| Reduced pro-inflammatory nitric oxide and IL-6 | (19) |
| Induced production of anti-inflammatory IL-10 in microglia | (19) |
| Effects of Monoacylglyceride Eicosapentaenoic Acid (1-EPG) | |
| Dose administration reduced reactivity of airway smooth muscles | (75) |
| Reduced expression NFκβ and COX-2 | (75) |
| Reduced trachael ring tension induced by U-46619 | (76) |
| Reduced severity of DSS-induced colitis | (77) |
| Reduced pro-inflammatory cytokines TNFα, IL-1β, and IL-6 | (77) |
In C2C12 myoblasts treated with EPA-EA, the expression of CB receptors and glucose-uptake receptors were increased. Additionally, EPA-EA-induced CB receptor expression was investigated for regulating insulin sensitivity via activation of the mitogen-activated protein kinase (MAPK) pathway. Interestingly, when C2C12 myoblasts were supplemented with EPA-EA, p38 MAPK phosphorylation was altered. Additionally, N-acyl phosphatidylethanolamine phospholipase mRNA levels increased with EPA-EA supplementation (Table 3) (55). Collectively, these findings propose that EPA-EA may be indirectly mediating its anti-inflammatory effects through increased levels of other fatty acid-derived eCBs. Ultimately, they may aid in the reduction of pro-inflammatory cytokines and increase insulin sensitivity through the MAPK pathway.
2.2. Role of EPA-EA in Cancer
EPA-EA has been shown to inhibit prostate cancer cell growth and induce cell death of LNCaP and PC3 cell lines. In these cells, EPA-EA was determined to be an agonist to CB1 and CB2 (Table 1). Similar to DHA-EA, the anti-proliferative effects of EPA-EA were significantly reduced in the presence of FAAH inhibitors (18). Additionally, in breast cancer cells (MCF-7), EPA-EA inhibited proliferation in a dose-dependent manner with an IC50 value of 1.5 μM (50). EPA-EA also demonstrated to activate PPARγ, which plays a key role in the autophagy process (Table 3). When PPARγ is activated, an autophagy process occurs in which activation of beclin-I is observed. When the autophagy process becomes inhibited, beclin-I interacts with Bcl-2. Remarkably, EPA-EA reduced the beclin-I and Bcl-2 interaction and ultimately inhibited the AKT-mTOR signaling pathway, which plays a key role in the autophagy and apoptotic processes of the cell cycle (50).
2.3. Metabolism of EPA-EA and Role of Metabolites
EPA-EA is metabolized by CYP450 to form corresponding epoxides that are found endogenously in rat tissues (Figure 2B). Specifically, CYP2J2, metabolized EPA-EA into five regioisomers called epoxyeicosatetraenoic acid-ethanolamides (EEQ-EAs). Of the five regioisomers produced, 17,18-EEQ-EA was the predominant metabolite formed (Table 3). Similar to 19,20-EDP-EA, 17,18 EEQ-EA significantly reduced pro-inflammatory NO and IL-6, whereas anti-inflammatory IL-10 production significantly increased in microglia cultures. Additionally, 17,18-EEQ-EA was also determined to be three- and six-fold more susceptible to FAAH and soluble epoxide hydrolase (sEH) hydrolysis, respectively, when compared to 19,20-EDP-EA. EPA-derived metabolites are more susceptible to breakdown compared to DHA-derived metabolites, which could be due to their structural differences. Interestingly, 17,18 EEQ-EA also inhibited platelet aggregation regulated vasodilation and angiogenesis, as well as activated CB1 and CB2 with an EC50 value of 18.5 nM and 1.4 nM, respectively (Table 1) (19).
2.4. Summary of DHA-EA and EPA-EA
In conclusion, endogenous DHA and EPA are non-oxidatively converted to DHA-EA and EPA-EA, respectively. These omega-3 eCBs are precursors to more potent bioactive molecules that interact through CB-independent and -dependent-pathways. For instance, DHA-EA and EPA-EA can undergo further downstream modifications through the canonical oxygenase pathway involving COX, LOX, and CYP450. The mechanism of action of these newly formed metabolites remains to be investigated. Thus far, DHA-EA and EPA-EA possess anti-proliferative as well as anti-inflammatory properties and may be key modulators of the inflammatory cascade. Additionally, DHA-EA and EPA-EA possess some affinity to CB and non-CB receptors (Table 1) (19, 20, 62). Although we focus on the nutritionally relevant omega-3 derived eCBs, from DHA and EPA, it is important to highlight reports that have demonstrated that similar analogues, such as α-linolenoyl ethanolamine (ALA-EA), possess pharmacological significance through activation of transient receptor potential vanilloid 1 (TRPV1) and not CB1 (28, 63). Therefore, there is a large class of omega-3 fatty acid derived eCB-like molecules that can modulate cell physiology through other non-CB receptors.
3. Omega-3 fatty acid DHA and EPA glycerol (DHG and EPG)
Another class of potential omega-3 eCBs with structural similarities to 2-AG have also been discovered. DHA-derived, docosahexaenoyl glycerol (DHG) and EPA-derived, 2–5,8,11,14,17-eicosapentaenoyl glycerol (EPG), have a glycerol ester linkage (Figure 1C). Both 2-DHG and 2-EPG were first reported in a study investigating 2-AG analogues. Since 2-AG induced a rapid, increase in intracellular free calcium levels, the effects of structural analogues were also assessed. When compared, 2-AG and 2-EPG both induced cAMP levels, that are partially mediated by CB1, with similar agonist activities. However, 2-DHG showed weak agonist activity at the CB1 (21). These initial studies set the precedence for investigating the endogenous production of 2-DHG and 2-EPG as well as their bioactive roles.
It is important to note that 2-AG isomerizes to 1-AG and is also hydrolyzed by active MAGL to form AA. Similarly, 2-DHG and 2-EPG are not thermodynamically stable and can isomerize to 1-DHG and 1-EPG. Furthermore, they are susceptible to become hydrolyzed to form DHA and EPA. Therefore, the effects observed with 2-DHG or 2-EPG might be partially be due to the unesterified DHA and EPA that is formed after the corresponding monoacyl-glycerol cleavage.
3.1. Endogenous Levels of 2-DHG and 2-EPG
Both 2-EPG and 2-DHG were found in mouse embryonic stem cells (mES) and embryoid bodies. In mES cells, there were comparable concentrations of 2-DHG and 2-EPG (43). Additionally, a high omega-3 and low omega-6 diet in humans produced a significant increase in 2-DHG levels, whereas 2-AG levels decreased. Furthermore, EPG and DHG levels have shown to increase in human milk upon maturation (Table ST1) (36). Strikingly, an increase in 2-DHG was correlated with a decrease in pain (Table 3 & ST1) (56). Despite the detection of 2-DHG and 2-EPG, the biological and physiological significance were not evident.
3.2. Bioactivity of 1-DHG
2-AG is one of the most well studied eCB. However, it isomerizes spontaneously into 1-AG under physiological conditions and is considered a potential mechanism of its deactivation (64–66). In fact, it has been reported that commercially available 2-AG contains approximately 10% 1-AG, thus the isomerization process is inevitable in biochemical research assays (66). Hence, testing of the potential effects of 2-AG is undermined due to the rate of isomerization and the chemical instability of these molecules (65, 66). 1-DHG is structurally similar to 2-DHG, containing a DHA attached to the sn-1 instead of the sn-2 position of the glycerol molecule via an ester bond. 1-DHG is also referred to as monoacylglyceride docosahexaenoic acid (MAG-DHA) (67). Its effects in pulmonary arterial hypertension (PAH) and cystic fibrosis have recently been investigated (Table 2).
PAH is a disease characterized by a progressive increase in pulmonary pressure and vascular resistance (68). It has been previously demonstrated that CYP450s in the lungs, including CYP2J2, CYP2C8 and CYP2C9, can metabolize AA, EPA and DHA into oxygenated eicosanoids, which have been reported to modulate vascular and airway smooth muscle tone (68–70). Thus, the ability for 1-DHG to inhibit vasoconstriction was first investigated using human pulmonary cells. In these studies, 1-DHG was found to reduce vasoconstriction in a dose-dependent manner, with an EC50 of 1.25 μM. In addition, 1-DHG was able to reduce tension in human pulmonary arteries by 50% at 3 μM . Interestingly, this tension was further reduced to 95% in the presence of a Rho-kinase inhibitor, H-1152 (67). Since 1-DHG reduced vasoconstriction modulated by Rho-kinase, further studies showed that 30 μM of 1-DHG was able to inhibit RhoA activity, a known activator of Rho-kinase (68). Furthermore, it was revealed that 1-DHG treatment reduced phosphorylation of downstream members of the Rho-kinase pathway (Table 2) (67, 68). Thus 1-DHG may represent a new pharmacological alternative in modulating vasoconstriction in patients with pulmonary hypertension.
Homeostatic response caused by omega-3 eCBs and their metabolites are not limited to cancer, inflammation or pain, as these molecules can act broadly in different diseases states as inflammation is common etiology in many diseases. For instance, cystic fibrosis is the most common autosomal, recessive, inherited disease with fatal outcome in Caucasians, and is characterized by persistent lung infections and losing the ability to breathe over time (71). Previously, cystic fibrosis patients have reported a fatty acid deficiency with an imbalance between omega-3 and omega-6 levels in their blood lipids (72, 73). Since omega-3 fatty acid supplements were found to reduce inflammation in cystic fibrosis patients, the effects of 1-DHG were investigated utilizing lung adenocarcinoma Calu-3 cells. At 3 μM, 1-DHG treatment was found to increase the mean DHA/AA ratio with at least a two-fold increase in DHA/AA ratio from control. Moreover, 1-DHG treatment also led to a reduction of pro-inflammatory markers, such as IL-6 and IL-8, induced by LPS. Notably, docosaepentaenoic glycerol (DPG) and EPG showed a more effective reduction (Table 2) (74). This study was the first to demonstrate the anti-inflammatory properties of 1-DHG and its potential in the management of cystic fibrosis. While there are some studies reported for 1-DHG, there are no current reports on 2-DHG.
3.3. Bioactivity of 1-EPG
Similar to DHA, EPA also has a monoacylglyceride derivative known as MAG-EPA or 1-EPG (Figure 1C). Previously, 1-EPG has shown to be anti-inflammatory through reduction of pro-inflammatory NF-κB and COX-2. Use of an asthma model has demonstrated that 1-EPG treatment reduced the reactivity of airway smooth muscles with an EC50 value of 0.77 nM. Interestingly, 1-EPG significantly reduced calcium sensitivity and decreased the omega-6/omega-3 ratio in the asthma model (Table 3) (75). These findings directly support the previous findings that1-EPG caused these effects, as omega-6 fatty acid levels are positively correlated with asthmatic symptoms. In related studies, the effects of 1-EPG on airway hyper-responsiveness were investigated. In guinea pig models, 1-EPG reduced tracheal ring tension with an IC50 = 1.03 μM. It was further reported that an EPA metabolite, 17,18-epoxyeicosatetraenoic acid (17,18-EEQ) had a greater effectiveness compared to its precursor 1-EPG (76). The findings of this study suggest that 1-EPG may have potential in the treatment of respiratory-related diseases, possibly through its metabolism to the highly bioactive 17,18-EEQ metabolite (76).
Since omega-3 supplementation causes a decrease in the production of inflammatory cytokines involved in ulcerative colitis pathogenesis, the effects of 1-EPG in ulcerative colitis were also investigated. In a colitis rat model, orally administered 1-EPG was found to reduce the severity of colitis, further suggesting that 1-EPG may prevent the acceleration of ulcerative colitis (77). To investigate the potential mechanism of 1-EPG in colitis, pro-inflammatory cytokines were measured. In colon tissue, an 81% reduction of NF-κβ was observed with 1-EPG treatment. As in previous studies, TNF-α, IL-1β, IL-6 and COX-2 expression levels were also decreased when treated with 1-EPG, suggesting that 1-EPG may act on a transcriptional level. Collectively, these studies suggest that 1-EPG is anti-inflammatory in several models, including asthma, airway hyper-responsiveness, and ulcerative colitis.
It is known that DHA and EPA have beneficial effects in cancer models in vitro and in vivo (78). Compared to 1-DHG, 1-EPG decreased cell growth of human colorectal carcinoma cells, where apoptosis was even induced in a dose-dependent manner (0.3 μM to 10 μM) by 1-EPG (79). In vivo mouse model studies demonstrated that 1-EPG reduced tumor sizes by 3-fold after daily administration. Moreover, 1-EPG treatments reduced VEGF and HIF-1α expression levels in tumors, both of which are commonly overexpressed in many cancers. 1-EPG downregulated the gene products of NF-κβ, VEGFR, and AKT within the colorectal carcinoma study, all of which are associated with angiogenesis, metastasis and proliferation of tumors (Table 3) (79). While these effects were observed in mice, a study is currently in progress to investigate whether the 1-EPG in cell cultures translates in humans. The effects of 1-EPG on prostate cancer on randomized, double-blind, placebo-controlled trial is currently being conducted as well, highlighting the potential far-reaching implications of eCB-based therapeutics (80).
4. N-acyl neurotransmitter derivatives of DHA and EPA: Dopamine and Serotonin
Neurotransmitters enable neurotransmission in the brain and are synthesized from amino acids precursors. Biogenic amines such as monoamine neurotransmitters and catecholamine neurotransmitters are crucial for transmitting signals across a chemical synapse from one neuron to the target cells. Recently, DHA and EPA derived conjugates of monoamine neurotransmitters have become of interest due to the potential dual physiological effects of omega-3 fatty acids and neurotransmitters. Herein, we discuss omega-3 derived N-acyl conjugates of neurotransmitters and their potential roles in immunomodulation and neurodegeneration.
4.1. Expression and biological effects of DHA-Dopamine and EPA-Dopamine (DHA-DA or EPA-DA)
N-arachidonoyl dopamine (NADA) was first reported as a novel CB1 ligand (81) (Figure 1D). Similar to NADA, DHA-dopamine (DHA-DA) is of interest due to its potential physiological properties that are linked to brain synapses and retinal neuroprotection (82). The synthesis and biological effects of omega-3 derived neurotransmitters, such as DHA-DA has been shown to possess analgesic and antioxidative effects (Table 2) (83, 84). In addition, DHA-DA decelerates the development of Parkinson’s disease symptoms and recently has shown to be anti-inflammatory (85). In rodent macrophages and microglia cells, DHA-DA dose-dependently, at 10 nM to 2.5 μM, suppressed the production of four pro-inflammatory markers: NO, MCP-1, macrophage-inflammatory protein 3α and IL-6. Other immunomodulatory effects by DHA-DA is suppressing levels of prostaglandin E2 (PGE2), a COX-2 product that promotes inflammation, pain and fever. Notably, PGE2 was suppressed dose dependently by DHA-DA up to 75% at 1 μM. Similarly, COX-2 transcript levels were also dose-dependently decreased by DHA-DA (86). However, DHA-DA did not affect NF-κB, a transcriptional regulator of COX-2. Notably, similar findings of DHA-DA in macrophages were consistent in BV-2 microglia cells, however COX-2 gene expression in LPS-activated microglial cells were unaffected (86). Collectively, this evidence suggests that DHA, the most abundant fatty acid in the brain, and dopamine, an important neurotransmitter in the brain, together form a lipid mediator conjugate that can modulate neuroinflammation.
In human breast cancer cell lines, DHA-DA and EPA-DA has been reported to induce cell death through its interaction with PPARγ. Both DHA-DA and EPA-DA dose-dependently reduced cell viability in MCF-7, SKBR3 and MDA-MB-231 cell lines. In MCF-7, SKBR3 and MDA-MB-231, DHA-DA dose- and time-dependently reduced cell viability in all cell lines with IC50 values of 25, 20 and 6 μM, respectively. Furthermore, DHA-DA did not appear to influence proliferation of non-tumorigenic breast MCF-10A cells. Notably, this data combined with the IC50 values of tumorigenic breast cancer cell lines indicate DHA-DA has greater effects on more aggressive cell types that others. Increased expression of PPARγ was measured and further studies demonstrated that activation of PPARγ by DHA-DA and EPA-DA mediate the antiproliferative effects in cancer cells. Notably, DHA-DA and EPA-DA also upregulated autophagic protein, Beclin-1, expression in these cells (87). Together, this suggests that DHA-DA and EPA-DA activation of PPARγ may have potential in adjuvant therapeutic interventions in breast cancer.
4.2. Expression and Biological effects of DHA-Serotonin and EPA-Serotonin (DHA-5HT or EPA-5HT)
It has been demonstrated that the endogenous production of N-acyl amides and serotonin levels are increased in intestinal tissues with omega-3 supplementation (88). In the first study, the serotonin conjugate of DHA, DHA-serotonin (DHA-5HT), significantly inhibited pro-inflammatory mediators, IL-6 and PGE2, at 2.5 μM in LPS-stimulated murine macrophages (Table 2). Similar to DHA-DA, gene expression of COX-2, IL-6 and NO synthase 2 were all suppressed by DHA-5HT at the transcriptional level. Furthermore, DHA-5HT treatment led to downregulation of co-stimulatory molecules needed for T cell activation, demonstrating that DHA-5HT reduces gene expression of mediators involved in inflammatory T cell responses. Moreover, microarray analysis found DHA-5-HT to reduce the gene expression of metalloproteinases, chemokines and pro-inflammatory interleukins such as IL-12b, IL-23a, IL-6, and IL-1β. Notably, these observations were consistent in LPS-stimulated RAW264.7 cells in which DHA-5HT treatments inhibited migration of macrophages by 52% and 77%, at 1 μB and 2 μM, respectively (Table 2) (89). DHA-5HT also reduced the production of MCP-1 and CCL-20, a chemokine released by pathogenic pro-inflammatory T helper 17 (Th17) cells, to that of DHA-DA (86). Collectively, this evidence suggests that DHA-5HT may have therapeutic potential for patients with intestinal immune-dysregulation of inflammatory T cells.
Recently the presence and effects of DHA-5HT in human tissues was demonstrated. LC-MS/MS analysis, found that both DHA-5HT and EPA-5HT were detected in addition to other N-acyl-5HT. Notably, out of the six N-acyl serotonins detected, DHA-5HT was most effective at suppressing pro-inflammatory cytokine IL-17 release in peripheral blood mononuclear cells (PMBCs) in a dose-dependent manner. DHA-5HT treatments decreased IL-17 production by PBMCs by 80% and 39% at 10 μM and 1 μM, respectively. Interestingly, DHA and serotonin separately did not influence IL-17 release, suggesting that the N-acyl serotonin moiety is vital for this physiological effect. Measuring CCL-20 production helped probe the immunomodulatory effects of DHA-5HT. Similar to IL-17, CCL-20 production was dose-dependently and significantly attenuated, by 39.5% and 45.95%, at 5 and 10 μM treatments, respectively (90). Hence, DHA-5HT regulates the production of two important Th17 mediators involved in pathogenesis of intestinal diseases and is a class of immunomodulatory omega-3 derived lipid mediators.
The mechanism of action of DHA-DA and DHA-5HT still remains unknown. However, similar conjugates such as, ALA-DA and ALA-5HT, can activate CB and TRPV1 receptors and inhibit FAAH degradation of AEA (84, 91). Thus, the potential mechanism of action of DHA-DA, DHA-5HT and the EPA analogues may follow similar receptor interactions. As eCBs also act through TRPV1 (92–94), they are termed as “endovanniloids”. In addition to mediating the pain associated from stimuli, TRPV1 exhibits pro- and anti-inflammatory effects (95–98). Recently, it has been postulated that there is a crosstalk between CB1 and TRPV1 receptors, which are co-localized in dorsal root ganglion. Therefore, the ECS and TRPV1 axis provides a promising target to develop pain and inflammation therapeutics.
5. Concluding Remarks
In the past few decades, a significant amount of work was performed to understand the role of omega-3 and omega-6 derived eCBs and their derivatives the overall physiology of the body. Despite structural similarities, common pathways for formation and enzymatic degradation, eCBs are intriguing lipid signaling molecules with varied interactions with several different types of receptors leading to varied physiological roles. Though previous studies focus on cannabinoid receptor activation, it is important to acknowledge that omega-3 and omega-6 eCBs could possibly activate other receptors as they are multi-functional molecules. Therefore, systems-based approaches are required to fully characterize these molecules with respect to receptor interactions and downstream signaling. Their on-demand signaling capabilities could suggest that they act at multiple receptors in tandem until they resolve the homeostatic challenge or are converted to new molecules with altered bioactivity through different pathways including eicosanoid synthesizing enzymes. All of these create possibilities for multiple modes of action of eCBs, create new areas of cross-talk to investigate. As shown, omega-3 and omega-6 fatty acid eCBs have demonstrated metabolism by LOX-, COX- and CYP450-mediated pathways, yet only LOX and CYP450 metabolites have been reported for DHEA and only CYP450 metabolites for EPEA (Figure 2). Thus, the physiological function of the LOX and CYP450 generated metabolites remains to be elucidated further to understand how these lipids modulate cell signaling under diseased and non-diseased states.
We must continue to focus on the influence of naturally produced omega-3 eCB derivatives to better understand how they display such ubiquitous biological roles. Referencing the molecular origins and fates of these molecules while discussing their physiological roles is critical in driving future research surrounding omega-3-derived eCBs. Many of the studies mentioned in this review limit our understanding of these molecules, as this basic characterization is biased towards measuring just CB receptor interaction and direct inflammatory responses. Hence, more research is needed to describe these molecules across multiple disease models.
Supplementary Material
Highlights.
Omega-3 fatty acid endocannabinoids are derived from omega-3 fatty acids and are found endogenously in the body.
Despite similar structural features, omega-3 fatty acid endocannabinoids and their derivatives demonstrate a perplexing diversity in function and receptor interactions that warrants further molecular characterization.
Acknowledgements
We want to thank Austin Weigle and Demetrios Maroutsos for edits of the manuscript. This work was partly supported by American Heart Association Scientist Development Grant 15SDG25760064 (A.D.), National Institutes of Health (NIH) Grant R01 GM1155884 (A.D.), and R03 DA042365 (A.D) and diversity supplement to Ms. Josephine Watson (R01GM115584-02S1)
Abbreviations:
- Δ9-THC
Δ9-tetrahydrocannabinol
- GPCR
G-Protein coupled receptor
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- eCB
endocannabinoid
- ECS
endocannabinoid system
- cAMP
cyclic adenosine monophosphate
- AA
arachidonic acid
- AEA
anandamide
- 2-AG
2-arachidonoyl-glycerol
- DHA
docosahexaenoic acid
- 2-DHG
2-docosahexaenoyl-glycerol
- DHA-EA
docosahexaenoyl ethanolamide
- DHA-DA
DHA-Dopamine
- DHA-5HT
DHA-Serotonin
- EPA
eicosapentaenoic acid
- EPA-EA
eicosapentaenoyl ethanolamide
- EPG
eicosapentaenoyl-glycerol
- EPA-DA
EPA-Dopamine
- EPA-5HT
EPA-Serotonin
- COX
cyclooxygenase
- LOX
lipoxygenase
- FAAH
fatty amide hydrolase
- sEH
soluble epoxide hydrolase
- MAGL
monoacylglycerol lipase
Footnotes
Conflict of Interest Statement
The authors declare no competing financial interest.
References
- 1.Andre CM, Hausman JF, Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front Plant Sci. 2016;7:19 Epub 2016/02/04. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams DI. The therapeutic effects of Cannabis and cannabinoids: An update from the National Academies of Sciences, Engineering and Medicine report. Eur J Intern Med. 2018;49:7–11. Epub 2018/01/09. doi: 10.1016/j.ejim.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 3.LOEWE S [Active principals of the cannabis and the pharmacology of the cannabinols]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1950;211(2):175–93. [PubMed] [Google Scholar]
- 4.Howlett AC. Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds. Life Sci. 1984;35(17):1803–10. [DOI] [PubMed] [Google Scholar]
- 5.Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27(4):429–36. [PubMed] [Google Scholar]
- 6.Howlett AC, Qualy JM, Khachatrian LL. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol. 1986;29(3):307–13. [PubMed] [Google Scholar]
- 7.Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13. [PubMed] [Google Scholar]
- 8.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–6. PubMed PMID: 2308954; PMCID: 53598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science. 1992;258(5090):1946–9. doi: DOI 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 12.Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993;90(16):7656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. [DOI] [PubMed] [Google Scholar]
- 14.Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73(1):96–105. [DOI] [PubMed] [Google Scholar]
- 15.Zelasko S, Arnold WR, Das A. Endocannabinoid metabolism by cytochrome P450 monooxygenases. Prostaglandins Other Lipid Mediat. 2015;116-117:112–23. Epub 2014/11/24. doi: 10.1016/j.prostaglandins.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Di Marzo V New approaches and challenges to targeting the endocannabinoid system. Nat Rev Drug Discov. 2018;17(9):623–39. Epub 2018/08/17. doi: 10.1038/nrd.2018.115. [DOI] [PubMed] [Google Scholar]
- 17.Alexander SP, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, Collaborators C. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br J Pharmacol. 2017;174 Suppl 1:S17–S129. doi: 10.1111/bph.13878. PubMed PMID: 29055040; PMCID: PMC5650667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown I, Cascio MG, Wahle KW, Smoum R, Mechoulam R, Ross RA, Pertwee RG, Heys SD. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis. 2010;31(9):1584–91. Epub 2010/07/25. doi: 10.1093/carcin/bgq151. PubMed PMID: 20660502; PMCID: PMC2930808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE, Johnson RW, Kilian KA, Holinstat M, Das A. Anti-inflammatory ω−3 endocannabinoid epoxides. Proc Natl Acad Sci U S A. 2017;114(30):E6034–E43. Epub 2017/07/07. doi: 10.1073/pnas.1610325114. PubMed PMID: 28687674; PMCID: PMC5544256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R, Fredman G, Krishnamoorthy S, Agrawal N, Irimia D, Piomelli D, Serhan CN. Decoding functional metabolomics with docosahexaenoyl ethanolamide (DHEA) identifies novel bioactive signals. J Biol Chem. 2011;286(36):31532–41. Epub 2011/07/16. doi: 10.1074/jbc.M111.237990 M111.237990 [pii] PubMed PMID: 21757729; PMCID: 3173121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiura T, Kodaka T, Nakane S, Miyashita T, Kondo S, Suhara Y, Takayama H, Waku K, Seki C, Baba N, Ishima Y. Evidence that the cannabinoid CB1 receptor is a 2-arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 1999;274(5):2794–801. [DOI] [PubMed] [Google Scholar]
- 22.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–7. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 23.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152(7):1092–101. Epub 2007/09/17. doi: 10.1038/sj.bjp.0707460. PubMed PMID: 17876302; PMCID: PMC2095107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syed SK, Bui HH, Beavers LS, Farb TB, Ficorilli J, Chesterfield AK, Kuo MS, Bokvist K, Barrett DG, Efanov AM. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am J Physiol Endocrinol Metab. 2012;303(12):E1469–78. Epub 2012/10/16. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 25.McHugh D, Page J, Dunn E, Bradshaw HB. Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol. 2012;165(8):2414–24. doi: 10.1111/j.1476-5381.2011.01497.x. PubMed PMID: 21595653; PMCID: PMC3423258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Console-Bram L, Ciuciu SM, Zhao P, Zipkin RE, Brailoiu E, Abood ME. N-arachidonoyl glycine, another endogenous agonist of GPR55. Biochem Biophys Res Commun. 2017;490(4):1389–93. Epub 2017/07/08. doi: 10.1016/j.bbrc.2017.07.038. PubMed PMID: 28698140; PMCID: PMC5576593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16(1):30–42. doi: 10.1038/nrn3876. PubMed PMID: 25524120; PMCID: PMC4471876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40(5):659–67. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 29.Bisogno T, Delton-Vandenbroucke I, Milone A, Lagarde M, Di Marzo V. Biosynthesis and inactivation of N-arachidonoylethanolamine (anandamide) and N-docosahexaenoylethanolamine in bovine retina. Arch Biochem Biophys. 1999;370(2):300–7. doi: 10.1006/abbi.1999.1410. [DOI] [PubMed] [Google Scholar]
- 30.Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc Natl Acad Sci U S A. 2001;98(11):6402–6. Epub 2001/05/17. doi: 10.1073/pnas.101119098. 101119098 [pii] PubMed PMID: 11353819; PMCID: 33480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, Hansen SH, Hansen HS. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781(4):200–12. Epub 2008/03/05. doi: 10.1016/j.bbalip.2008.01.006. S1388-1981(08)00035-8 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Wood JT, Williams JS, Pandarinathan L, Janero DR, Lammi-Keefe CJ, Makriyannis A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J Lipid Res. 2010;51(6):1416–23. doi: 10.1194/jlr.M002436. PubMed PMID: 20071693; PMCID: 3035504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossmeisl M, Jilkova ZM, Kuda O, Jelenik T, Medrikova D, Stankova B, Kristinsson B, Haraldsson GG, Svensen H, Stoknes I, Sjovall P, Magnusson Y, Balvers MG, Verhoeckx KC, Tvrzicka E, Bryhn M, Kopecky J. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: possible role of endocannabinoids. PLoS One. 2012;7(6):e38834. doi: 10.1371/journal.pone.0038834. PubMed PMID: 22701720; PMCID: 3372498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Carlson ME, Kuchel GA, Newman JW, Watkins BA. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. Int J Obes (Lond). 2016;40(1):129–37. Epub 2015/07/29. doi: 10.1038/ijo.2015.135. PubMed PMID: 26219414; PMCID: PMC4722239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buczynski MW, Svensson CI, Dumlao DS, Fitzsimmons BL, Shim JH, Scherbart TJ, Jacobsen FE, Hua XY, Yaksh TL, Dennis EA. Inflammatory hyperalgesia induces essential bioactive lipid production in the spinal cord. J Neurochem. 2010;114(4):981–93. doi: 10.1111/j.1471-4159.2010.06815.x. PubMed PMID: 20492349; PMCID: 3994888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaitán AV, Wood JT, Zhang F, Makriyannis A, Lammi-Keefe CJ. Endocannabinoid Metabolome Characterization of Transitional and Mature Human Milk. Nutrients. 2018;10(9). Epub 2018/09/12. doi: 10.3390/nu10091294. PubMed PMID: 30213124; PMCID: PMC6165354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HY, Moon HS, Cao DH, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochemical Journal. 2011;435:327–36. doi: 10.1042/Bj20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011;435(2):327–36. Epub 2011/02/02. doi: 10.1042/BJ20102118. BJ20102118 [pii] PubMed PMID: 21281269; PMCID: 3169088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim HY, Spector AA, Xiong ZM. A synaptogenic amide N-docosahexaenoylethanolamide promotes hippocampal development. Prostaglandins Other Lipid Mediat. 2011;96(1–4):114–20. doi: 10.1016/j.prostaglandins.2011.07.002. PubMed PMID: 21810478; PMCID: PMC3215906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HY, Spector AA. Synaptamide, endocannabinoid-like derivative of docosahexaenoic acid with cannabinoid-independent function. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):121–5. doi: 10.1016/j.plefa.2012.08.002. PubMed PMID: 22959887; PMCID: 3541447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HY, Spector AA. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol Aspects Med. 2018;64:34–44. Epub 2018/03/27. doi: 10.1016/j.mam.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Lee J-W, Huang BX, Kwon H, Rashid MA, Kharebava G, Desai A, Patnaik S, Marugan J, Kim H-Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nature communications. 2016;7:13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang S, Fu Y, Williams J, Wood J, Pandarinathan L, Avraham S, Makriyannis A, Avraham HK. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS One. 2007;2(7):e641. doi: 10.1371/journal.pone.0000641. PubMed PMID: 17653268; PMCID: 1919431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashid MA, Katakura M, Kharebava G, Kevala K, Kim HY. N-Docosahexaenoylethanolamine is a potent neurogenic factor for neural stem cell differentiation. J Neurochem. 2013;125(6):869–84. doi: 10.1111/jnc.12255. PubMed PMID: 23570577; PMCID: 3775276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashid MA, Kim HY. N-Docosahexaenoylethanolamine ameliorates ethanol-induced impairment of neural stem cell neurogenic differentiation. Neuropharmacology. 2016;102:174–85. doi: 10.1016/j.neuropharm.2015.11.011. PubMed PMID: 26586023; PMCID: 4698216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kharebava G, Rashid MA, Lee JW, Sarkar S, Kevala K, Kim HY. N-docosahexaenoylethanolamine regulates Hedgehog signaling and promotes growth of cortical axons. Biology open. 2015;4(12):1660–70. doi: 10.1242/bio.013425. PubMed PMID: 26545965; PMCID: 4736029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balvers MG, Verhoeckx KC, Plastina P, Wortelboer HM, Meijerink J, Witkamp RF. Docosahexaenoic acid and eicosapentaenoic acid are converted by 3T3-L1 adipocytes to N-acyl ethanolamines with anti-inflammatory properties. Biochim Biophys Acta. 2010;1801(10):1107–14. Epub 2010/07/06. doi: 10.1016/j.bbalip.2010.06.006S1388-1981(10)00144-7. [pii] [DOI] [PubMed] [Google Scholar]
- 48.Meijerink J, Plastina P, Vincken JP, Poland M, Attya M, Balvers M, Gruppen H, Gabriele B, Witkamp RF. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: evidence for a new link between fish oil and inflammation. Br J Nutr. 2011;105(12):1798–807. Epub 2011/02/08. doi: 10.1017/S0007114510005635. S0007114510005635 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Meijerink J, Poland M, Balvers MG, Plastina P, Lute C, Dwarkasing J, van Norren K, Witkamp RF. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. Br J Pharmacol. 2015;172(1):24–37. doi: 10.1111/bph.12747. PubMed PMID: 24780080; PMCID: 4280965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I, Lanzino M, De Amicis F, Sisci D, Mauro L, Aquila S, Catalano S, Bonofiglio D, Andò S. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J Cell Physiol. 2013;228(6):1314–22. doi: 10.1002/jcp.24288. [DOI] [PubMed] [Google Scholar]
- 51.Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14(17):2209–11. doi: 10.1097/01.wnr.0000097048.56589.47. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10(7):870–9. Epub 2007/06/10. doi: 10.1038/nn1916. PubMed PMID: 17558404; PMCID: PMC2234438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raichlen DA, Foster AD, Gerdeman GL, Seillier A, Giuffrida A. Wired to run: exercise-induced endocannabinoid signaling in humans and cursorial mammals with implications for the ‘runner’s high’. J Exp Biol. 2012;215(Pt 8):1331–6. doi: 10.1242/jeb.063677. [DOI] [PubMed] [Google Scholar]
- 54.Koltyn KF, Brellenthin AG, Cook DB, Sehgal N, Hillard C. Mechanisms of exercise-induced hypoalgesia. J Pain. 2014;15(12):1294–304. doi: 10.1016/j.jpain.2014.09.006. PubMed PMID: 25261342; PMCID: 4302052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Carlson ME, Watkins BA. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Frontiers in physiology. 2014;5:100. doi: 10.3389/fphys.2014.00100. PubMed PMID: 24711795; PMCID: 3968752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsden CE, Zamora D, Makriyannis A, Wood JT, Mann JD, Faurot KR, MacIntosh BA, Majchrzak-Hong SF, Gross JR, Courville AB, Davis JM, Hibbeln JR. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain. 2015;16(8):707–16. doi: 10.1016/j.jpain.2015.04.007. PubMed PMID: 25958314; PMCID: 4522350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54(1):1–7. Epub 2007/06/06. doi: 10.1016/j.neuropharm.2007.05.020. PubMed PMID: 17631919; PMCID: PMC2219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalli J, Colas RA, Serhan CN. Novel n-3 immunoresolvents: structures and actions. Sci Rep. 2013;3:1940. doi: 10.1038/srep01940. PubMed PMID: 23736886; PMCID: PMC3672887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas BF, Adams IB, Mascarella SW, Martin BR, Razdan RK. Structure-activity analysis of anandamide analogs: relationship to a cannabinoid pharmacophore. J Med Chem. 1996;39(2):471–9. doi: 10.1021/jm9505167. [DOI] [PubMed] [Google Scholar]
- 60.Adams IB, Ryan W, Singer M, Thomas BF, Compton DR, Razdan RK, Martin BR. Evaluation of cannabinoid receptor binding and in vivo activities for anandamide analogs. J Pharmacol Exp Ther. 1995;273(3):1172–81. [PubMed] [Google Scholar]
- 61.Matias I, Carta G, Murru E, Petrosino S, Banni S, Di Marzo V. Effect of polyunsaturated fatty acids on endocannabinoid and N-acyl-ethanolamine levels in mouse adipocytes. Biochim Biophys Acta. 2008;1781(1–2):52–60. Epub 2007/11/17. doi: 10.1016/j.bbalip.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Alharthi N, Christensen P, Hourani W, Ortori C, Barrett DA, Bennett AJ, Chapman V, Alexander SPH. n-3 polyunsaturated N-acylethanolamines are CB. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(11):1433–40. Epub 2018/08/07. doi: 10.1016/j.bbalip.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, Ermund A, Sterner O, Zygmunt PM, Högestätt ED. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280(46):38496–504. Epub 2005/08/04. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- 64.van der Stelt M, van Kuik JA, Bari M, van Zadelhoff G, Leeflang BR, Veldink GA, Finazzi-Agrò A, Vliegenthart JF, Maccarrone M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J Med Chem. 2002;45(17):3709–20. [DOI] [PubMed] [Google Scholar]
- 65.Fanelli F, Mezzullo M, Belluomo I, Di Lallo VD, Baccini M, Ibarra Gasparini D, Casadio E, Mastroroberto M, Vicennati V, Gambineri A, Morselli-Labate AM, Pasquali R, Pagotto U. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol Metab. 2017;6(5):406–15. Epub 2017/03/21. doi: 10.1016/j.molmet.2017.03.005. PubMed PMID: 28462075; PMCID: PMC5404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dócs K, Mészár Z, Gonda S, Kiss-Szikszai A, Holló K, Antal M, Hegyi Z. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front Cell Neurosci. 2017;11:39 Epub 2017/02/20. doi: 10.3389/fncel.2017.00039. PubMed PMID: 28265242; PMCID: PMC5316530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caroline Morin SF, Eric Rousseau. Docosahexaenoic Acid Monoacylglyceride Decreases Endothelin-1 Induced Ca2+ Sensitivity and Proliferation in Human Pulmonary Arteries. American Journal of Hypertension. 2012;25(7):756–63. doi: 10.1038/ajh.2012.45. [DOI] [PubMed] [Google Scholar]
- 68.Caroline Morin SF, Eric Rousseau. 19,20-EpDPE, a bioactive CYP450 metabolite of DHA monoacylglyceride, decreases Ca2+ sensitivity in human pulmonary arteries. American Journal of Physiology and Heart Circulation. 2011;301(4):1311–8. doi: 10.1152/ajpheart.00380.2011. [DOI] [PubMed] [Google Scholar]
- 69.Caroline Morin MS, Echave Vincent, Rizcallah Edmond, Rousseau Eric. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. American Journal of Physiology and Heart Circulation. 2008(296):130–9. doi: 10.1152/ajplung.90436.2008. [DOI] [PubMed] [Google Scholar]
- 70.Caroline Morin MS, Vincent Echave, Roula Albadine, Eric Rousseau. 17,18-epoxyeicosatetraenoic Acid Targets PPARγ and p38 Mitogen-Activated Protein Kinase to Mediate Its Anti-inflammatory Effects in the Lung. American Journal of Respiratory Cell and Molecular Biology. 2010;43(5):564–75. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- 71.Caroline Morin AC, Eric Rousseau, Marco Sirois, Chantal Sirois, Edmond Rizcallah, Samuel Fortin. Proresolving Action of Docosahexaenoic Acid Monoglyceride in Lung Inflammatory Models Related to Cystic Fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2015;53(4):574–83. doi: 10.1165/rcmb.2014-0223OC. [DOI] [PubMed] [Google Scholar]
- 72.Christophe ER A. Current knowledge on fatty acids in cystic fibrosis. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1996;55(3):129–38. [DOI] [PubMed] [Google Scholar]
- 73.Strandvik B Fatty acid metabolism in cystic fibrosis. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;82(4):121–9. doi: 10.1016/j.plefa.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Morin C, Blier PU, Fortin S. Eicosapentaenoic acid and docosapentaenoic acid monoglycerides are more potent than docosahexaenoic acid monoglyceride to resolve inflammation in a rheumatoid arthritis model. Arthritis Res Ther. 2015;17:142 Epub 2015/05/29. doi: 10.1186/s13075-015-0653-y. PubMed PMID: 26022389; PMCID: PMC4624173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morin C, Fortin S, Cantin AM, Rousseau É. MAG-EPA resolves lung inflammation in an allergic model of asthma. Clin Exp Allergy. 2013;43(9):1071–82. doi: 10.1111/cea.12162. [DOI] [PubMed] [Google Scholar]
- 76.Khaddaj-Mallat R, Rousseau É. MAG-EPA and 17,18-EpETE target cytoplasmic signalling pathways to reduce short-term airway hyperresponsiveness. Pflugers Arch. 2015;467(7):1591–605. Epub 2014/08/13. doi: 10.1007/s00424-014-1584-1. [DOI] [PubMed] [Google Scholar]
- 77.Morin C, Blier PU, Fortin S. MAG-EPA reduces severity of DSS-induced colitis in rats. American journal of physiology Gastrointestinal and liver physiology. 2016;310(10):G808–21. Epub 2016/03/24. doi: 10.1152/ajpgi.00136.2015. [DOI] [PubMed] [Google Scholar]
- 78.Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108(3):486–92. Epub 2013/01/08. doi: 10.1038/bjc.2012.586. PubMed PMID: 23299528; PMCID: PMC3593545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morin C, Rodríguez E, Blier PU, Fortin S. Potential Application of Eicosapentaenoic Acid Monoacylglyceride in the Management of Colorectal Cancer. Mar Drugs. 2017;15(9). Epub 2017/09/04. doi: 10.3390/md15090283. PubMed PMID: 28869531; PMCID: PMC5618422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guertin MH, Robitaille K, Pelletier JF, Duchesne T, Julien P, Savard J, Bairati I, Fradet V. Effects of concentrated long-chain omega-3 polyunsaturated fatty acid supplementation before radical prostatectomy on prostate cancer proliferation, inflammation, and quality of life: study protocol for a phase IIb, randomized, double-blind, placebo-controlled trial. BMC Cancer. 2018;18(1):64 Epub 2018/01/10. doi: 10.1186/s12885-017-3979-9. PubMed PMID: 29321047; PMCID: PMC5763552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tiziana Bisogno DM, Bobrov Mikhail, Gretskaya Natalia, Bezuglov Vladimir, Petrocellis Luciano, Di Marzo Vincenzo. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Journal of Biochemistry. 2000(351):817–24. [PMC free article] [PubMed] [Google Scholar]
- 82.Vladimir Bezuglov MB, Natalia Gretskaya, Alla Gonchar, Galina Zinchenko, Dominique Melck, Tiziana Bisogno, Vincenco Di Marzo, Dmitry Kuklev, Jean-Claude Rossie, Jean-Pierre Vidal, Thierry Durand. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorganic & Medicinal Chemistry Letters. 2001;11(4):447–9. doi: 10.1016/s0960-894x(00)00689-2. [DOI] [PubMed] [Google Scholar]
- 83.Bezuglov V, Bobrov M, Gretskaya N, Gonchar A, Zinchenko G, Melck D, Bisogno T, Di Marzo V, Kuklev D, Rossi JC, Vidal JP, Durand T. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg Med Chem Lett. 2001;11(4):447–9. [DOI] [PubMed] [Google Scholar]
- 84.Bisogno T, Melck D, Bobrov MYu, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351 Pt 3:817–24. PubMed PMID: 11042139; PMCID: PMC1221424. [PMC free article] [PubMed] [Google Scholar]
- 85.Bobrov AL M, Andrianova EL, Gretskaya N, Zinchenko G, Frumkina L, Khaspekov L, Bezuglov V. Antioxidant and Neuroprotective properties of N-docosahexaenoyl Dopamine. Biophysics and Biochemistry. 2006;142(4):425–7. doi: 0007-4888/06/1424 0425. [DOI] [PubMed] [Google Scholar]
- 86.Ya Wang PP, Jean-Paul Vincken, Renate Janse, Michiel Balvers, Jean Paul ten Klooster, Harry Gruppen, Renger Witkamp, Jocelijn Meijerink. N-docosahexaenoyl Dopamine, an Endocannabinoid-like Conjugate of Dopamine and the n-3 Fatty Acid Docosahexaenoic Acid, Attenuates Lipopolysaccharide-Induced Activation of Microglia and Macrophages via COX-2. American Chemical Society Neuroscience. 2016;15(8):548–57. doi: 10.1021/acschemneuro.6b00298. [DOI] [PubMed] [Google Scholar]
- 87.Daniela Rovito CG, Pierluigi Plastina, Ines Barone, Francesca De Amicis, Loredana Mauro, Pietro Rizza, Marilena Lanzino, Stefania Catalano, Daniela Bonofiglio, Sebastiano Andò. Omega-3 DHA- and EPA-dopamine conjugates induce PPARγ-dependent breast cancer cell death through autophagy and apoptosis. Biochimica et Biophysica Acta. 2015;1850(11):2185–95. doi: 0.1016/j.bbagen.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Verhoeckx KC, Voortman T, Balvers MG, Hendriks HF, M Wortelboer H, Witkamp RF. Presence, formation and putative biological activities of N-acyl serotonins, a novel class of fatty-acid derived mediators, in the intestinal tract. Biochim Biophys Acta. 2011;1811(10):578–86. Epub 2011/07/21. doi: 10.1016/j.bbalip.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Mieke Poland JK, Zheng Wang, Raymond Pieters, Mark Boekschoten, Renger Witkamp, Jocelijn Meijerink. Docosahexaenoyl serotonin, an endogenously formed n-3 fatty acid-serotonin conjugate has anti-inflammatory properties by attenuating IL-23-IL-17 signaling in macrophages. Biochimica et Biophysica Acta. 2016;1861(12):2020–8. doi: 10.1016/j.bbalip.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 90.Ya Wang MB, Henk Hendriks, Tessa Wilpshaar, Tjarda van Heek, Renger Witkamp, Jocelijn Meijerink. Docosahexaenoyl serotonin emerges as most potent inhibitor of IL-17 and CCL-20 released by blood mononuclear cells from a series of N-acyl serotonins identified in human intestinal tissue. Molecular and Cell Biology of Lipids. 2017;1862(9):823–31. doi: 10.1016/j.bbalip.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 91.Ortar G, Cascio MG, De Petrocellis L, Morera E, Rossi F, Schiano-Moriello A, Nalli M, de Novellis V, Woodward DF, Maione S, Di Marzo V. New N-arachidonoylserotonin analogues with potential “dual” mechanism of action against pain. J Med Chem. 2007;50(26):6554–69. Epub 2007/11/21. doi: 10.1021/jm070678q. [DOI] [PubMed] [Google Scholar]
- 92.Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–52. doi: 10.1038/sj.bjp.0704327. PubMed PMID: 11606325; PMCID: 1573017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143(2):247–50. doi: 10.1038/sj.bjp.0705920. PubMed PMID: 15313881; PMCID: 1575333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morales P, Hurst DP, Reggio PH. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog Chem Org Nat Pr. 2017;103:103–31. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsuji F, Aono H. Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals (Basel). 2012;5(8):837–52. Epub 2012/01/01. doi: 10.3390/ph5080837ph5080837. [pii] PubMed PMID: 24280677; PMCID: 3763671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Wang DH. TRPV1 ablation aggravates inflammatory responses and organ damage during endotoxic shock. Clin Vaccine Immunol. 2013;20(7):1008–15. Epub 2013/05/03. doi: 10.1128/CVI.00674-12CVI.00674-12. [pii] PubMed PMID: 23637043; PMCID: 3697449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33(13):5603–11. Epub 2013/03/29. doi: 10.1523/JNEUROSCI.1806-12.2013. 33/13/5603 [pii] PubMed PMID: 23536075; PMCID: 3690366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida A, Furube E, Mannari T, Takayama Y, Kittaka H, Tominaga M, Miyata S. TRPV1 is crucial for proinflammatory STAT3 signaling and thermoregulation-associated pathways in the brain during inflammation. Sci Rep-Uk. 2016;6. doi: Artn 26088 10.1038/Srep26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andrew P Somlyo AVS. Ca2+ Sensitivity of Smooth Muscle and Nonmuscle Myosin II: Modulated by G Proteins, Kinases and Myosin Phosphatase. Physiological Reviews. 2003;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.