Abstract
Background
Osteochondral lesions of talus (OLT) are among the most common ankle problems. Platelet-rich plasma (PRP) and prolotherapy (PrT) are 2 successful injection-based techniques for treatment of chronic musculoskeletal problems. The aim of the present study was to compare PRP and PrT injections for the management of OLT.
Material/Methods
This was a retrospective cohort study of 49 patients with OLT symptoms of more than 6 months who had been refractory to 3 months of treatment using conservative methods. The patients were divided into 2 groups: PrT injections (PrT group, n=27) or PRP injections (PRP group, n=22). The patients were given 3 injections of 4 mL solution into periarticular and intra-articular ankle joint spaces. After treatment, patients were evaluated via Visual Analogue Scale (VAS), American Orthopedic Foot and Ankle Society Score (AOFAS), and Ankle Osteoarthritis Scale (AOS) at baseline and 21-, 90-, 180-, and 360-day follow-up periods.
Results
Both PRP and PrT treatments resulted in greater improvement in pain and ankle functions at follow-up periods extending to 1 year (P<0.001) and there was no difference between the groups for the outcomes at follow-up periods (P>0.05). Excellent or good outcomes were reported by 88.8% of the patients in PrT group and 90.9% of the patients in PRP group.
Conclusions
Both PRP and PrT are efficient and safe methods in treatment of OLT. PrT offers advantages of less cost and minimal invasiveness.
MeSH Keywords: Injections, Platelet-Rich Plasma, Talus
Background
Osteochondral lesions of the talus (OLT) are a common ankle problem characterized by degeneration of cartilage, resulting in joint pain and destruction [1]. These lesions can result in pain, function loss, disability,and reduction in quality of life. Ankle pain, occasional swelling, weakness, stiffness, and instability of the ankle are among the typical complaints of patients with OLT [2]. Management of symptomatic lesions has been problematic [3]. Available treatment methods might not prevent joint degeneration. In some patients who are not treated in early stages, large defects, cystic lesions, or instable lesions could develop [4]. In some patients, sequela of early stage foot ankle osteoarthritis could form, which in turn could lead to excess pain and movement restriction in the foot ankle in the longer terms [4]. In the literature, OLT incidence was reported to be 17% to 79% in patients with acute ankle fractures [5].
Lesions from early stage (cystic lesion) to full-thickness chondral lesions could be treated with conservative methods such as immobilization, restriction of weight bearing, and physiotherapy [6]. Advanced stage lesions (from displaced lesions to free loose fragments) or early stage lesions recalcitrant to conservative treatment are usually treated with surgical methods [3,6,7]. The most successful surgical methods are cartilage replacement procedures (autologous chondrocyte implantation, allograft transplantation, osteochondral autograft transplantation and mosaicplasty) or bone marrow stimulation techniques (microfracture, drilling and abrasion arthroplasty) [8]. There is an abundance of studies in the literature pertaining to these methods. Most of these methods are invasive and costly procedures. However, not all patients benefit from these procedures due to problems such as excessive pain, inability to cartilage adherence, lower strength of new cartilage, or mechanic symptoms [9,10].
Recently, injection methods have gained popularity in the treatment of osteodegenerative problems and many studies involving these methods have been conducted [11,12]. Corticosteroid injections are among the important alternatives. There are no reports of the use of corticosteroid injections in OLT to the best of our knowledge. However, corticosteroid injections have been used for the treatment of similar indications such as knee and finger osteoarthritis to reduce inflammation and alleviate the pain, thereby providing a comfortable treatment [13]. It has been mentioned in published reports that beneficial effects of corticosteroids are only temporary, and treatment can result in side effects such as connective tissue damage and osteomyelitis [13]. Similarly, hyaluronic acid is an injection therapy successfully used in knee and foot ankle osteoarthritis with its stimulation of cartilage and tissue healing [14]. Mei-Dan et al. [15] compared hyaluronic acid injections and platelet-rich plasma (PRP) for the treatment of talus OLT and found that both methods significantly improved pain score and functional scores relative to baseline. The authors concluded that PRP was more efficient and mentioned it as the most successful injection therapy of OLT.
Prolotherapy (PrT) and PRP are injection methods successfully used in degenerated and damaged joint structures including tendons, cartilage and other connective tissues [15–17]. Many studies have been conducted about PRP and PrT, and most of these studies have reported satisfactory outcomes. Mei-Dan et al. [15] used PRP for the treatment of OLT and reported successful results. However, effectiveness of prolotherapy in the treatment of OLT has not been evaluated so far. We hypothesized that prolotherapy injections could be as effective as other similar indications in the treatment of OLT.
The present retrospective study was carried out to evaluate the hypothesis that both PRP and PrT injections are effective to reduce pain and improve function in the treatment of OLT.
Material and Methods
Research design and patients
The present study was a retrospective cohort study with 2 equal groups receiving different treatments. Study protocols were approved by the Local Ethics Committee. An informed consent was signed by each patient enrolled in the study.
Patients whose ages varied between 18 and 70 years, who had at least 6 months of symptomatic OLT refractory (patients who had pain, stiffness, disability, and dissatisfaction after treatment) to at least 3 months of standard care modalities (temporary immobilization, use of analgesics and anti-inflammatory drugs, partial weight bearing and orthotic provision) and who had grade I, II, or III lesions in their standard ankle radiographies were included in the study. Grading of lesions was performed based on Berndt and Harty classification using their ankle radiographies [18]. Magnetic resonance imaging (MRI) was used to verify the diagnosis and to determine whether extra pathologies other than OLT were present.
Patients with rheumatic or systemic diseases, patients who had active or chronic infection in the treatment area, previous operation history on ankle, other ankle problems accompanying OLT which may cause pain and loss of function in the ankle and pregnant patients were excluded from the study.
A total of 74 patients who had chronic OLT and applied to our center from January 2016 to January 2018 period were included in the present study. Ten patients were excluded: 2 patients had stage IV instable lesions, 1 patient was under 18 years of age, 2 patients had previous operation histories, 5 patients had additional ligament-joint pathologies in their MRI examinations. The patients with instable lesions were referred to other centers since we did not have experience and materials to perform foot ankle arthroscopy. Seven patients were unable to complete the treatment protocols, 1 patient refused PrT injection after the first injection session. Similarly, 1 patient refused PRP treatment after the first injection session. Contact was lost with 2 patients at the second and third follow-ups. One case had hypotension, 1 case was diagnosed a hematological disorder after the first injection of PRP and 1 case had extreme pain after the first injection of PRP and they refused to participate in further study protocols. To reduce the effect of treatment selection bias and potential confounding in this cohort study, we performed rigorous adjustment for the differences in baseline characteristics using propensity-score matching. The PrT group consisted of 27 patients who received 3 PrT injections. The PRP group consisted of 22 patients who received 3 PRP injections (Figure 1).
Figure 1.
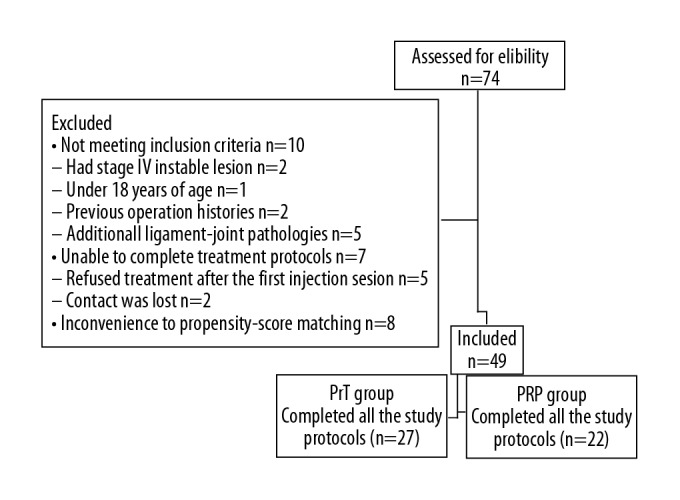
Flowchart of the study.
PRP and PrT preparation
PRP was prepared using GPS III Platelet Separation System (Biomet Biologics, Warsaw, IN, USA) according to the system instructions. A total of 45 mL venous blood was drawn from each patient and mixed with 5 mL of citrate for inhibition of clotting. The total solution of 50 mL was centrifuged in a specially designed disposable tube for 15 minutes at 3200 rpm. At the end of the procedure, 5 mL of PRP was obtained. Then 4 mL was used for injection (2 mL for intra-articular and 2 mL for tibial edge and talar dome adjacent to the joint surface) without any buffering or activating agent, and the remaining 1 mL of PRP was used for calculating the platelet concentration.
We used 4 mL of PrT solution without any activating agent for PrT intervention (2 mL 25% dextrose for intra-articular, 1.8 mL 15% dextrose in combination with 0.2 mL lidocaine for tibial edge and talar dome adjacent the joint surface). We used the same combination of PrT (25% dextrose intra-articular, 15% dextrose in combination with lidocaine as local anesthetic) as was used in most previous studies [1,16,17].
Intervention
One of the authors (DG) with 10 years of clinical experience in orthopedic surgery carried out all injection procedures. Each protocol consisted of 3 sessions (one session in 3 weeks). A 22-gauge needle was used for injections. All injections were performed under ultrasonography guidance and under aseptic conditions. In OLT, because of the bone marrow edema, periosteum reaction and soft tissue injury, a tenderness frequently occurs at tibial edge and talar dome adjacent to ankle joint [19]. In order to reduce pain and contribute to healing in these regions, we used a total of 4 mL solution (1 mL to painful areas at the tibial edge and 1 mL to talar dome adjacent to ankle joint and 2 mL intra-articularly (slowly infiltrated on the medial aspect of the ankle joint) was injected to 3 injection points in every patient, while the ankle was plantar flexed (Figure 2). Injection of patients with posterolateral lesion was made by posterolateral approach [20]. After injections, we recommended that patients do ankle flexion-extension motion for full solution coverage in the ankle. All participants were reminded at each visit to avoid nonsteroidal anti-inflammatory drugs (if the pain was unbearable for the patients, they were allowed to use 500 mg of acetaminophen up to 4 times per day) and to limit overuse of the ankle for the first 3 days during the treatment period.
Figure 2.
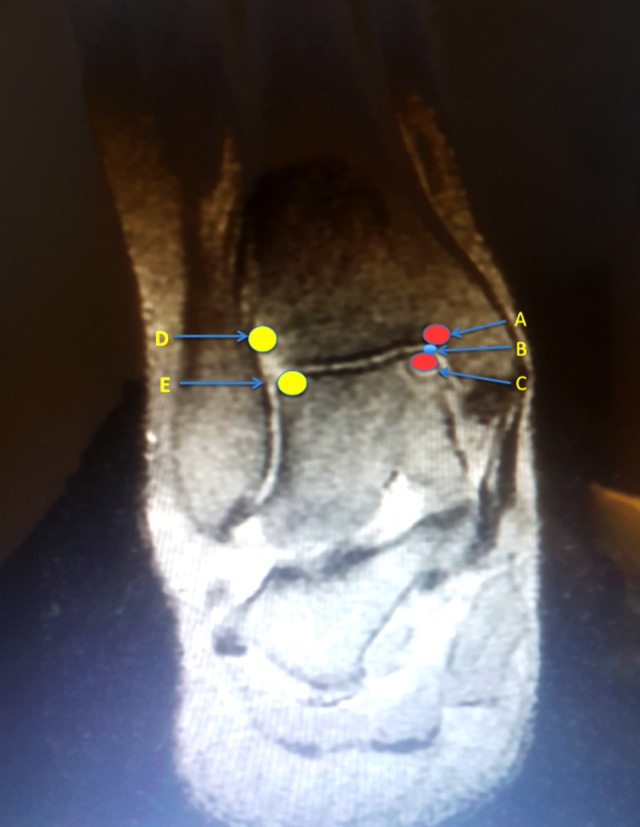
The injection points. A) Medial joint surface of tibia. B) Intraarticular injection point. C) Medial joint surface of talus. D) Lateral joint surface of tibia. E) Lateral joint surface of talus. Injection point A, B, and C used for posteromedial lesions; Injection points B, D, and E used for anterolateral lesions.
Assessment of outcomes
Pain was evaluated using a Visual Analogue Scale (VAS), (0=asymptomatic and 10=severe pain). American Orthopedic Foot and Ankle Society Score (AOFAS) was used to measure subjective and objective components of ankle including pain, function and alignment [21]. Ankle Osteoarthritis Scale (AOS) was used to evaluate patient’s disease-specific symptoms due to cartilage damage and osteoarthritic changes. AOS had 2 subscales evaluating pain and disability, with higher scores representing higher pain or difficulty [22].
The clinical outcomes were considered as “excellent”, “good”, “fair”, or “poor”. If there was no ankle pain after daily activities or sport activities, it was defined as an “excellent result”. Pain levels of ≤50% of the original ankle pain were defined as a “good result” while pain levels between 50% and 75% of the original ankle pain were defined as a “fair result” and pain levels ≥75% of the original ankle pain were defined as “poor result” [21].
Sample size
For the present study, calculated necessary minimum subject number to reveal significant differences in G*power program (Version 3.1.2) using VAS score of both groups was 21, with 80% power, 5% type I error, and effect size of 0.8.
Statistical analyses
Descriptive analyses were performed to provide information on general characteristics of the study population. Quantitative data were expressed as arithmetic means, standard deviations, frequencies or percentages. Two-way repeated measures ANOVA was used for time comparison of groups and group effects. Independent samples t-test was used to compare the data with continuous distribution. A P-value <0.05 was considered significant. Analyses were carried out using SPSS 19 software (IBM SPSS Statistics 19, SPSS Inc., IBM Co., Somers, NY, USA).
Results
Forty-nine patients (PrT group: n=27 and PRP group: n=22) were included in the study results. Demographic characteristics of the 2 groups were similar (Table 1).
Table 1.
General characteristics of variables.
| Variables | Total | Group | P | |
|---|---|---|---|---|
| PrT | PRP | |||
| n | 49 | 27(55.1) | 22(44.9) | – |
| Gender (Male/Female) | 14 (28.6)/35 (71.4) | 8 (29.6)/19 (70.4) | 6 (27.2)/16 (72.7) | 0.856 |
| Side (right/left) | 25 (51)/24 (49) | 15 (55.6)/12 (44.4) | 10 (45.4)/12 (54.6) | 0.482 |
| Etiology (idiopathic/traumatic) | 30 (61.2)/19 (38.8) | 15 (55.6)/12 (44.4) | 15 (68.2)/7 (31.8) | 0.367 |
| Location (posteromedial/anterolateral) | 46 (93.9)/3 (6.1) | 25 (92.6)/2 (7.4) | 21 (95.4)/1 (4.5) | 0.678 |
| Age (years) | 56.08±11.30 | 57.74±11.09 | 54.05±11.48 | 0.259 |
| Time of symptoms (months) | 24.82±19.38 | 26.19±16.84 | 23.14±22.41 | 0.589 |
| Lesion size (cm2) | 1.53±0.74 | 1.57±0.81 | 1.49±0.65 | 0.709 |
| Severity of the disease | Grade I: n=10(37%) Grade II: n=9 (33%) Grade III: n=8(29.6%) |
Grade I: n=8 (36,3%) Grade II: n=8 (36,3%) Grade III: n=6 (27.2%) |
||
Data are shown as mean ± standard deviation or frequency, percentage. P=independent samples t-test or chi-square test were used.
The mean platelet number in whole blood specimen was 256.9±55.7×103/mL. Mean platelet number in PRP samples was 1007.1±268.2×103/mL, which meant an increase of 4 times in the platelet numbers compared to whole blood samples.
Mean lesion size was 1.54±0.72 cm2 in PRP group, and 1.64±0.9 cm2 in PrT group. In the PRP group there were 8 grade I lesions (36.3%), 8 grade II lesions (36.3%), and 6 grade III lesions (27.2%). In the PrT group there were 10 grade I lesions (37.0%), 9 grade II lesions (33.0%), and 8 grade III lesions (29.6%).
All included patients completed a minimum of 12-months of follow-ups. Pre-injection values of the groups for VAS, AOFAS, and AOS were similar (P1=0.169, 0.101, and 0.177, respectively) (Tables 2–4). VAS scores of both groups were significantly improved compared to pretreatment values (P<0.001) and there was no significant difference between the groups at the follow-up periods (P1=0.099, 0.914, 0.894, 0.811 for 21-, 90-, 180-, and 360-day follow-up periods, respectively). AOS scores of 2 groups significantly improved compared to pretreatment values (P<0.001), and there was no significant difference between the 2 groups at the follow-up periods (P1=0.129, 0.837, 0.567, 0.981 for 21-, 90-, 180-, and 360-day follow-up periods, respectively). AOFAS scores of the 2 groups were also significantly improved compared to pretreatment values (P<0.001), and there was no significant difference between the 2 groups at the follow-up periods (P1=0.187, 0.851, 0.805, 0.643, respectively), (Tables 2–4).
Table 2.
VAS Scores of two study groups in different follow-up periods.
| Measurements | Group | P1 | |
|---|---|---|---|
| PrT | PRP | ||
| VAS_0 | 7.15±1.46 (a) | 7.73±1.42 (a) | 0.169 |
| VAS_21 days | 3.96±1.58 (b) | 4.68±1.36 (b) | 0.099 |
| VAS_3 months | 2.50±1.76 (c) | 2.55±0.96 (c) | 0.914 |
| VAS_6 months | 1.65±1.67 (d) | 1.59±1.22 (d) | 0.894 |
| VAS_12 months | 1.30±1.79 (d) | 1.41±1.40 (d) | 0.811 |
| P2 | <0.001 | <0.001 | |
ANOVA was used for repeated measures. Means with the same letters (in the same column) are not statistically different, P1 – between-subject effect, P2 – within-subject effect. VAS scores of both groups were significantly improved compared to pretreatment values, and there was no significant difference between the groups at the follow-up periods.
Table 3.
AOS scores of two study groups in different follow-up periods.
| Measurements | Group | P1 | |
|---|---|---|---|
| PrT | PRP | ||
| VAS_0 | 129.37±20.00 (a) | 137.41±20.88 (a) | 0.177 |
| VAS_21 days | 75.15±23.27 (b) | 86.45±27.97 (b) | 0.129 |
| VAS_3 months | 51.41±28.33 (c) | 49.91±20.54 (c) | 0.837 |
| VAS_6 months | 36.89±25.79 (d) | 33.27±15.56 (d) | 0.567 |
| VAS_12 months | 29.89±25.86 (e) | 30.05±19.54 (d) | 0.981 |
| P2 | <0.001 | <0.001 | |
ANOVA was used for repeated measures. Means with the same letters (in the same column) are not statistically different, P1 – between-subject effect, P2 – within-subject effect. AOS scores of 2 groups significantly improved compared to pretreatment values, and there was no significant difference between the 2 groups at the follow-up periods.
Table 4.
AOFAS scores of two study groups in different follow-up periods.
| Measurements | Total | Group | P1 | |
|---|---|---|---|---|
| PrT | PRP | |||
| AOFAS_0 | 34.71±17.80 (a) | 38.48±18.02 (a) | 30.09±16.79 (a) | 0.101 |
| AOFAS_21 days | 65.51±11.73 (b) | 67.52±11.23 (b) | 63.05±12.12 (b) | 0.187 |
| AOFAS_3 months | 79.35±9.99 (c) | 79.59±11.33 (c) | 79.05±8.31 (c) | 0.851 |
| AOFAS_6 months | 85.14±9.34 (d) | 85.44±10.69 (d) | 84.77±7.6 (d) | 0.805 |
| AOFAS_12 months | 88.69±12.38 (e) | 89.44±13.93 (e) | 87.77±10.42 (d) | 0.643 |
| P2 | <0.001 | <0.001 | <0.001 | |
ANOVA was used for repeated measures. Means with the same letters (in the same column) are not statistically different, P1 – between-subject effect, P2 – within-subject effect. AOFAS scores of the 2 groups also significantly improved compared to pretreatment values, and there was no significant difference between the 2 groups at the follow-up periods.
Twenty-four patients (88.8%) in the PrT group reported excellent or good outcomes (excellent: n=20 and good: n=4) and 3 patients (11.11%) reported fair or poor outcomes (fair: n=1 and poor: n=2). Twenty patients (90.9%) in the PRP group reported excellent or good outcomes (excellent: n=18 and good: n=2), while 2 patients (9.09%) reported fair or poor outcomes (fair: n=1 and poor: n=1).
Average lesion size was significantly lower in patients with excellent or good outcomes (1.43±0.68 cm2 and 1.42±0.63 cm2 for PrT and PRP groups, respectively) compared to patients with fair or poor outcomes (2.6±1.21 (PrT), 2.25±0.21 for PrT and PRP groups, respectively) (P<0.001).
The average cost of PrT to the hospital was 30 Turkish Liras (TL) ($6.8) per session, and average cost of PRP to the hospital was 250 TL ($56.8) per session.
Patients did not suffer from any side effects such as infection, fever, hematoma, or rupture. Only 3 patients reported extreme pain 1 or 2 days after injection in the prolotherapy group, which was alleviated after 2 days of non-weight bearing.
Discussion
OLT is an important ankle problem, which considerably decreases patient’s satisfaction and comfort. Although many treatment modalities have been described in the literature, there has been no consensus yet over the optimal procedure [3,8,23]. In the present study, efficiencies of PRP and PrT were compared. The results showed that both treatments were effective, and no difference was found between these methods in terms of follow-up parameters.
In the treatment of OLT, conservative methods are the primary choice. Some patients can be successfully treated by these methods. However, symptoms of some patients are recalcitrant to these treatments and, therefore, surgery is inevitable [3,6,7]. It has been reported that success of conservative methods was lower than that of surgical ones in treatment of OLT [24,25]. Arthroscopic debridement and curettage are widely used surgical methods especially at earlier stages and for smaller lesions. With proper patient selection, success rates with these methods can be up to 80% [3,26]. However, in some patients, the symptoms recur after a short time period and revision surgeries become inevitable. On the other hand, low success rates have been reported with this method in larger defects and in instable and cystic lesions. Some authors have stated that fibrocartilaginous repair tissue forming in defect areas was devoid of durability and mechanical properties of articular hyaline cartilage [6,27]. More invasive methods, such as autologous chondrocyte implantation or osteoarticular transfer system, have gained popularity with higher success rates of up to 90% [28]. These methods are very expensive and require 2 surgical procedures, which involves extra surgical complication risks. Immune host response, donor site morbidity, and infections are other problems associated with these invasive techniques [29]. Some studies have reported that efficiencies of these invasive methods were not superior to microfracture. In addition, there is limited evidence that the cartilage harvested from knee can withstand to forces sustained by talus [30,31]. Many similar invasive surgical methods with varying levels of invasiveness are available. In contrast, there is only limited evidence related to proliferative injection methods. Therefore, surgery seems to be inevitable for these patients. In the present study, 2 major injection methods were used in the treatment of OLT and success rates of up to 80% were obtained in patients using PRP or PrT injections. Considering higher complication risks and costs of surgery, injection methods with higher treatment success seem to be preferable.
Due to concerns related to graft incorporation, donor site morbidity or post-surgical recovery, weight-bearing is restricted for a certain period of time after surgical interventions and this leads to a considerable amount of labor loss. Healthy ligament, bone, and cartilage structures could be injured during surgical operations, which might reduce quality of life for patients after these procedures [9,10,28]. In the present study, all patients were allowed to bear full weight and to do their jobs 3 days after the procedures. None of the patient’s quality of life worsened after the injections. These findings were similar to those of Mei-Dan et al. who used hyaluronic acid and PRP for OLT [15]. Therefore, proliferative methods such as prolotherapy and PRP could be considered as a first-line treatment option for patients with grade I–III lesions.
PrT is a proliferative injection method gaining popularity in the treatment of musculoskeletal problems. Although PrT has been successfully used in similar indications, including knee osteoarthritis and chondromalacia patella, it has not been previously used in OLT. Knee osteoarthritis and chondromalacia patella have similar pathophysiologic mechanisms to those of OLT such as degenerative process, cartilage degeneration, and ligament injury. Yildiz et al. [25] used extra- and intra-articular injection of PrT in treatment of 44 recreational athletes with chondromalacia patella and obtained significant improvements in knee functions, joint balance, and coordination. Rabago et al. [29] used PrT for patients with mild to severe knee osteoarthritis. They obtained significant improvements in knee functions, pain intensity, and stiffness after an average of 2.5 years of follow-up periods [29]. Results of the present study were similar to those in the literature dealing with the use of PrT in the treatment of osteodegenerative diseases. PrT as a less invasive, simple, and inexpensive method, could be successfully used in the treatment of OLT.
PRP has been increasingly used for osteodegenerative problems to provide essential growth factors that induce differentiation and proliferation of mesenchymal stem cells into degenerated structures [32]. Animal studies have shown that PRP provided healing and strengthening effects on degenerated cartilage and resulted in better recovery as measured by the International Cartilage Repair Society (ICRS) scores [23]. PRP was previously used in the treatment of OLT using PRGF (plasma rich in growth factors) technique, which has 2–3 times the blood platelets count without white blood cells [15]. Mei-Dan et al. [15] compared PRP and hyaluronic acid in the treatment of OLT. At 28-week follow-ups, intra-articular injections with these 2 methods were found to be effective compared to pre-injection values. However, PRP provided significantly better outcomes than hyaluronic acid [15]. In the present study, a different PRP technique was used which provided 3–4 times the blood platelets count and involved injection not only to intra-articular space but also to tibial edge and talar dome, which are painful areas because of bone marrow edema and periosteal reaction. We performed ultrasonography guided injections to increase safety and efficiency. The clinical outcomes in the present study were slightly better than Mei-Dan et al. study outcomes, which could be due to injection technique and preparation method. PRP injections were also successfully used in previous published studies in the treatment of ligament injury, tendinopathies and other soft tissue disorders [33]. According to our experience, with its lower solution requirement, PRP can be applied to smaller joints using peri- and pre-articular injections with better results. PRP preparation methods might differ among studies. In the present study, 90.9% of the patients in PRP group had excellent or good outcomes. Although success rate with this method varied based on different preparation methods, it had satisfaction levels similar to what was reported by Mei-Dan et al. Therefore, future research efforts are needed to better evaluate and refine PRP as a treatment option for OLT.
In the present study, 90.9% of the patients in PRP group and 88.8% of the patients in PrT group reported excellent or good outcomes, and no significant difference was found between the 2 groups. There was limited evidence regarding the efficiencies of the injection methods in the treatment of OLT. Therefore, we used the most successful method for comparison. Unlike corticosteroids, both methods (PRP and PrT) induced proliferation and tissue regeneration in the applied region [13]. PRP is costlier than PrT, and has some difficulties in preparation of PRP. In addition, it requires another invasive procedure including blood drawing, and lacks optimized and standardized preparation protocols [2,34,35]. Blood is extremely essential for human life and its supply is highly restricted. Therefore, only a limited amount of PRP can be obtained from each patient. Its effect might be limited in the patients who have other accompanying ligament and tendon pathologies. PrT is preferable to PRP because it is a less invasive process and has high treatment success with lower costs.
Success of the treatment modalities varies depending upon the stage of lesion, patient’s age, and duration of symptoms. Older patients or advanced stage OLTs have poorer results, while younger patients or early stage lesions have more satisfactory results [7]. Similar to these reports, average lesion size of patients with excellent or good outcomes in both groups (1.42±0.65 cm2) were significantly lower compared to patients with fair or poor outcomes (2.46±0.88 cm2). These findings support the proposition that injection methods should be used in patients with early stage and small sized lesions [7].
In the present study, 3 patients (11.11%) in the PrT group and 2 patients (9.09%) in the PRP group reported fair or poor outcomes. We concluded that surgery was needed for these patients. Similar low percentages for fair or poor outcomes were also reported by Mei-Dan et al. [15] in both PRP and hyaluronic acid treatment groups. These results showed that a high percentage of patients could benefit from proliferative injection methods. Nevertheless, only 12-month follow-ups in the study were not enough to have a good understanding of long-term effects of this treatment modality and to reveal what proportion of patients who might fail and require surgery. In the literature, patients with failed injection treatments went on to surgery. Therefore, additional studies with longer follow-ups are needed.
In a high percentage of patients, other joint and ligament pathologies might accompany OLT [36]. Similar to reports in the literature, the ratio of accompanying ligament and joint injuries was high in our OLT patient population. In order to keep groups equal and to be able to see the effect of injection methods on OLT, we excluded these patients. The ankle ligaments provide significant contributions to joint stabilization [15,37]. In some patients, ankle joint stabilization could be disturbed as a result of ligament injury, leading to secondary OLT. In these patients, both resulting OLT and its cause can be treated at the same time using these proliferative injection methods. Injection methods can also be used for patients who received previous surgeries and suffered from pain. Mei-Dan et al. [15] used PRP and hyaluronic acid injections for 5 symptomatic patients who had previously undergone arthroscopy with micro-fracture or drilling, and obtained satisfactory results.
The most significant limitations of the present study were its small sample size, short follow-up period, lack of a control group, and its retrospective design. Another limitation in both groups was that needles used in injection caused focal bleeding, which might have increased inflammatory response and interfered with healing mechanisms [38].
Conclusions
Results of the present study supported the use of both PRP and PrT injections in the treatment of OLT. The 2 methods can be used to obliterate OLT symptoms and improve physical abilities of patients. However, PrT offers advantages of lower cost and minimal invasiveness.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Roach R, Mcbride DJ, Maffulli N. Osteochondral lesions of the talus. Minerva Ortop Traumatol. 2002;53(3):157–63. [Google Scholar]
- 2.Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 2002;23(7):651–54. doi: 10.1177/107110070202300712. [DOI] [PubMed] [Google Scholar]
- 3.Haene R, Qamirani E, Story RA, et al. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105–10. doi: 10.2106/JBJS.J.02010. [DOI] [PubMed] [Google Scholar]
- 4.Elias I, Jung JW, Raikin SM, et al. Osteochondral lesions of the talus: Change in MRI findings over time in talar lesions without operative intervention and implications for staging systems. Foot Ankle Int. 2006;27(3):157–66. doi: 10.1177/107110070602700301. [DOI] [PubMed] [Google Scholar]
- 5.McGahan PJ, Pinney SJ. Current concept review: Osteochondral lesions of the talus. Foot Ankle Int. 2010;31(1):90–101. doi: 10.3113/FAI.2010.0090. [DOI] [PubMed] [Google Scholar]
- 6.Chew KTL, Tay E, Wong YS. Osteochondral lesions of the talus. Ann Acad Med Singapore. 2008;37:63–68. [PubMed] [Google Scholar]
- 7.Van Bergen CJ, de Leeuw PA, van Dijk CN. Potential pitfall in the micro fracturing technique during the arthroscopic treatment of an osteochondral lesion. Knee Surg Sports Traumatol Arthrosc. 2009;17(2):184–87. doi: 10.1007/s00167-008-0594-y. [DOI] [PubMed] [Google Scholar]
- 8.Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26:583–89. doi: 10.1177/107110070502600801. [DOI] [PubMed] [Google Scholar]
- 9.Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg [Am] 1999;81(9):1229–35. doi: 10.2106/00004623-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Parisien JS, Vangsness T. Operative arthroscopy of the ankle. Three years’ experience. Clin Orthop Relat Res. 1985;199:46–53. [PubMed] [Google Scholar]
- 11.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 12.Seven MM, Ersen O, Akpancar S, et al. Effectiveness of prolotherapy in the treatment of chronic rotator cuff lesions. Orthop Traumatol Surg Res. 2017;103(3):427–33. doi: 10.1016/j.otsr.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91–97. doi: 10.1177/107110079801900207. [DOI] [PubMed] [Google Scholar]
- 14.Di Matteo B, Filardo G, Kon E. Platelet-rich plasma: Evidence for the treatment of patellar and Achilles tendinopathy – a systematic review. Musculoskelet Surg. 2015;99:1–9. doi: 10.1007/s12306-014-0340-1. [DOI] [PubMed] [Google Scholar]
- 15.Mei-Dan O, Carmont MR, Laver L, et al. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534–41. doi: 10.1177/0363546511431238. [DOI] [PubMed] [Google Scholar]
- 16.Akpancar S, Seven MM, Tuzun HY, et al. Current concepts of prolotherapy in orthopedic surgery. Arch Trauma Res. 2017;6(2):e40447. [Google Scholar]
- 17.Ersen O, Koca K, Akpancar S, et al. A randomized-controlled trial of prolotherapy injections in the treatment of plantar fasciitis. Turk J Phys Med Rehab. 2017;64(1):59–65. doi: 10.5606/tftrd.2018.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41:988–1020. [PubMed] [Google Scholar]
- 19.Nencini S, Ivanusic JJ. The physiology of bone pain. how much do we really know? Front Physiol. 2016;7:157. doi: 10.3389/fphys.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varenika V, Harter J, Chu E, Steinbach L. The posterolateral approach for fluoroscopy-guided tibiotalar joint injection. Skeletal Radiol. 2017;46(8):1113–15. doi: 10.1007/s00256-017-2650-5. [DOI] [PubMed] [Google Scholar]
- 21.Kitaoka HB, Alexander IJ, Adelaar RS, et al. Clinical rating systems for the ankle-hindfoot, midfoot, hallux and lesser toes. Foot Ankle Int. 1994;15(7):349–53. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 22.Domsic RT, Saltzman CL. Ankle osteoarthritis scale. Foot Ankle Int. 1998;19:466–71. doi: 10.1177/107110079801900708. [DOI] [PubMed] [Google Scholar]
- 23.Milano G, Sanna Passino E, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: An experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18:971–80. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: Long-term results. Am J Sports Med. 2008;36(9):1750–62. doi: 10.1177/0363546508316773. [DOI] [PubMed] [Google Scholar]
- 25.Yildiz Y, Apaydin AH, Seven MM, Orscelik A. The effects of prolotherapy (hypertonic dextrose) in recreational athletes with patellofemoral pain syndrome. J Exp Integr Med. 2016;6(2):53–56. [Google Scholar]
- 26.Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8:233–42. viii–ix. doi: 10.1016/s1083-7515(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 27.Bohay DR, Anderson JG. Osteochondral defects of the talar dome: Debridement and drilling. Tech Foot Ankle Surg. 2004;3(1):40–44. [Google Scholar]
- 28.La Prade RF, Botker JC. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004;20:e69–73. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Rabago D, Mundt M, Zgierska A, Grettie J. Hypertonic dex- trose injection (prolotherapy) for knee osteoarthritis: Long term outcomes. Complement Ther Med. 2015;23(3):388–95. doi: 10.1016/j.ctim.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 30.El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: A retrospective review. J Bone Joint Surg Am. 2011;93(17):1634–40. doi: 10.2106/JBJS.J.00900. [DOI] [PubMed] [Google Scholar]
- 31.Reddy S, Pedowitz DI, Parekh SG, et al. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80–85. doi: 10.1177/0363546506290986. [DOI] [PubMed] [Google Scholar]
- 32.Wroblewski AP, Melia HJ, Wright VJ. Application of platelet-rich plasma to enhance tissue repair. Oper Tech Orthop. 2010;20:98–105. [Google Scholar]
- 33.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA. 2010;303:144–49. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 34.Baksh N, Hannon CP, Murawski CD, et al. Platelet-rich plasma in tendon models: A systematic review of basic science literature. Arthroscopy. 2013;29(3):596–607. doi: 10.1016/j.arthro.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Kesikburun S, Tan AK, Yılmaz B, et al. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41(11):2609–16. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 36.Link SC, Erickson SJ, Timins ME. MR imaging of the ankle and foot: Normal structures and anatomic variants that may simulate disease. Am J Roentgenol. 1993;161(3):607–12. doi: 10.2214/ajr.161.3.8352117. [DOI] [PubMed] [Google Scholar]
- 37.Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: A systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–46. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen MM, Altman RD, Hollstrom R, et al. Safety and efficacy of intra-articular sodium hyaluronate (Hyalgan) in a randomized, double-blind study for osteoarthritis of the ankle. Foot Ankle Int. 2008;29(7):657–63. doi: 10.3113/FAI.2008.0657. [DOI] [PubMed] [Google Scholar]


