Abstract
Study Objectives
We compared resident physician work hours and sleep in a multicenter clustered-randomized crossover clinical trial that randomized resident physicians to an Extended Duration Work Roster (EDWR) with extended-duration (≥24 hr) shifts or a Rapidly Cycling Work Roster (RCWR), in which scheduled shift lengths were limited to 16 or fewer consecutive hours.
Methods
Three hundred two resident physicians were enrolled and completed 370 1 month pediatric intensive care unit rotations in six US academic medical centers. Sleep was objectively estimated with wrist-worn actigraphs. Work hours and subjective sleep data were collected via daily electronic diary.
Results
Resident physicians worked fewer total hours per week during the RCWR compared with the EDWR (61.9 ± 4.8 versus 68.4 ± 7.4, respectively; p < 0.0001). During the RCWR, 73% of work hours occurred within shifts of ≤16 consecutive hours. In contrast, during the EDWR, 38% of work hours occurred on shifts of ≤16 consecutive hours. Resident physicians obtained significantly more sleep per week on the RCWR (52.9 ± 6.0 hr) compared with the EDWR (49.1 ± 5.8 hr, p < 0.0001). The percentage of 24 hr intervals with less than 4 hr of actigraphically measured sleep was 9% on the RCWR and 25% on the EDWR (p < 0.0001).
Conclusions
RCWRs were effective in reducing weekly work hours and the occurrence of >16 consecutive hour shifts, and improving sleep duration of resident physicians. Although inclusion of the six operational healthcare sites increases the generalizability of these findings, there was heterogeneity in schedule implementation. Additional research is needed to optimize scheduling practices allowing for sufficient sleep prior to all work shifts.
Clinical Trial: Multicenter Clinical Trial of Limiting Resident Work Hours on ICU Patient Safety (ROSTERS), https://clinicaltrials.gov/ct2/show/NCT02134847
Keywords: sleep, work hours, fatigue, medical education, actigraphy, sleep diary
Statement of Significance.
This operational trial, conducted in six pediatric intensive care units across the country, showed that rosters eliminating scheduled extended-duration shifts reduced weekly work hours and improved sleep duration of resident physicians. These findings extended evidence from a previous single-site study, as the results were consistent even though the rosters were implemented differently across sites. Knowledge gaps remain about optimal scheduling practices that ensure sufficient opportunity for resident physician sleep prior to all work shifts. Sufficient sleep is necessary for patient safety and the health and safety of the resident physician.
Introduction
The nature of healthcare delivery requires clinical coverage 24 hr per day, 7 days per week. Consequently, healthcare workers’ schedules often include night and rotating shifts, early morning start times, and other nonstandard work hours. Shift workers report disturbed sleep and excessive sleepiness more frequently than day workers [1], and 40% of healthcare practitioners, nurses, and others in healthcare support report insufficient sleep [2]. There is a compelling need for the design of schedules that enable sufficient sleep in settings that require safety-sensitive 24 hr operations.
Extended-duration work shifts (≥24 hr) have been the cornerstone of resident-physician training. Resident physicians in the United States are sanctioned to work shifts lasting 24 or more continuous hours, and up to 88 hr per week, averaged across 4 weeks, limiting their time for activities of daily living and sleep. In a single-site randomized clinical trial to test whether the elimination of extended-duration work shifts could decrease weekly work hours and increase sleep duration, the work and sleep of first-year resident physicians (PGY1s) working an Extended Duration Work Roster (EDWR) were compared with the same PGY1s scheduled to a Rapid Cycling Work Roster (RCWR), in which scheduled shift duration was limited to no more than 16 consecutive hours in medical and cardiac intensive care units. Work hours decreased from 85 to 65 per week under the RCWR, sleep duration increased almost 1 hr per night, and electro-oculographically documented nighttime attentional failures decreased by 50% [3].
Based primarily on these results and others, the Accreditation Council of Graduate Medical Education limited PGY1s to shifts of 16 or fewer continuous hours of work from 2011 to 2017, but citing a lack of data among more senior resident physicians, permitted resident physicians to continue working extended-duration work shifts up to 28 hr after their first postgraduate year. We therefore conducted the first multicenter clinical trial of senior resident physicians (PGY2 and higher) to compare the work hours and sleep obtained under EDWR with those under RCWRs, with scheduled shift lengths limited to no more than 16 consecutive hours in the latter condition.
Methods
Full details of the study design, including collection of patient safety and performance data, are available elsewhere [4–6]. Briefly, the Randomized Order Safety Trial Evaluating Resident-physician Schedules (ROSTERS) study was a multicenter clustered-randomized, crossover clinical trial designed to evaluate the effectiveness of eliminating resident physicians’ traditional shifts of 24 hr or longer.
Many academic medical centers nationwide were not eligible to participate, as they had previously eliminated shifts scheduled for longer than 16 hr for resident-physicians working in their Pediatric Intensive Care Units (PICUs). Six sites were initially selected for participation, and five of those (83%) completed the trial. The originally selected sixth site withdrew from the study due to a change in leadership, and the first replacement site selected was unable to meet study timelines. A second replacement selected for the sixth site then completed the study. Overall, the completion rate was six out of eight sites (75%) that were selected for participation in the trial.
Six PICUs participated from July 2013 to March 2017: Boston Children’s Hospital; Children’s Hospital Colorado; University of Iowa Stead Family Children’s Hospital; Seattle Children’s Hospital; Cincinnati Children’s Hospital Medical Center; and University of Virginia Children’s Hospital. The units’ initial study condition was randomly assigned to either an EDWR, with regularly scheduled 24–28 hr extended-duration work shifts, or to a RCWR that limited resident physicians’ scheduled work shifts to no more than 16 consecutive hours, including regular overnight shifts. Each condition had a 4-month wash-in interval following by an 8-month data collection interval. Ethical approval was obtained at each academic medical center, as well as at Sutter Health (Data Coordinating Center) and Partners Human Research Committee (Clinical Coordinating Center). Study investigators obtained a Certificate of Confidentiality from the National Institutes of Health to protect the privacy of research participants.
Recruitment
All PGY2 and higher resident physicians working in the PICU over the study interval were invited to participate in the study. Volunteers provided written consent and were offered an incentive (e.g. iPad or cash equivalent) for participation.
Data collection interval
Actigraphy and eDiary data were collected during each participant’s rotation in the PICU. Individual rotations in the PICU lasted approximately 1 month, and resident physicians could complete multiple rotations in the PICU during the study.
Actigraphy
During the rotation, resident-physician volunteers continuously wore wrist Motionlogger actigraphs (Ambulatory Monitoring, Inc., Ardsley, NY) to collect rest/activity patterns. The Motionlogger is a battery-operated device and is the size of a watch. Participants were instructed to wear it on the wrist of their nondominant hand. Sleep was estimated for each day using the Action-W version 2.0 software (Ambulatory Monitoring, Inc., Ardsley, NY; UCSD algorithm with rescoring) [7–9].
eDiary
Resident physicians completed daily sleep/wake electronic logs (“eDiary”) as part of their morning routine. The logs provided a daily assessment of work hours and sleep duration and were also used to assist with interpretation of actigraphy data (e.g. confirming sleep intervals).
Questionnaire
On the baseline survey, resident physicians self-reported demographic information (e.g. height and weight) and were screened for sleep apnea using the Berlin questionnaire [10]. Resident physicians completed end-of-rotation surveys to report hours spent in patient care and rate their work experience on each schedule. Responses to multiple-choice questions were combined to create three scores that summarized work experience. These included the extent to which their training offered an opportunity to obtain knowledge and skills (15 questions, range 0 to 60), expectations of the residency (13 questions, range 13 to 65), and day-to-day activities of work (5 questions, range 0–30; see Supplementary Table 1).
Resident workload
As a measure of resident workload, ICU patients per resident-physician (IPRP) were calculated as the average daily patient census over the average resident-physicians present on the unit [6].
Analysis
Both actigraphy and eDiary data were divided into consecutive 6 am–6 am intervals for analyses of total sleep time and work hours. To be eligible for analysis, participants had to have a minimum of fourteen 6 am–6 am intervals of continuous actigraphy or 14 intervals of eDiary data. For actigraphy, 6 am to 6 am data intervals were considered usable if they contained at least 22 hr of data. For eDiary, 6 am to 6 am data intervals were reviewed visually and compared with actigraphy data to determine whether the sleep and work data reported were complete. Repeated interval-specific outcomes were averaged within rotations then multiplied by 7 to generate weekly averages. Data are reported as mean ± standard deviation.
Not all resident physicians contributed equally in the data set. Our statistical approach, which used random intercepts to account for within-participant correlation of repeated outcomes across rotations, accounted for unequal numbers of observations for each resident physician. We used generalized linear models to estimate the effects of schedule. Fixed effects included schedule, site, and randomization order, as well as baseline characteristics found to be unbalanced by schedule. Linear mixed models were used for the continuous outcomes, with variances and covariances estimated by the restricted maximum likelihood method. The distributions of the continuous outcomes were examined graphically for normality. Mixed logistic models were used for the dichotomous outcomes. Augmented models including a site-schedule interaction were used to assess evidence for modification of the effect of schedule by site. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC), with Proc Mixed used for continuous outcomes and Proc Glimmix for binary outcomes. All significance levels reported were two-sided and alpha level was set at 0.05.
Results
Participants
A total of 302 individual resident physicians participated in the study, with 51 who enrolled twice, four who enrolled three times and three who enrolled four times for a total of 370 rotations. Twenty-four resident physicians provided data in both the EDWR and RCWR conditions. No resident physician repeated more than twice within condition. 99.3% (300/302) of resident physicians completed the demographics section of the baseline questionnaire. The mean age was 29.4 ± 2.3 (range 25–42) years and 62.1% were female. The Berlin Questionnaire was completed for 312 rotations and 5.5% (17/312) screened at high risk for sleep apnea. Demographics, apnea risk, and year of residency did not differ by schedule conditions (p ≥ 0.38). Body mass index was slightly higher in the RCWR group than the EDWR group, on average. Although the difference is not clinically important, it was significantly different (23.3 vs. 23.2 kg/m2, p < 0.01; Table 1).
Table 1.
Resident-physician characteristics by schedule type
| Characteristic | Overall (n = 362) |
Schedule | P | |
|---|---|---|---|---|
| EDWR | RCWR | |||
| (n = 171) | (n = 191) | |||
| Gender | 0.97 | |||
| Female | 225 (62.1) | 105 (61.4) | 120 (62.8) | |
| Male | 137 (37.9) | 66 (38.6) | 71 (37.2) | |
| Age, years | 29.4 ± 2.3 | 29.3 ± 2.19 | 29.5 ± 2.4 | 0.38 |
| Race | 0.90 | |||
| White | 294 (81.4) | 138 (81.2) | 156 (81.7) | |
| Nonwhite | 67 (18.6) | 32 (18.8) | 35 (18.3) | |
| Ethnicity | 0.44 | |||
| Hispanic/Latino | 23 (6.6) | 13 (7.9) | 10 (5.4) | |
| Not Hispanic/Latino | 326 (93.4) | 152 (92.1) | 174 (94.6) | |
| Year of residency program | 0.95 | |||
| PY2 | 235 (64.9) | 112 (65.5) | 123 (64.4) | |
| PY3 | 127 (35.1) | 59 (34.5) | 68 (35.6) | |
| High risk for apnea* | 17 (5.5) | 9 (6.3) | 8 (4.7) | 0.54 |
| Body mass index, kg/m2 | 23.2 ± 3.4 | 23.2 ± 3.4 | 23.3 ± 3.5 | <0.01 |
Data shown as n(%) or mean ± SD, per rotation.
p Values from generalized mixed model adjusted for site and randomization order.
*Based on the Berlin questionnaire.
†362 rotations with ≥14 days of actigraphy and/or eDiary data.
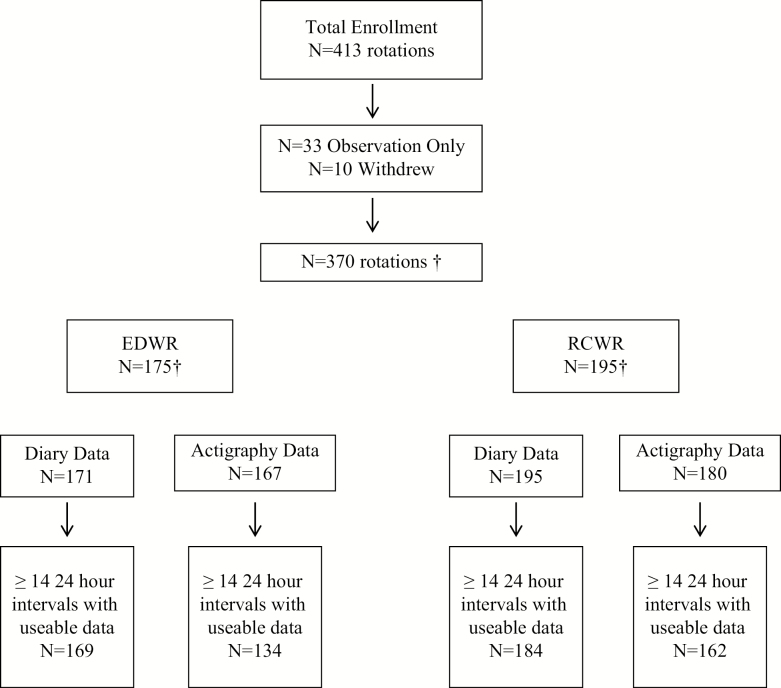
There were sufficient actigraphy data in 296/370 rotations data to include in the analysis, 134 in the EDWR and 162 in the RCWR (Figure 1). Similarly, 353/370 rotations had sufficient eDiary data, 169 in the EDWR and 184 in the RCWR. By schedule condition, the difference in the rate of rotations with valid actigraphy or diary data was not statistically significant (p = 0.09 and p = 0.34, respectively). Representative examples of raster plots of work and sleep data are shown in Figure 2.
Figure 1.
Enrollment and status of participation in the study. Three hundred seventy rotations contributed to the actigrpahy and diary data. †A total of 302 unique residents participated in the study, with 51 enrolled twice, four enrolled three times, and three enrolled four times.
Figure 2.
Two examples from each site of work and sleep data plotted from resident physicians’ eDiaries. Gray bars depict work and black bars depict sleep. There was variation in schedules, work, and sleep among resident physicians and sites.
Work hours
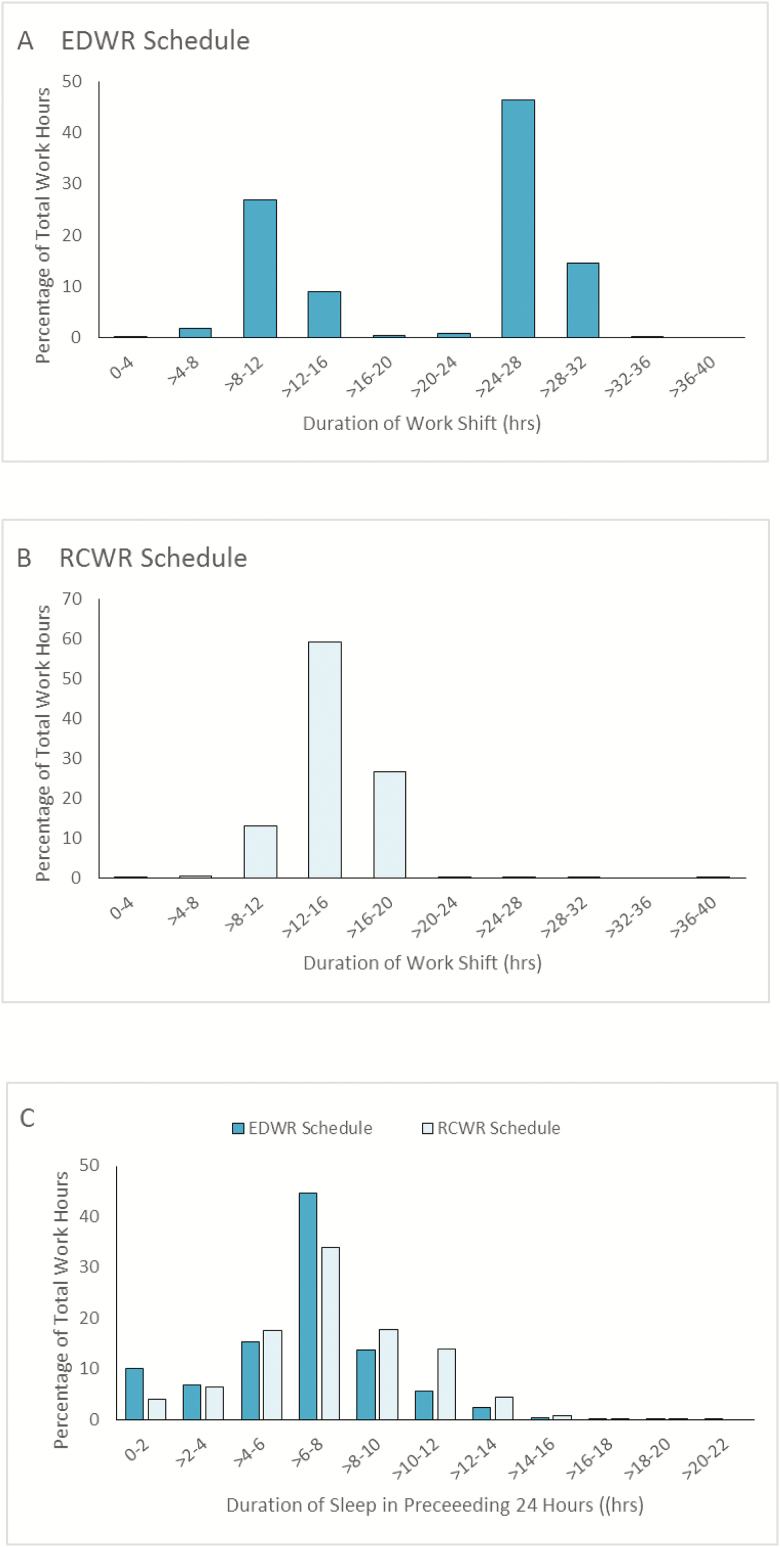
Resident physicians worked significantly more hours per week on average in the EDWR (68.4 ± 7.4) than they did in the RCWR condition (61.9 ± 4.8; p < 0.0001). In the EDWR conditions, resident physicians reported 2566 work shifts, 38% of which were extended-duration (≥24 hr) and 61% of the total work hours occurred during these shifts (Figure 3A). Overall, 15% of EDWR work hours occurred during shifts longer than 28 hr, but the number of these shifts varied greatly by site (p < 0.0001), ranging from 0.5% to 25% of shifts. During the RCWR condition, 0.3% of work hours occurred during extended-duration shifts; 73% of work hours occurred within shifts of 16 or fewer continuous consecutive hours (Figure 3B). In contrast, during the EDWR condition only 38% of work hours occurred on shifts of 16 hr or fewer in duration. The mean number of hours spent participating in direct patient care was on average higher in the EDWR than RCWR conditions (59.6 ± 16.1 and 55.9 ± 14.7, respectively; p = 0.008). In a subset of rotations with 28 or more continuous days of diary data (51 EDWR rotations and 70 RCWR rotations), the maximum weekly work hours, averaged over 4 weeks, were higher on EDWR (69.3 ± 6.0 hr) than on RCWR (63.2 ± 3.8 hr; p < 0.0001). None of the rotations had maximum work hours, averaged over 4 weeks, of more than 80 hr per week.
Figure 3.
Proportion of total work hours plotted against the duration of the shift during the EDWR (A) and the RCWR (B) and the percentage of total work hours that occurred after various amounts of sleep in the preceding 24 hr (C).
We examined the number of hours resident physicians had off between shifts in each scheduling condition by calculating the percentage who had 10 or fewer hours between shifts. There were significantly more short between-shift intervals (<10 hr) during the RCWR (8.4%) when compared with the EDWR (0.3%; p < 0.0001). Three sites did not have any short between-shift intervals on the EDWR. During the RCWR, the percentage of short between-shift intervals varied across sites, ranging from 1% to 14%. Table 2 shows the rate of short between-shift intervals at each site.
Table 2.
Rate of short between-shift intervals (<10 hr)
| Site | EDWR | RCWR | P |
|---|---|---|---|
| A | 0.2 ± 1.0 | 1.3 ± 2.7 | 0.03 |
| B | 1.6 ± 4.7 | 2.6 ± 4.2 | 0.45 |
| C | 0 ± 0 | 12.2 ± 8.4 | <0.0001 |
| D | 0 ± 0 | 14.3 ± 9.5 | <0.0001 |
| E | 0 ± 0 | 2.7 ± 4.6 | 0.01 |
| F | 0.6 ± 1.7 | 14.2 ± 5.9 | <0.0001 |
| Overall | 0.4 ± 2.0 | 8.2 ± 8.6 | <0.0001 |
Sleep
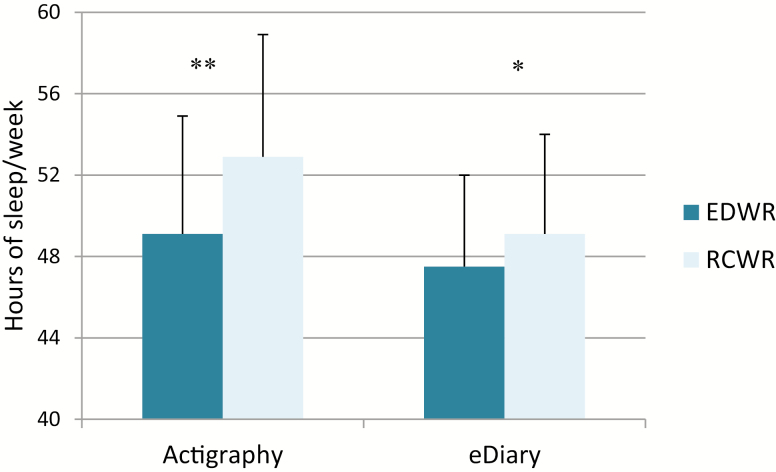
Resident physicians obtained significantly more sleep per week during the RCWR than during the EDWR as measured objectively with actigraphy (52.9 ± 6.0 versus 49.1 ± 5.8 hr, p < 0.0001) and subjectively via the eDiary (49.1 ± 4.9 versus 47.5 ± 4.5 hr, p = 0.008; Figure 4). The percentage of 24-hr intervals with less than 4 hr of actigraphically-measured sleep was 25% on the EDWR and 9% on the RCWR (p < 0.0001). Subjectively, resident physicians reported less than 4 hr of sleep in 23% and 13% of 24 hr intervals on the EDWR and RCWR, respectively (p < 0.0001). During the EDWR, 10% of work hours were preceded by 2 or fewer hours of sleep in the preceding 24 hr, when compared with 4% during the RCWR (p < 0.0001; Figure 3C). Although the percentage of 24 hr intervals with less than 7 hr of actigraphically measured sleep was similar on the EDWR and on the RCWR (43% and 46%, respectively, p = 0.11), resident physicians subjectively reported less than 7 hr of sleep in 47% and 52% of 24 hr intervals on the EDWR and RCWR, respectively (p < 0.0001).
Figure 4.
Weekly hours of sleep were significantly higher during the RCWR schedule as compared to the EDWR schedule (*p = 0.0005; **p < 0.0001).
During the EDWR, resident physicians reported napping on 31% of extended-duration shifts (307/978). On shifts of all lengths, resident physicians reported napping on 13% (316/2525) of shifts on the EDWR and on 5% (147/3032) of shifts during the RCWR (p < 0.0001). Of those shifts with any nap time, the mean duration of total nap time was similar during the EDWR and RCWR, 1.6 ± 1.2 and 1.6 ± 1.32 hr, respectively (p = 0.99).
Ratings of work experience
Two hundred ninety resident physicians completed an end-of-rotation survey for 355/370 (96%) rotations. Resident physicians completed 169 questionnaires following EDWR rotations and 186 questionnaires following RCWR. Twenty-four resident physicians completed an end-of-rotation survey in both conditions. There was no difference between the EDWR and RCWR rotations in how resident physicians rated the opportunity to obtain skills/knowledge (p = 0.50) or the negative effects on day-to-day activities (p = 0.27). Resident physicians rated the quality of their work experience more negatively in the RCWR. Following the EDWR, 11% of resident physicians rated the work experience as poor/fair compared with 30% of resident physicians following the RCWR (p = 0.0001). The educational experience on the rotation was also significantly different; 17% of EDWR resident physicians rated the educational experience as poor/fair compared with 38% following the RCWR (p = 0.0001; Table 3).
Table 3.
Work experience by schedule
| Characteristic | Overall† (n = 350) |
Schedule | ||
|---|---|---|---|---|
| EDWR | RCWR | P | ||
| (n = 167) | (n = 183) | |||
| To what extent did the training this year offer you the opportunity to obtain skills and knowledge? (range 0–60)* | 45.0 ± 8.3 | 45.2 ± 8.2 | 44.8 ± 8.3 | 0.50 |
| Day to day activities that were negatively affected (range 0–25)† | 8.6 ± 3.9 | 8.4 ± 4.1 | 8.8 ± 3.8 | 0.27 |
| Experience was what you were expecting from your residency (13–65)* | 39.0 ± 8.2 | 40.2 ± 8.3 | 37.8 ± 8.1 | 0.004 |
| Overall, work experience over the past month was: | 0.0001 | |||
| Fair/poor | 73 (21.0) | 19 (11.4) | 54 (29.8) | |
| Good/very good/excellent | 275 (79.0) | 148 (88.6) | 127 (70.2) | |
| Overall, educational experience over the past month was: | 0.0001 | |||
| Fair/poor | 98 (28.0) | 29 (17.4) | 69 (37.7) | |
| Good/very good/excellent | 252 (72.0) | 138 (82.6) | 114 (62.3) | |
Data shown as n(%) or mean ± SD, per rotation.
p Values from generalized mixed model adjusted for site and randomization order.
*Higher scores represent a more positive experience.
†Higher scores represent a more negative experience.
Association among work schedules, sleep, and ratings of work experience
There was no association between rate of short between-shift intervals and self-reported average weekly sleep duration (p = 0.14). There was a modest association between the rate of short between-shift intervals and the odds of reporting a fair or poor work experience or educational experience [for each 1% increase in the rate of short between-shift intervals OR (95% CI): 1.05 (1.01, 1.10) and 1.04 (1.01, 1.08), respectively].
Mean ICU patients per resident physician (IPRP) was 25% higher on the RCWR (8.5 ± 2.8) than on the EDWR (6.8 ±2.2; p < 0.001) [6]. For each additional intensive care unit patient per resident physician, the odds or reporting a fair or poor work or educational experience was significantly greater (2.05, 95% CI: 1.48, 2.85 and 1.79, 95% CI: 1.36, 2.37, respectively).
Discussion
Compared with the traditional EDWR, work hours were reduced 10% per week and objectively measured hours of sleep per week increased 8% when pediatric resident physicians worked a RCWR in the PICU. The RCWR called for shifts of 16 or fewer consecutive hours. In practice, 73% of work hours occurred within shifts of 16 or fewer hours, compared with only 38% of work hours occurring with the first 16 hr on duty during the EDWR.
Both the EDWR and RCWR were designed to be compliant with the 2011 Accreditation Council for Graduate Medical Education (ACGME) work-hour guidelines. Although the 2011 guidelines limited first-year resident physicians to shifts of 16 or fewer consecutive hours, PGY2 and higher resident physicians continued to be allowed to work up to 28 consecutive hours and 80 hr per week, averaged over 4 weeks [11]. In both the EDWR and RCWR conditions, average work hours were, respectively, 15% and 23% lower than the maximum allowed. Although the mean maximum hours, averaged over 4 weeks, were higher on the EDWR than on the RCWR, none of the rotations, in either condition, exceeded the ACGME 80-hr limit when averaged over 4 weeks. Resident physicians exceeded the 28 hr limit, in violation of ACGME guidelines, on 9% of shifts in the EDWR and 0.1% of shifts on the RCWR. Scheduled shift durations need to be substantially lower than the maximum limits, such as the 16-hr limit imposed on the RCWR, to minimize the probability that resident physicians will exceed ACGME limits by working longer hours than scheduled due to off-nominal situations, or to ensure the continued care of their patients.
During the EDWR, resident physicians were scheduled to 24- to 28-hr extended-duration overnight shifts every 4 to 5 days, with shorter shifts in between, and during the RCWR resident physicians were scheduled for shifts of 16 or fewer consecutive hours with periodic overnight shifts [4]. Extensive efforts were made to standardize certain features of the RCWR schedule (e.g. avoiding recurrent night shifts, ensuring sufficient days off each month). However, differences in site characteristics, operational necessity, and the manner in which site principal investigators and program directors chose to implement the schedule within the study guidelines resulted in differences in the way the six participating academic medical centers implemented the EDWR and RCWR. Five of the six sites worked a 4-day RCWR rotation, whereas one site worked a 5-day rotation [4]. The duration of the extended-duration shifts also varied between sites. Almost one-quarter of the EDWR shifts at one site were longer than 28 hr, whereas less than one-half percent of the EDWR extended-duration shifts were that length at another site. Although the heterogeneity in the implementation and results highlight the variations that can arise from attempting to employ “standard” schedules in operational healthcare environments and the need for greater enforcement of ACGME work hour limits, the inclusion of six sites from around the country, including a mix of larger and smaller academic centers, provides greater generalizability of our findings.
When schedule changes are implemented to reduce consecutive working hours, undesirable scheduling changes may occur, such as reducing the number of hours off between shifts and increasing workload [4]. When given 10 or fewer hours off between shifts, it is difficult to obtain the necessary 7–9 hr of sleep, given commute times and the other tasks of daily living. These short between-shift intervals occurred 28 times more frequently on the RCWR than on the EDWR, and varied from 1% to 14% of RCWR shifts at the six sites. Resident physicians in this study reported less satisfaction with their work and educational experience when rates of short between-shift intervals and workload were substantially increased (by 28-fold and 25%, respectively). Similarly, in nurses, between-shift intervals of <11 hr have been associated with significantly increased risk of sick leave [12], the occurrence of shift work disorder, pathological fatigue [13], higher stress [14], poor sleep quality, and reduced satisfaction with work hours [15].
Despite this shortcoming of the way some sites implemented the RCWR, weekly sleep duration increased nearly 4 hr overall in the RCWR when compared with the EDWR. The increase of actigraphically estimated sleep duration by 3.8 hr per week in this trial importantly equates to just over 30 min more sleep per night and was comparable to the 3.5 hr increase observed with reduction and redistribution of scheduled work hours of UK physicians in compliance with the European Working Time Directive [16]. The improvement in sleep duration was smaller, however, than the 5.8 hr of increased weekly sleep duration associated with the elimination of extended-duration shifts in a single-site clinical trial [17], in which between-shift intervals were scheduled to be greater than 10 to 14 hr, depending on the duration of the prior work shift, as recommended by the National Academy of Medicine [18]. Together, these findings indicate the efficacy of reduced consecutive working hours in increasing physician sleep durations, but also highlight the potential additional benefits that can be obtained by protecting sufficient time for sleep between shifts.
Two consensus groups recently determined that adults require 7–9 hr of sleep each day [19, 20]. On average, resident physicians in both the EDWR and RCWR met this requirement, when averaged over the entire rotation; however, approximately one-third of the 24 hr intervals in both rotation conditions had less than 6 hr of sleep. Sleep deficiency of this magnitude is associated with significant cumulative performance deficits and adverse health outcomes [21, 22].
In addition to the increase in weekly sleep duration during the RCWR, the resident physicians were more rested while caring for patients. The percentage of work hours preceded by two or fewer hours of sleep in the last 24 hr was reduced by 60% during the RCWR. Reducing the frequency of acute sleep deprivation, which has been associated with attentional failures and increased surgical complications [3, 23], is a strength of the RCWR. Resident physicians on the RCWR had significantly improved performance on the psychomotor vigilance task (PVT), compared with the EDWR, with fewer PVT attentional failures and faster mean reaction time [5]. They also rated themselves as significantly more alert on the RCWR [5]. In addition to the type of scheduled worked, physician workload is also an important component to consider [6]. Simultaneously increasing workload while eliminating resident-physicians’ extended work shifts increased serious medical errors [6]. Further research is needed to identify physician workloads necessary for patient safety.
Despite the increase in sleep and reduction in hours of weekly work, resident physicians rated quality of their educational experience and the quality of their work experience lower on the RCWR. The resident physicians, however, rated their opportunity to obtain skills/knowledge similarly between conditions. This finding suggests that the training opportunities for resident physicians remained similar; the decrease in work hours did not directly correspond to a decrease in time spent in patient care. Although work hours decreased 10% in the RCWR condition, the reported patient care hours decreased by only 6%, while workload increased by 25% [6]. Although statistical evaluation revealed only a weak association between short between-shift intervals and ratings of fair or poor work or education experience, more research is necessary to further understand what specific aspects of a resident’s experience, including actual learning, may be altered by schedules with shorter shift durations. Additionally resident-physician workload should be further explored as a factor influencing the resident work and educational experience.
This multicenter clinical trial expanded on our previous single-site trial of the elimination of extended-duration shifts in resident physicians [17]. Due to the operational conditions across six different hospitals, the EDWR and RCWR were not implemented identically at all sites. Nevertheless, the RCWR was shown to reduce work hours and increase sleep. Further research needs to be accomplished to optimize the shift duration and interval between shifts to allow for sufficient sleep prior to all work shifts.
Supplementary Material
Acknowledgments
We thank the Data Safety and Monitoring Board members for their oversight: Donald L. Bliwise, Barry Markovitz, Eva Petkova, Wasima N. Rida, and Ramesh C. Sachdeva. We thank the National Heart, Lung, and Blood Institute for their support: Carol J. Blaisdell, Peyvand Ghofrani, Lora A. Reineck, Robert A. Smith, Michael Twery, Gail G. Weinmann, and Colin O. Wu. Thank you also to the resident physicians, attending physicians, nurses, and clinical pharmacists of the participating Pediatric Intensive Care Units for their ongoing support.
Thank you to the following ROSTERS team members:
Clinical Coordinating Center: Justin D. Buie and Joshua T. Stephens. Data Coordinating Center: Lynn Harvey, Vicki Li, and Eric Vittinghoff. Colorado: Bradley Brainard, Tristan Bakke Dear, Tondeleyo Gonzalez, Jonathan D. Haywood, Heather Hoch, Brian M. Jackson, Ayoub Lahlou, Kathryn J. Yucha, Karen Meyer, Tolulope Oyewumi, Kimberly Ralston, Nabeel Sawaged, Beth E. Smith, and Vanitha K. Varre. Iowa: Safa Abukhalil, Ihab Ahmed, Safa Abdelwahid Mohamed Ahmed, Shilpa C. Balikai, Maria Ana C. Canaya-Voskov, Janice M. Jeter, Sameer Kamath, Crystal Tuley, Jessica G. Moreland, Vani C. Movva, Geoffrey Ounda Obel, Angie Platt, Thomas D. Scholz, Ruthann Schrock, Amy Stier, Alexandra Paige Davis Volk, and Jin Zhou. Massachusetts: Oluwafunmilola Alabi, Joseph Asemota, Abimbola Chris-Olaiya, Virginia Leon, Alexandra Male, Siyu Ma, Adeolu O. Oladunjoye, Olubunmi O. Oladunjoye, MBBS, Saki Onda, Kimberly Ralston, Bhavya Atul Shah, Lisa Tse, and Sandra Wooldridge. Ohio: Juanita Dudley, Tatiana Elson, Narinderpal Kaur, Samuel Lee, Najima Mwase, Narissa Puran, Ndidi Unaka, MD, Andrew M. Warner, Robin Widing, and Hector R. Wong. Virginia: Indu Aggarwal, Fatimah Begom, Kateryna Bilanovych, Ashley C. Eason, Nasir Farhat, Nicole M. Frank, Robin L. Kelly, Evan B. Kudron, Abigail V. W. Kumral, Brock D. Libby, Jules Mukunde Katotola, Trevor Pollock, Justin Rizer, Isaac A. Shields, Terrell D. Smith, Carolyn Spilman, Albert T. Tang, Linlin Wang, Weonpo Yarl, Hong Zhu, and Jenna V. Zschaebitz. Washington: Mouammar M. Abouagila, Ibrahim K. Abukenda, Canan Akture, Jennifer Jane Gile, Carol Mendivil, Anas L. Najjar, Gowri Rajendran, Shahar Robinzon, Erin M. Sullivan, and Nastassya West. Other: David Gozal, Leila Kheirandish-Gozal, and Sharon M. Unti.
ROSTERS Study Group Authors
Clinical Coordinating Center: Laura K. Barger, Charles A. Czeisler, Melissa A. St. Hilaire, Elizabeth B. Klerman, Christopher P. Landrigan,* Steven W. Lockley, Conor S. O’Brien, Andrew J.K. Phillips, Salim Qadri, Shadab A. Rahman, Jason P. Sullivan, and Natalie C. Viyaran. Data Coordinating Center: Terri Blackwell, Dana R. Kriesel, and Katie L. Stone. Colorado: Angela S. Czaja, Ann C. Halbower, Adam Rosenberg, and Kenneth P. Wright Jr. Iowa: Gretchen Cress, Gwen E. Erkonen, and Jeffrey L. Segar. Massachusetts: Lindsey B. Armstrong, Ben D. Albert, Erin A. Bressler, Dennis Daniel, Christopher P. Landrigan,* Bradley S. Podd, Amy L. Sanderson, Theodore C. Sectish, Patrick A. Upchurch, and Traci A. Wolbrink. Ohio: Sue E. Poynter. Virginia: Jeannean Carver and Pearl L. Yu. Washington: Maneesh Batra, Reid W.D. Farris, Horacio O. de la Iglesia, John K. McGuire, and Michael V. Vitiello. Other: Phyllis C. Zee.
*Dr. Christopher Landrigan fulfilled two roles: ROSTERS Study Multiple Principal Investigator (with Dr. Charles Czeisler) of the Clinical Coordinating Center and Site Principal Investigator at Boston Children’s Hospital.
Contributor Information
ROSTERS Study Group:
Laura K Barger, Charles A Czeisler, Melissa A St Hilaire, Elizabeth B Klerman, Christopher P Landrigan, Steven W Lockley, Conor S O’Brien, Andrew J K Phillips, Salim Qadri, Shadab A Rahman, Jason P Sullivan, Natalie C Viyaran, Terri Blackwell, Dana R Kriesel, Katie L Stone, Angela S Czaja, Ann C Halbower, Adam Rosenberg, Kenneth P Wright, Jr, Gretchen Cress, Gwen E Erkonen, Jeffrey L Segar, Lindsey B Armstrong, Ben D Albert, Erin A Bressler, Dennis Daniel, Christopher P Landrigan, Bradley S Podd, Amy L Sanderson, Theodore C Sectish, Patrick A Upchurch, Traci A Wolbrink, Sue E Poynter, Jeannean Carver, Pearl L Yu, Maneesh Batra, Reid W D Farris, Horacio O de la Iglesia, John K McGuire, Michael V Vitiello, and Phyllis C Zee
Funding
ROSTERS is supported by National Heart, Lung, and Blood Institute (U01-HL-111478 and U01-HL-111691). Dr. Klerman was supported by NIH K24-HL-105664, R01-HL-128538, R01-HL-114088, R01-GM-105018, R21-HD-086392, P01-AG-009975 and NSBRI HFP-02802, HFP-0006, and HFP-04201. Drs. Barger, Lockley, and Czeisler were supported in part by funding from the National Institute of Occupational Safety and Health R01-OH-010300. The funders were not involved in the design and conduct of the study, the collection, preparation, or interpretation of the data, or the preparation or approval of the manuscript.
Conflict of interest statement. Dr. Barger is on the scientific advisory board for CurAegis Technologies. She has received consulting fees from University of Pittsburgh, Sygma, Insight and Puget Sound Pilots. Mr. Sullivan reports no conflicts. Ms. Blackwell reports grants from NIH during the conduct of the study; grants from Merck Sharp & Dohme Corp, outside the submitted work. Mr. O’Brien reports no conflicts. Dr. St. Hilaire reports personal fees from The MathWorks, Inc., outside the submitted work, and has received honoraria as an invited speaker from the Providence Sleep Research Interest Group, honoraria and travel funds for grant review activities from the NIH, and travel funds from the American Academy of Neurology. Dr. Rahman holds patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in individuals exposed to light at night. Dr. Rahman owns equity in Melcort Inc. and has provided paid consulting services to Sultan & Knight Limited, Bambu Vault LLC. Dr. Rahman has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., Seoul Semiconductor Co. LTD. Dr. Phillips is an investigator on a project supported by the CRC for Alertness, Safety, and Productivity at Monash University. He also holds a patent (US20150080756A1) for estimating arousal states from ambulatory recordings using sleep/wake models. Mr. Qadri reports no conflicts. Dr. Wright Jr reports grants from National Institutes of Health, during the conduct of the study; personal fees from Circadian Therapeutics, LTD, grants, personal fees, non-financial support and other from CurAegis Technologies, personal fees from Kellogg’s, non-financial support from Somalogic, Inc., American Academy of Sleep Medicine, American College of Chest Physicians, personal fees from American College of Sports Medicine, personal fees from American Diabetes Association, personal fees from Associated Professional Sleep Societies, and from Obesity Medicine Association, and grants from Philips Inc. outside the submitted work. Dr. Segar reports no conflicts. Dr. McGuire reports no conflicts. Dr. Vitiello reports no conflicts. Dr. de la Iglesia reports no conflicts. Dr. Poynter reports no conflicts. Dr. Yu reports no conflicts. Dr. Zee reports grants from National Institutes of Health, during the conduct of the study; personal fees from Merck, grants and personal fees from Eisai, grants and personal fees from Philips, personal fees from Sanofi, grants from Jazz, grants from Technogel, grants and personal fees from Harmony Biosciences, grants from Apnimed, grants from X—a Division of Alphabet, Inc., other from Teva, outside the submitted work; In addition, Dr. Zee has a patent U.S. Serial Nos: 62/038,700 & PCT/US2015/045273 pending, and a patent U.S. Serial No: 62/515,361 pending. Dr. Sanderson no conflicts. Dr. Halbower reports she has a patent In-Ear Sensing Systems and Methods for Biological Signal Monitoring pending. Dr. Lockley reports commercial interests from the last 3 years (2015–2018), unrelated to the work, that are listed below. Dr. Lockley has received consulting fees from the Atlanta Falcons, Atlanta Hawks, Delos Living LLC, Noble Insights, OpTerra Energy Services Inc., Pegasus Capital Advisors LP, Serrado Capital, Slingshot Insights, and Team C Racing. He has current consulting contracts with Akili Interactive, Apex 2100 Ltd, BHP Billiton, Consumer Sleep Solutions, Headwaters Inc., Hintsa Performance AG, Light Cognitive, Lighting Science Group Corporation, Mental Workout, McCullough Hill Leary PS, Paul, Weiss, Rifkind, Wharton & Garrison LLP, PlanLED, Six Senses, Stantec and Wyle Integrated Science and Engineering. Dr. Lockley has received unrestricted equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and F.LUX Software LLC; has equity in iSLEEP, Pty; royalties from Oxford University Press; honoraria plus travel, accommodation and/or meals for invited seminars, conference presentations or teaching from BHP Billiton, Estee Lauder, Informa Exhibitions (USGBC), and Teague; travel, accommodation and/or meals only (no honoraria) for invited seminars, conference presentations or teaching from IES, Lightfair, USGBC, DIN and SLTBR. Dr. Lockley has completed investigator-initiated research grants from Biological Illumination LLC and has an ongoing investigator-initiated grant from F. Lux Software LLC; he is a Program Leader for the non-profit CRC for Alertness, Safety and Productivity, Australia, through an adjunct faculty position at Monash University and unpaid Member of the Scientific Advisory Board for the non-profit Midwest Lighting Institute. Dr. Lockley holds a process patent for “Systems and methods for determining and/or controlling sleep quality,” which is assigned to the Brigham and Women’s Hospital per Hospital policy. Dr. Lockley has also served as a paid expert in legal proceedings related to light and health. Dr. Landrigan reports grants from Patient-Centered Outcomes Research Institute, during the conduct of the study; personal consulting fees and other equity from I-PASS Patient Safety Institute, personal consulting fees from Children’s Hospital Association, personal fees from Virgin Pulse, outside the submitted work. Dr. Stone reports grants from Merck & Co., outside the submitted work. Dr. Czeisler reports grants from Cephalon Inc., Jazz Pharmaceuticals Plc. Inc., National Football League Charities, Optum, Philips Respironics, Inc. Regeneron Pharmaceuticals, ResMed Foundation, San Francisco Bar Pilots, Sanofi S.A., Sanofi-Aventis, Inc, Schneider Inc., Sepracor, Inc, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Sysco Corp., Takeda Pharmaceuticals Co. Ltd. Teva Pharmaceuticals Industries, Ltd., and Wake Up Narcolepsy; and consulting fees from Bose Corporation, Boston Red Sox, Columbia River Bar Pilots, Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics Inc. Teva Pharma Australia, Vanda Pharmaceuticals, Inc., Washington State Board of Pilotage Commissioners, lecture fees from Ganésco Inc. and Zurich Insurance Company, Ltd.; and fees for serving as a member of an advisory board for Institute of Digital Media and Child Development and the Klarman Family Foundation. In addition, Dr. Czeisler holds a number of process patents in the field of sleep/circadian rhythms (e.g. photic resetting of the human circadian pacemaker) and holds an equity interest in Vanda Pharmaceuticals, Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon Inc. Since 1985, Dr. Czeisler has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Casper Sleep Inc., Comair/Delta Airlines, Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, Purdue Pharma, LP, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC, and United Parcel Service (UPS). Dr. Czeisler received royalties from the New England Journal of Medicine; McGraw Hill; Houghton Mifflin Harcourt/Penguin; and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1. Luckhaupt SE, et al. . The prevalence of short sleep duration by industry and occupation in the National health interview survey. Sleep. 2010;33(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shockey TM, et al. . Short sleep duration by occupation group - 29 states, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66(8):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lockley SW, et al. ; Harvard Work Hours, Health and Safety Group Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351(18):1829–1837. [DOI] [PubMed] [Google Scholar]
- 4. Blackwell T, et al. . ROSTERS Study Group. Design and recruitment of the randomized order safety trial evaluating resident-physician schedules (ROSTERS) study. Contemp Clin Trials. 2019;80:22–33. [DOI] [PMC free article] [PubMed]
- 5. Rahman SA, et al. . Attentional failures are correlated with serious medical errors in resident physicians. SLEEP. 42(Abstract supplement):A390.
- 6. Landrigan CP, et al. . Schedule re-design and patient safety: the randomized order safety trial evaluating resident-physician schedules (ROSTERS). SLEEP. 42(Abstract supplement):A400.
- 7. Action-W User’s Guide, Version 2.0 [computer program]. Ardsley, NY: Ambulatory Monitoring, Inc.. [Google Scholar]
- 8. Motionlogger® User’s Guide: Act Millenium [computer program]. Ardsley, NY: Ambulatory Monitoring, Inc. [Google Scholar]
- 9. Jean-Louis G, et al. . Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105(2):185–191. [DOI] [PubMed] [Google Scholar]
- 10. Netzer NC, et al. . Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. [DOI] [PubMed] [Google Scholar]
- 11. Accreditation Council for Graduate Medical Education Common Program Requirements 2011. https://www.acgme.org/Portals/0/PDFs/Common_Program_Requirements_07012011[2].pdf. Accessed April 10, 2018.
- 12. Vedaa Ø, et al. . Short rest between shift intervals increases the risk of sick leave: a prospective registry study. Occup Environ Med. 2017;74(7):496–501. [DOI] [PubMed] [Google Scholar]
- 13. Flo E, et al. . Short rest periods between work shifts predict sleep and health problems in nurses at 1-year follow-up. Occup Environ Med. 2014;71(8):555–561. [DOI] [PubMed] [Google Scholar]
- 14. Vedaa Ø, et al. . Sleep detriments associated with quick returns in rotating shift work: a diary study. J Occup Environ Med. 2017;59(6):522–527. [DOI] [PubMed] [Google Scholar]
- 15. Dahlgren A, et al. . Quick returns and night work as predictors of sleep quality, fatigue, work-family balance and satisfaction with work hours. Chronobiol Int. 2016;33(6):759–767. [DOI] [PubMed] [Google Scholar]
- 16. Cappuccio FP, et al. ; Warwick EWTD Working Group Implementing a 48 h EWTD-compliant rota for junior doctors in the UK does not compromise patients’ safety: assessor-blind pilot comparison. QJM. 2009;102(4): 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Landrigan CP, et al. . Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–1848. [DOI] [PubMed] [Google Scholar]
- 18. Ulmer C, Wolman DM, Johns MME, editors. Institute of Medicine Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 19. Hirshkowitz M, et al. . National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 20. Watson NF, et al. . Joint consensus statement of the American academy of sleep medicine and sleep research society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. 2015;11(8):931–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. [DOI] [PubMed] [Google Scholar]
- 22. St Hilaire MA, et al. . Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rothschild JM, et al. . Risks of complications by attending physicians after performing nighttime procedures. JAMA. 2009;302(14):1565–1572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.