Abstract
Background
The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a prognostic factor in patients who have some types of malignant tumors. The aim of this study was to investigate the prognostic significance of the HALP score in patients with small cell lung cancer (SCLC) before first-line treatment with etoposide.
Material/Methods
A retrospective study included 178 patients with SCLC who received first-line chemotherapy with etoposide between September 2015 and May 2019. The baseline clinical characteristics and blood parameters were recorded. Univariate and multivariate analysis and Kaplan-Meier plots were used to identify the factors associated with progression-free survival (PFS).
Results
The optimal cut-off values of the HALP score was determined by X-tile software to be 25.8. Univariate and multivariate analysis showed that in 178 patients, the HALP score, body mass index (BMI), and serum albumin levels had no prognostic significance. In the patient age group <65 years, a BMI ≥24 kg/m2 was an independent prognostic factor (HR, 1.943; 95% CI, 1.251–3.018) (P=0.003). In the patient age group ≥65 years, a HALP score >25.8 was an independent positive prognostic factor for outcome following first-line treatment with etoposide (HR, 0.483; 95% CI, 0.270–0.865) (P=0.014).
Conclusions
In patients <65 years with SCLC who underwent first-line treatment with etoposide, a BMI ≥24 kg/m2 an independent prognostic factor, and in patients ≥65 years, a HALP score >25.8 was an independent predictor of improved outcome, associated with increased PFS.
MeSH Keywords: Body Mass Index, Nutrition Assessment, Small Cell Lung Carcinoma, Treatment Outcome
Background
Non-small cell lung cancer (NSCLC) is the most common primary malignancy of the lung and comprises adenocarcinoma and squamous cell carcinoma. Small cell lung cancer (SCLC) is less common, representing between 10–15% of all primary lung cancers, but is a rapidly growing malignancy with a poor prognosis [1]. Rapid tumor growth can result in systemic changes that demonstrate nutritional changes [2]. Recent studies on nutritional oncology have shown that cancer can lead to malnutrition through several metabolic pathways, and chemotherapy also initiates proteolysis and lipolysis at the tissue level [3]. In the field of cancer treatment, the nutritional status of patients and the behavioral characteristics of the tumor have received increasing attention [4].
Measurements of body mass index (BMI) and serum albumin as classic indicators of nutritional status and have been previously studied as potential indicators of prognosis in patients with cancer [5,6]. Recently, the hemoglobin, albumin, lymphocyte, and platelet (HALP) score has been described as a prognostic factor in patients with several types of malignant tumors, including in gastrointestinal cancer [7], and genitourinary cancer [8]. There have been several studies that have investigated the relationship between the prognostic nutritional index (PNI) and patient outcome in SCLC [9,10]. However, the role of the HALP score in SCLC remains to be investigated.
Therefore, this study aimed to investigate the prognostic significance of the HALP score in patients with SCLC before undergoing etoposide-based first-line treatment in terms of progression-free survival (PFS). This study also aimed to compare the prognostic value of the HALP score, the BMI, and albumin levels in two patient age groups, including patients <65 years and ≥65 years.
Material and Methods
Ethical approval and informed consent
This study was approved by the Ethics Committee of Anhui Provincial Hospital. All patients had a histologically confirmed diagnosis of small cell lung cancer (SCLC) and were from Anhui Provincial Hospital. All study participants were informed of the study and provided informed consent.
Patients
The study included patients who were diagnosed with SCLC from September 2015 to May 2019 at Anhui Provincial Hospital. The patients were not treated surgically and were retrospectively reviewed. Initially, 542 patients, were identified from which 178 patients met the study inclusion criteria. Patients were included in the study who had a histologically confirmed diagnosis of SCLC that did not include combined tumor types, imaging was performed to stage the tumors, and patients received first-line chemotherapy with etoposide combined with platinum, and had treatment progression before May 2019. Patients were excluded from the study if they had hematological disease, diseases of the immune system diseases, hepatitis virus infections, or long-term glucocorticoid therapy.
Clinical data
Clinical data collected including age, gender, body mass index (BMI), tumor stage, first-line chemotherapy treatment regimens, first evaluation results, type of radiotherapy, and tumor progression. Patients were classified into the following three groups according to their body mass index (BMI) values: underweight (BMI <18.5 kg/m2); normal weight (BMI 18.5–24 kg/m2); and overweight (BMI ≥24 kg/m2). Hematologic parameters, including serum albumin, hemoglobin, and lymphocytes and platelets were collected within a week before the first dose of chemotherapy. According to the cut-off value of albumin, patients were divided into the low albumin group (≤40 g/L) and the high albumin group (>40 g/L). The hemoglobin, albumin, lymphocyte, and platelet (HALP) score was calculated according to the following formula: hemoglobin (g/L)×albumin (g/L)×lymphocytes (/L)/platelets (/L).
The progression-free survival (PFS), which was the main endpoint, was defined as the time from randomization to disease progression, or death, during first-line treatment.
Laboratory tests
Patients were at a resting state during the early morning when blood samples were collected. The blood samples were tested using an XE-5000 automated fluorescence flow cytometer (Sysmex, Kobe, Japan) and a Beckman AU5800 (Beckman Coulter, Brea, CA, USA) automatic blood analyzer. All samples were tested within two hours of blood sampling.
Statistical analysis
Data analysis was performed using SPSS version 19.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad Prism version 6.0 software (GraphPad Software Inc., San Diego, CA, USA). The optimal cut-off values of the HALP score was determined using X-tile software version 3.6.1 (Yale University, New Haven CT, USA) [11]. The chi-squared (χ2) test was used to compare rates. The two-tailed Student’s t-test and analysis of variance (ANOVA) were used to compare data with a normal distribution. The Kaplan-Meier method was used for survival analysis, and the log-rank test was used for statistical comparisons. Cox regression analysis was used for univariate and multivariate analysis. A P-value <0.05 was considered to be statistically significant.
Results
Clinical characteristics
The clinical characteristics of 178 patients were analyzed and showed that the mean age was 61.24±9.27 years (median age, 62 years). There were 107 patients who were <65 years old and 71 patients who were ≥65 years old. All patients received first-line treatment with etoposide-based chemotherapy (Table 1).
Table 1.
Clinical characteristics of 178 patients with small cell lung cancer (SCLC).
| Clinical characteristics | Cases (n) | % |
|---|---|---|
| Gender | ||
| Female | 36 | 20.22 |
| Male | 142 | 79.78 |
| Age (years) | ||
| <65 | 107 | 60.11 |
| ≥65 | 71 | 39.89 |
| X±s | 61.24±9.27 | |
| Body mass index (kg/m2) | ||
| BMI <18.5 | 12 | 6.74 |
| BMI 18.5–24 | 101 | 56.74 |
| BMI ≥24 | 65 | 36.52 |
| Stage | ||
| Limited disease (LD) | 50 | 28.09 |
| Extensive disease (ED) | 128 | 71.91 |
| First-line chemotherapeutic regimen | ||
| Etoposide + luoplatinum | 107 | 60.11 |
| Etoposide + cisplatin or carboplatin | 71 | 39.89 |
| Radiotherapy in first-line therapy | ||
| Yes | 70 | 39.33 |
| No | 108 | 60.67 |
| First evaluation results | ||
| CR | 5 | 2.81 |
| PR | 110 | 61.80 |
| SD | 32 | 17.98 |
| PD | 31 | 17.41 |
| Progress-free survival (months) | ||
| <6.0 | 87 | 48.88 |
| ≥6.0 | 91 | 51.12 |
| X±s | 6.56±3.53 | |
| Median (IQR) | 6.05 (3.69–9.01) | |
| Reasons for the progress of first-line treatment | ||
| Lesions increase | 101 | 56.74 |
| Distant metastasis | 77 | 43.26 |
| Albumin (g/L) | ||
| ≤40 | 89 | 50.00 |
| >40 | 89 | 50.00 |
CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease; IQR – interquartile range.
The cut-off value for the hemoglobin, albumin, lymphocyte, and platelet (HALP) score
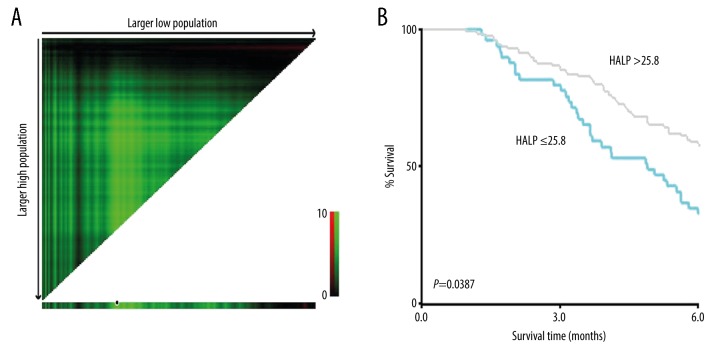
The optimal cut-off value for the HALP score was analyzed and calculated as 25.8 using X-tile software (survival time: cut-off at PFS=6 months). Therefore, patients were divided into low HALP group (HALP score ≤25.8) (n=48) and the high HALP group (HALP score >25.8) (n=130) (Figure 1).
Figure 1.
(A, B) Cut-off values for the hemoglobin, albumin, lymphocyte, and platelet (HALP) score determined by X-tile software.
The association between the HALP score and clinical characteristics
The chi-squared test demonstrated the difference between the pretreatment HALP score and clinical characteristics. The HALP score showed no differences regarding gender, age, body mass index (BMI), tumor stage, chemotherapy regimen, and results of the first evaluation groups. However, patients with a high HALP score had also received radiotherapy, had high albumin levels, and a significantly increased progression-free survival (PFS) of ≥6 months. The results also showed that patients with an increased HALP score were more likely to have tumor metastasis (Table 2).
Table 2.
Association between clinical features and the hemoglobin, albumin, lymphocyte, and platelet (HALP) score in 178 patients with small cell lung cancer (SCLC).
| Clinical features | The HALP score | ||
|---|---|---|---|
| ≤25.8 | >25.8 | P-value | |
| Gender | |||
| Female | 13 | 23 | 0.166 |
| Male | 35 | 107 | |
| Age (years) | |||
| <65 | 32 | 75 | 0.278 |
| ≥65 | 16 | 55 | |
| Body mass index (kg/m2) | |||
| BMI <18.5 | 5 | 7 | 0.110 |
| BMI 18.5–24 | 31 | 70 | |
| BMI ≥24 | 12 | 53 | |
| Stage | |||
| Limited disease (LD) | 9 | 41 | 0.092 |
| Extensive disease (ED) | 39 | 89 | |
| Chemotherapeutic regimen | |||
| Etoposide + luoplatinum | 30 | 77 | 0.693 |
| Etoposide + cisplatin or carboplatin | 18 | 53 | |
| Radiotherapy | |||
| Yes | 11 | 59 | 0.006 |
| No | 37 | 71 | |
| Results of the first evaluation | |||
| ORR (CR+PR) | 28 | 87 | 0.288 |
| SD+PD | 20 | 43 | |
| PFS (months) | |||
| <6 | 32 | 55 | 0.004 |
| ≥6 | 16 | 75 | |
| Reasons for the progress | |||
| Lesions increase | 34 | 67 | 0.021 |
| Distant metastasis | 14 | 63 | |
| Albumin (g/L) | |||
| ≤40 | 40 | 49 | <0.001 |
| >40 | 8 | 81 | |
PFS – progression-free survival; ORR – objective response rate; CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease.
Kaplan-Meier analysis in nutritional parameters
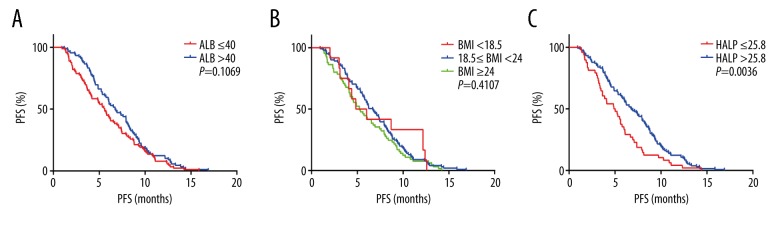
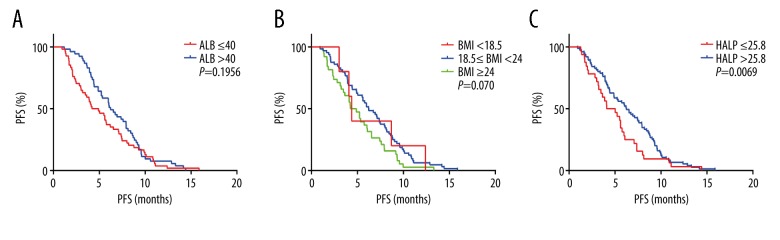
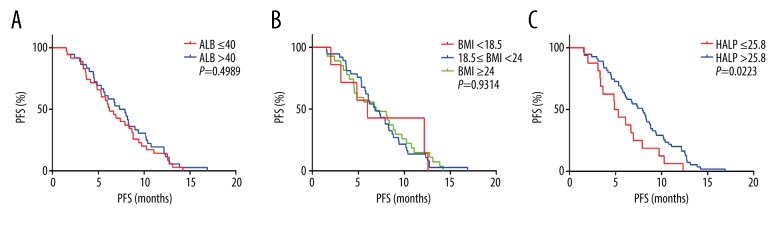
In all 178 patients, Kaplan-Meier analysis showed that the PFS of the high HLAP score group was significantly longer than that of the low HLAP score group (P=0.0036). The PFS of the high albumin and high BMI groups showed no significant differences (Figure 2). Because age was an important factor that affected nutritional status, all 178 patients were divided into two age groups, <65 years (n=107) and ≥65 years (n=71). The PFS of the patient group with a high HLAP score group was longer than the low HLAP score group regardless of age, which was similar in the 107 patients <65 years (P=0.0069) and in the 71 patients ≥65 years (P=0.0223). However, the high albumin and BMI groups showed no significant difference in the PFS in the different age groups (Figures 3, 4).
Figure 2.
Kaplan-Meier curves for progression-free survival (PFS) in all 178 patients with small cell lung cancer (SCLC) according to the albumin (A), body mass index (B) and the hemoglobin, albumin, lymphocyte, and platelet (HALP) score (C).
Figure 3.
Kaplan-Meier curves for progression-free survival (PFS) in 107 patients with SCLC (age, <65 years) according to the albumin (A), body mass index (B) and the hemoglobin, albumin, lymphocyte, and platelet (HALP) score (C).
Figure 4.
Kaplan-Meier curves for progression-free survival (PFS) in 71 patients with small cell lung cancer (SCLC) (age, ≥65 years) according to the albumin (A), body mass index (B) and the hemoglobin, albumin, lymphocyte, and platelet (HALP) score (C).
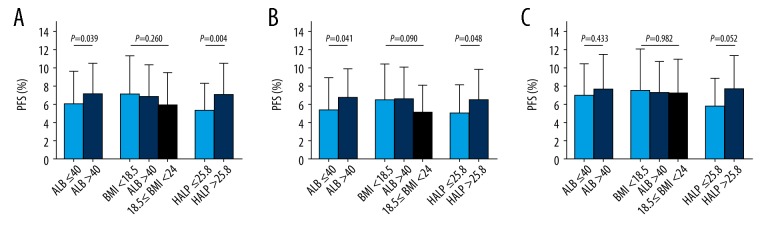
The mean PFS in patients with different nutritional parameters
The two-tailed Student’s t-test and analysis of variance (ANOVA) compared the mean PFS between the patient groups according to the nutritional parameter groups. In all 178 patients, the PFS showed no statistical difference in the three BMI groups. However, in the low albumin group the PFS was significantly shorter compared with the high albumin group at 6.01±3.58 months and 7.10±3.42 months, respectively (P=0.039). In the groups with the low HALP score, the PFS was significantly shorter compared with the high HALP score group, 5.30±3.08 months and 7.02±3.59 months, respectively (P=0.004) and in the 107 patients <65 years, the results were similar to the 71 patients ≥65 years and the high albumin group (P=0.041) and high HALP score group (P=0.048) showed increased PFS. In the 71 patients ≥65 years, the PFS showed no significant difference between the different nutritional parameter groups (Figure 5).
Figure 5.
Comparison of the mean progression-free survival (PFS) between the different parameters. Comparison of the mean progression-free survival (PFS) in 178 patients with small cell lung cancer (SCLC) (A). Comparison of the mean PFS in 107 patients with SCLC age <65 years (B). Comparison of the mean PFS in 71 patients with SCLC age ≥65 years (C). Two-tailed Student’s t-test and analysis of variance (ANOVA) for normal distribution were used to compare the data.
The univariate and multivariate analysis of nutritional parameters
In all 178 patients, univariate analysis identified age, tumor stage, radiotherapy, and the HALP score to be significantly associated with PFS. Multivariate analysis showed that age ≥65 years (HR, 0.725; 95% CI, 0.532–0.986) (P=0.041), and treatment with radiotherapy (HR, 0.510; 95% CI, 0.370–0.704) (P<0.001) were independent prognostic factors, predicting longer PFS. Metastatic disease (HR, 1.487; 95% CI, 1.055–2.095) (P=0.024) was an independent risk factor (Table 3). In the 107 patients <65 years, multivariate analysis showed that a BMI ≥24 kg/m2 (compared with BMI 18.5–24 kg/m2) was an independent risk factor (HR, 1.943; 95% CI, 1.251–3.018) (P=0.003) (Table 4). However, in the 71 patients ≥65 years, multivariate analysis showed that a HALP score >25.8 was an independent protective factor that increased PFS in patients with SCLC undergoing etoposide-based first-line treatment (HR, 0.483; 95% CI, 0.270–0.865) (P=0.014) (Table 5).
Table 3.
Univariate and multivariate analysis of progression-free survival (PFS) in 178 patients with small cell lung cancer (SCLC).
| Variable | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P-value | HR (95% CI) | P-value | |
| Gender | |||
| Female | 0.527 | – | – |
| Male | |||
| Age (years) | |||
| <65 | 0.041 | Reference | 0.041 |
| ≥65 | 0.725 (0.532–0.986) | ||
| Stage | |||
| Limited disease (LD) | 0.001 | Reference | 0.024 |
| Extensive disease (ED) | 1.487 (1.055–2.095) | ||
| Chemotherapy regimen | |||
| Etoposide + cisplatin or carboplatin | 0.341 | – | – |
| Etoposide + luoplatinum | |||
| Radiotherapy | |||
| No | <0.001 | Reference | <0.001 |
| Yes | 0.510 (0.370–0.704) | ||
| Body mass index (BMI) (kg/m2) | |||
| BMI 18.5–24 | Reference | – | – |
| BMI <18.5 | 0.851 | ||
| BMI ≥24 | 0.212 | ||
| Albumin (g/L) | |||
| ≤40 | 0.109 | Reference | 0.587 |
| >40 | 0.917 (0.672–1.252) | ||
| The HALP score | |||
| ≤25.8 | 0.004 | Reference | 0.168 |
| >25.8 | 0.777 (0.544–1.112) | ||
HR – hazard ratio; CI – confidence interval; HALP – hemoglobin, albumin, lymphocyte, and platelet.
Table 4.
Univariate and multivariate analysis of progression-free survival (PFS) in 107 patients (age, <65 years) with small cell lung cancer (SCLC).
| Variable | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P-value | HR (95% CI) | P-value | |
| Gender | |||
| Female | 0.842 | – | – |
| Male | |||
| Stage | |||
| Limited disease (LD) | <0.001 | Reference | 0.023 |
| Extensive disease (ED) | 1.809 (1.086–3.012) | ||
| Chemotherapy regimen | |||
| Etoposide + cisplatin or carboplatin | 0.785 | – | – |
| Etoposide + luoplatinum | |||
| Radiotherapy | |||
| No | <0.001 | Reference | 0.004 |
| Yes | 0.513 (0.326–0.806) | ||
| Body mass index (BMI) (kg/m2) | |||
| BMI 18.5–24 | Reference | Reference | |
| BMI <18.5 | 0.937 | 0.712 (0.276–1.840) | 0.483 |
| BMI ≥24 | 0.024 | 1.943 (1.251–3.018) | 0.003 |
| Albumin (g/L) | |||
| ≤40 | 0.198 | Reference | 0.621 |
| >40 | 0.901 (0.595–1.363) | ||
| The HALP score | |||
| ≤25.8 | 0.071 | Reference | 0.455 |
| >25.8 | 0.841 (0.533–1.325) | ||
HR – hazard ratio; CI – confidence interval; HALP – hemoglobin, albumin, lymphocyte, and platelet.
Table 5.
Univariate and multivariate analysis of progression-free survival (PFS) in 71 patients (age, ≥65 years) with small cell lung cancer (SCLC).
| Variable | Univariate analysis | Multivariate analysis | |
|---|---|---|---|
| P-value | HR (95% CI) | P-value | |
| Gender | |||
| Female | 0.224 | – | – |
| Male | |||
| Stage | |||
| Limited disease (LD) | 0.308 | – | – |
| Extensive disease (ED) | |||
| Chemotherapy regimen | |||
| Etoposide + cisplatin or carboplatin | 0.169 | Reference | 0.129 |
| Etoposide + luoplatinum | 1.482 (0.892–2.464) | ||
| Radiotherapy | |||
| No | 0.004 | Reference | 0.002 |
| Yes | 0.435 (0.258–0.734) | ||
| Body mass index (BMI) (kg/m2) | |||
| BMI 18.5–24 | Reference | – | – |
| BMI <18.5 | 0.865 | ||
| BMI ≥24 | 0.712 | ||
| Albumin (g/L) | |||
| ≤40 | 0.502 | – | – |
| >40 | |||
| The HALP score | |||
| ≤25.8 | 0.025 | Reference | 0.014 |
| >25.8 | 0.483 (0.270–0.865) | ||
HR – hazard ratio; CI – confidence interval; HALP – hemoglobin, albumin, lymphocyte, and platelet.
Discussion
Small cell lung cancer (SCLC) has neuroendocrine tumor characteristics, and although etoposide-based chemotherapy is effective, acquired drug-resistance can develop [12]. Previous studies have shown the prognostic role of nutritional indicators for patient outcome [13], and of chemotherapy [14,15], treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) [16] and anti-PD-1/PD-L1 immunotherapy [17]. This study showed the prognostic role of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and body mass index (BMI) in prognosis in patients with SCLC during etoposide-based first-line treatment.
The BMI provides an important measure of the health of an individual and their nutritional status. Previous studies have focused on the relationship between BMI and health, especially in endocrine disease [18] and cardiovascular disease [19]. However, recent studies have shown an association between BMI and the risk of cancer [20], and the efficacy of cancer treatment [21]. This study showed that a BMI ≥24 kg/m2 was an independent risk factor for patients with SCLC <65 years of age. Inomata et al. [21] found that a BMI <21 kg/m2 was one of the independent factors significantly associated with reduced overall survival (OS) in patients with recurrent SCLC treated with amrubicin. A previous study showed that in patients with SCLC who received third-line chemotherapy, BMI <22 kg/m2 was a prognostic factor associated with a reduced time to progression (TTP) [22]. The findings from the present study showed that a BMI ≥24 kg/m2 was a prognostic risk factor in SCLC, which may have been identified because this study compared patients who were overweight with patients of normal weight, and the cut-off value, therapeutic regimen, and tumor stage were different. Also, etoposide had low aqueous solubility [23], and in overweight patients, after etoposide enters the human body, this drug is more likely to be distributed in adipose tissue. Therefore, the drug concentration of etoposide in tumor tissue was lower in overweight patients compared with patients of normal weight, which might have altered the therapeutic effect.
The HALP score is a comprehensive index that reflects components of the nutritional and immune status of patients, which had been shown to have a prognostic role in gastrointestinal cancers, including gastric cancer [24], esophageal squamous cell cancer [25], advanced colorectal cancer [7], and genitourinary cancers, including bladder cancer [8], and renal cell carcinoma [26]. However, to our knowledge, there have been no previously reported studies on the prognostic significance of the HALP score in patients with SCLC. This study showed that a HALP score >25.8 was an independent prognostic factor in patients older than 65 years, who had increased PFS following etoposide-based first-line treatment. Previous studies showed that in other tumors, a high HALP score predicted good therapeutic outcomes and prognosis [7,8,24–26], which supported the findings of this study. This study showed in patients <65 years with SCLC, BMI was a prognostic marker. Hsu et al. [27] also found that BMI was a prognostic factor in patients ≤45 years who had advanced-stage non-small cell lung cancer (NSCLC). However, the role of the HALP score as a prognostic marker in patients with NSCLC who undergo etoposide-based first-line treatment requires further study.
This study had several limitations. The cut-off value for the HALP score was determined by X-tile software from the baseline blood parameters of 178 patients involved in this study. Also, this was a retrospective study that was conducted at a single center, and further prospective clinical studies are required that include patients with SCLC from multiple centers to validate the findings from the present study.
Conclusions
The study aimed to investigate the prognostic significance of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score in patients with small cell lung cancer (SCLC) before first-line treatment with etoposide. A body mass index (BMI) ≥24 kg/m2 was an independent prognostic factor in patients with SCLC who were <65 years of age who were given etoposide-based first-line treatment. However, a HALP score of >25.8 was an independent prognostic factor in patients with SCLC who were ≥65 years of age.
Acknowledgments
The authors thank all the patients who participated in this study.
Footnotes
Source of support: This study was supported by the Key Research and Development Projects from the Science and Technology Department of Anhui Province (No. 1704a0802148) and the Natural Science Foundation of Anhui Province (No. 1908085MH260)
Conflict of interest
None.
References
- 1.Zimmerman S, Das A, Wang S, et al. 2017–2018 Scientific Advances in Thoracic Oncology: Small cell lung cancer. J Thorac Oncol. 2019;14(5):768–83. doi: 10.1016/j.jtho.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Wegiel B, Vuerich M, Daneshmandi S, Seth P. Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Front Oncol. 2018;8:284. doi: 10.3389/fonc.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiessel DL, Baracos VE. Barriers to cancer nutrition therapy: Excess catabolism of muscle and adipose tissues induced by tumour products and chemotherapy. Proc Nutr Soc. 2018;77(4):394–402. doi: 10.1017/S0029665118000186. [DOI] [PubMed] [Google Scholar]
- 4.Laviano A, Di Lazzaro L, Koverech A. Nutrition support and clinical outcome in advanced cancer patients. Proc Nutr Soc. 2018;77(4):388–93. doi: 10.1017/S0029665118000459. [DOI] [PubMed] [Google Scholar]
- 5.Filliatre-Clement L, Broseus J, Muller M, et al. Serum albumin or body mass index: Which prognostic factor for survival in patients with acute myeloblastic leukaemia? Hematol Oncol. 2019;37(1):80–84. doi: 10.1002/hon.2543. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Guo M, Zhang N, Wang G. Association of body mass index and outcomes following lobectomy for non-small cell lung cancer. World J Surg Oncol. 2018;16(1):90. doi: 10.1186/s12957-018-1394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7(44):72076–83. doi: 10.18632/oncotarget.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng D, Zhang CJ, Gong YQ, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8(1):794. doi: 10.1038/s41598-018-19146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go SI, Jeon H, Park SW, et al. Low pre-treatment nutritional index is significantly related to poor outcomes in small cell lung cancer. Thoracic Cancer. 2018;9(11):1483–91. doi: 10.1111/1759-7714.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin S, Cao S, Xu S, et al. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum-based chemotherapy. Clin Respir J. 2018;12(9):2433–40. doi: 10.1111/crj.12925. [DOI] [PubMed] [Google Scholar]
- 11.Camp RL, Dolled-Filhart M, Rimm DL. A new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–59. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 12.Steffens CC, Elender C, Hutzschenreuter U, et al. Treatment and outcome of 432 patients with extensive-stage small cell lung cancer in first, second and third line – Results from the prospective German TLK cohort study. Lung Cancer. 2019;130:216–25. doi: 10.1016/j.lungcan.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 13.Sepesi B, Gold KA, Correa AM, et al. The influence of body mass index on overall survival following surgical resection of non-small cell lung cancer. J Thoracic Oncol. 2017;12(8):1280–87. doi: 10.1016/j.jtho.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo Y, Eo W, Kim S, et al. Can nutritional status predict overall survival in patients with advanced non-small cell lung cancer? Nutr Cancer. 2019 doi: 10.1080/01635581.2019.1598564. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Takada K, Shimokawa M, Akamine T, et al. Association of low body mass index with poor clinical outcomes after resection of non-small cell lung cancer. Anticancer Res. 2019;39(4):1987–96. doi: 10.21873/anticanres.13309. [DOI] [PubMed] [Google Scholar]
- 16.Ono T, Igawa S, Ozawa T, et al. Evaluation of osimertinib efficacy according to body surface area and body mass index in patients with non-small cell lung cancer harboring an EGFR mutation: A prospective observational study. Thorac Cancer. 2019;10(4):880–89. doi: 10.1111/1759-7714.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Loubon C, Fernandez-Molina M, Singh G, Correa R. Obesity and its cardiovascular effects. Diabetes Metab Res Rev. 2019;35(4):e3135. doi: 10.1002/dmrr.3135. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Jin G, Yu C, et al. Cancer incidence in relation to body fatness among 0.5 million men and women: Findings from the China Kadoorie Biobank. Int J Cancer. 2019 doi: 10.1002/ijc.32394. Int J Cancer. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inomata M, Hayashi R, Tokui K, et al. Lactate dehydrogenase and body mass index are prognostic factors in patients with recurrent small cell lung cancer receiving amrubicin. Tumori. 2016;102(6):606–9. doi: 10.5301/tj.5000435. [DOI] [PubMed] [Google Scholar]
- 22.Inomata M, Hayashi R, Tokui K, et al. Outcome and prognostic factors in patients with small cell lung cancer who receive third-line chemotherapy. Tumori. 2014;100(5):507–11. doi: 10.1700/1660.18164. [DOI] [PubMed] [Google Scholar]
- 23.Tian QT, Ding CY, Song SS, et al. New tanshinone I derivatives S222 and S439 similarly inhibit topoisomerase I/II but reveal different p53-dependency in inducing G2/M arrest and apoptosis. Biocem Pharmacol. 2018;154:255–64. doi: 10.1016/j.bcp.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Chen XL, Xue L, Wang W, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget. 2015;6(38):41370–82. doi: 10.18632/oncotarget.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong L, Hu L. The value of the combination of hemoglobin, albumin, lymphocyte and platelet in predicting platinum-based chemoradiotherapy response in male patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2017;46:75–79. doi: 10.1016/j.intimp.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Peng D, Zhang CJ, Tang Q, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urology. 2018;18(1):20. doi: 10.1186/s12894-018-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CL, Chen KY, Shih JY, et al. Advanced non-small cell lung cancer in patients aged 45 years or younger: Outcomes and prognostic factors. BMC Cancer. 2012;12:241. doi: 10.1186/1471-2407-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]