Abstract
Hypercalcaemia, renal dysfunction, anaemia and bone lesions (CRAB) are a constellation of signs and symptoms that are collectively referred to as the CRAB features. When present together, multiple myeloma (MM) should be at the top of the differential diagnosis. We present a 69-year-old man who presented with severe body aches and bone pain in his ribs and pelvis, associated with fatigue and constipation. He was found to have hypercalcaemia, acute kidney injury, anaemia and numerous lytic lesion on chest imaging. Physical examination and imaging were unremarkable for any enlarged lymph nodes. The patient was initially suspected to have multiple myeloma, however, serum and urine protein electrophoresis, and serum free light chain assays were negative. The patient was ultimately diagnosed with diffuse large B cell lymphoma based on a bone marrow biopsy. This case highlights the fact that presence of hypercalcaemia, renal dysfunction, anaemia and bone lesions are not usually specific for MM.
Keywords: oncology, cancer intervention
Background
Diffuse large B cell lymphoma (DLBCL) is the most common non-Hodgkin’s lymphoma (NHL), and usually presents as palpable lymph nodes or as a mass, and may be associated with fatigue, night sweats and weight loss, symptoms that are termed ‘B’ symptoms.1–3 On the other hand, multiple myeloma (MM) most commonly presents with hypercalcaemia, renal dysfunction, anaemia and bone lesions (CRAB), a collection of signs and symptoms that are collectively referred as the CRAB presentation.4 Up until 2014, the CRAB features were thought to be enough to diagnose MM. However, in 2014 the international myeloma group added some new criteria including finding more than 60% plasma cells on a bone marrow biopsy, and the ratio of involved versus uninvolved serum free light chain ration >100. This was performed to include cases that were previously considered smouldering myeloma. The CRAB features however are not typical of any other condition. Although hypercalcaemia occurs in up to 20% of patients of DLBCL sometime in the course of their disease, it is not common at presentation.5 6 Lytic lesions and anaemia are also not typical of most DLBCL at presentation, though they may be present.7 8 However, the presence of all the CRAB features together is more rare as a presenting feature of DLBCL, and only a few cases have been reported previously.7 8 It is important to also note that this is a clinically distinct entity from primary bone lymphoma (PBL), which usually presents with a single bone lesion.
Case presentation
A 69-year-old man with a medical history of hypertension and hyperlipidaemia, was referred by his primary care physician to our hospital. The patient presented with a 15-day history of severe fatigue, anorexia, 5 pounds unintentional weight loss, generalised body aches that were most prominent in his ribs bilaterally, and lumbosacral region. He was also severely constipated and had not had a bowel movement for over a week. He was seen in a clinic 1 week ago and was given pain medications. He was thought to be having a viral infection considering his fatigue. The patient subsequently presented to his primary care physician’s office again for persistent fatigue and had basic lab work performed there. He was found to have an acute kidney injury and hypercalcaemia, and was referred to our hospital for further workup and management. At presentation, the patient was found to have normal vital signs and physical examination was remarkable for an older man who appeared severely dehydrated and in moderate discomfort. His chest examination was remarkable for diffuse tenderness in multiple ribs at multiple sites, and he also had tenderness in the lower spine. The cardiovascular, respiratory, neurological and gastrointestinal (GI) examinations were otherwise unremarkable. The patient did not have any palpable lymphadenopathy in any part of his body
Investigations
Initial lab work performed on admission showed a haemoglobin of 1.01 from 1.48 g/L at baseline, a calcium of 14.2 mg/dL, with an ionised calcium of 1.6 mmol/L, a creatinine of 1.3 mg/dL from baseline of 0.7 mg/dL. All other components of the complete blood count and comprehensive metabolic panel were unremarkable. Other pertinent labs included an erythrocyte sedimentation rate (ESR) of 84 mm/hour, C reactive protein of 125 mg/L, parathormone (PTH) of 14 pg/mL, lactate dehydrogenase (LDH) of 811 U/L. Vitamin D levels were normal.
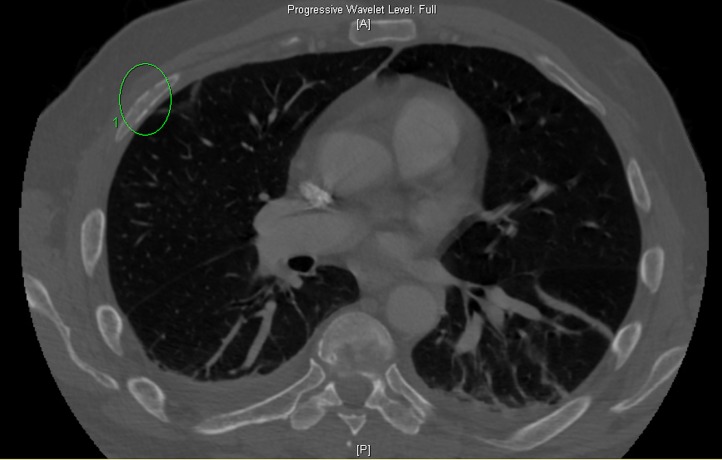
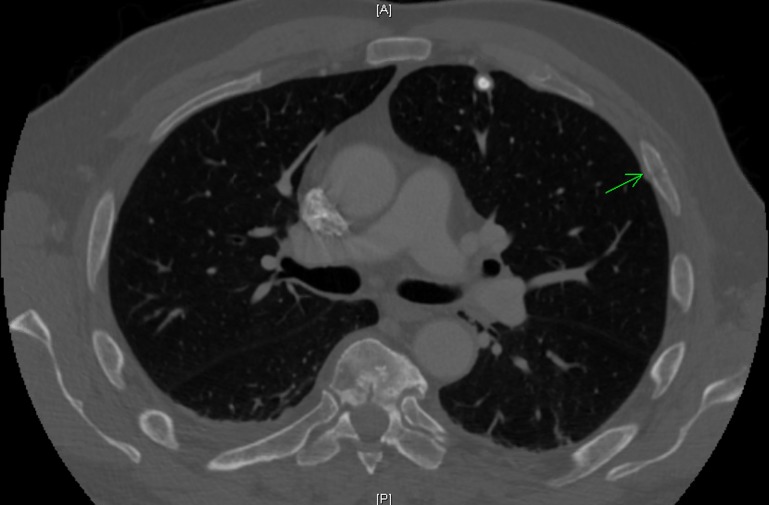
The patient had an initial chest X-ray and abdominal X-ray that were unremarkable. However, due to severe pain in multiple ribs, a CT scan of the chest was performed which showed a diffuse mottled appearance of multiple bones with areas of cortical destruction, highly suggestive of MM, with other metastatic diseases also a consideration (figure 1). Also, a non-displaced left fourth rib pathologic fracture was present (figure 2). The abdomen and pelvis CT scan read as lytic lesions throughout the bones with a pathologic compression fracture of the L5 vertebral body and areas of cortical destruction in the left iliac bone which was thought to be consistent with MM given his clinical picture, with metastatic disease also a possibility.
Figure 1.
CT scan of the chest showed a diffuse mottled appearance of multiple bones with areas of cortical destruction, highly suggestive of multiple myeloma, with other metastatic diseases also a consideration.
Figure 2.
CT scan of the chest also showed a nondisplaced left fourth rib pathologic fracture.
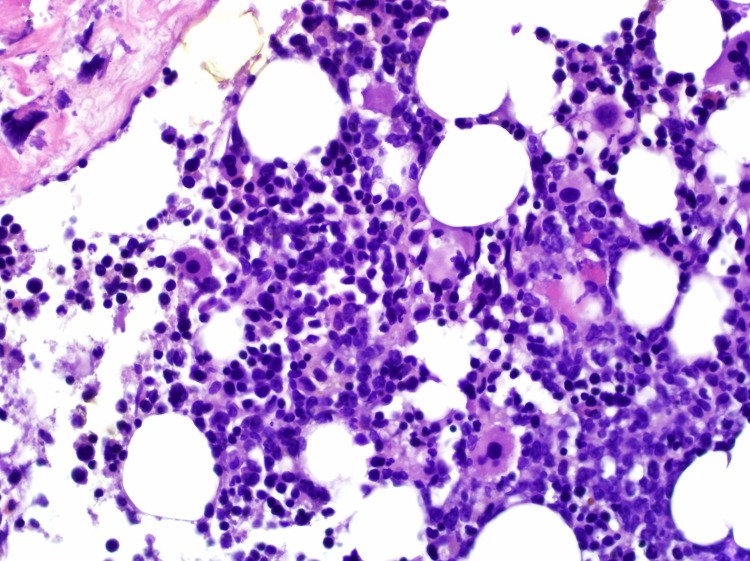
The patient subsequently had serum and urine protein electrophoresis with immune fixation studies which did not reveal any monoclonal paraproteins or light chains. At this point, it was decided to perform a bone marrow biopsy to confirm the diagnosis, which surprisingly came back positive for large B cell lymphoma (BCL) of germinal centre origin infiltrating the bone marrow (figure 3). The bone marrow core biopsy showed hypercellular marrow for age with 40%–50% of the cells that were large with distinct nucleoli and slightly irregular nuclear contours. Immunohistochemistry was positive for CD45, CD20, PAX5 and CD10 with focal weak positivity for MUM-1. No rearrangement of MYC, BCL2 and BCL6 genes was noted, which suggested the lymphoma was not a high-grade BCL. The lymphoma was of germinal centre origin. The patient had a positron emission tomography scan for staging, which revealed innumerable hypermetabolic lesion skull, ribs, vertebrae and pelvic bones, and also hypermetabolic activity in some lymph nodes, most prominently in the perigastric, celiac plexus, left peri aortic, bilateral hilar and right pericardial lymph nodes. Based on this, the patient was diagnosed with stage IV DLBCL, with the involvement of lymph nodes of multiple regions.
Figure 3.
400× image of the bone marrow core biopsy showing hypercellular marrow for age with 40%–50% of the cells that were large with distinct nucleoli and slightly irregular nuclear contours.
Differential diagnosis
Considering the patient’s anaemia, bone lesion and hypercalcaemia, MM was very high on the differential initially. The patient had a serum creatinine of 1.3 mg/dL, and though this was less than 2 mg/dL and as such did not qualify for the R in the CRAB features, it was nonetheless almost double of the patient’s creatinine of 0.7 mg/dL at his baseline.
Even when the serum and urine protein electrophoresis came back negative, the patient was thought to be having a non-secretory myeloma. Another metastatic malignancy was also one of the possibilities as the patient previously did have borderline elevated prostate specific antigen (PSA), and had issues with prostatic hypertrophy. However, bone marrow biopsy eventually confirmed the diagnosis of a DLBCL at stage IV.
Treatment
The patient was initially managed with intravenous fluids, zoledronic acid and calcitonin for his hypercalcaemia. He was also given pain medications and stool softeners. The patient symptoms improved somewhat, however, he continued to have severe fatigue. He has subsequently seen by oncology, who started him on R cyclophosphamide, doxorubicin, vincristine (oncovin), and prednisone (CHOP) therapy inpatient.
Outcome and follow-up
The patient’s treatment course was complicated by significant GI bleeding, and anaemia and the patient was re-hospitalised for that, however, the patient recovered from this. The patient had already completed six cycles of his planned chemotherapy, with a significant response and only mild residual disease left with a repeat PET scan scheduled in the near future.
Discussion
DLBCL is the most prevalent histological subtype of NHL. It accounts for around 25% of NHL cases.9–11 DLBCL is also overall the most common lymphoma in the developed world.5 9 12 It is more common in men overall, and the median age of presentation of this condition is 64 years. Familial aggregation of patients with DLBCL and other NHL subtypes has been noted.13 14 DLBCL usually arises from mature B cells and is made of cells that resemble centroblasts or immunoblasts, both two distinct types of activated B cells. It can also arise through the transformation of different types of low-grade BCL. It is also an AIDS-defining malignancy in HIV positive patients and is thought to arise from chronic B cell stimulation along with T cell immunodeficiency.
DLBCL most commonly presents as an enlarging lymphoid mass that usually signifies an enlarging lymph node. This is most commonly seen in the neck or the abdomen, and less frequently in the mediastinum too. Extranodal involvement is also common and occurs in up to 40% of patient.1 This can arise in virtually every organ system.1 15 The bone marrow can be involved in up to 30% of cases of extranodal disease,3 16 though a large number of these patients have a discordant type of lymphoma. In the case of extranodal disease, the presentation can be very variable and depends on the organ system involved. Almost 30% of the patients with DLBCL also have weight loss, fever and drenching night sweats at presentation.2 3 These are termed the B symptoms
Occasionally, DLBCL can involve the bone too. This can occur in two settings. The first case is with a condition termed PBL, which entails primary involvement of the bone with lymphoma usually a BCL. It usually presents with bone lesions and hypercalcaemia.17 This is an uncommon condition and accounts for only 1% of NHLs.18 19 It usually has a better outcome with treatment.20 However, our patient had numerous bone lesions spread throughout the body and as such did not qualify as a primary BCL. The other scenario is when patients have NHL, most commonly DLBCL, they can have hypercalcaemia and lytic bone lesions in advanced stages. However, it is rare for NHLs to present with hypercalcaemia and lytic bone lesions like our patient as an initial presentation. Through our literature review, we came across seven such cases reported previosuly.7 8 21–24 These are presented in table 1. One case described by Ruiz Argüelles was in Spanish and not available to us.24 Iravani et al also described a case series of four patients who presented with hypercalcaemia, but they were diagnosed with a precursor B cell lymphoblastic lymphoma.25
Table 1.
Previously described cases of diffuse large B cell lymphoma presenting with hypercalcaemia and lytic bone lesions
| Case | Age/sex | Hypercalcaemia, mg/dL | Lytic bone lesions | Kidney injury creatinine mg/dL | Anaemia g/L | Clinical lymphadenopathy | Mechanism of hypercalcaemia | Diagnosis | Treatment | Outcome |
| Matsuhashi et al 7 | 24/M | Hypercalcaemia | Numerous | Elevated creatinine | 0.74* | No | RANKL and MIP-1a | BM biopsy DLBCL stage IVB |
RCHOP | Remission but relapsed and died |
| Takasaki et al 8 | 50/F | 18.3* | Numerous | 2.6* | - | Inguinal, cervical | Elevated PTHrP | LN biopsy DLBCL stage IV |
RCHOP | Remission but relapsed |
| Mandal et al 21 | 49/M | 16.3* | Numerous | 3.4 (Reference 0.5–1.5) | 0.5 (Reference 1.3-1.7) |
Cervical | Unknown | LN biopsy DLBCL |
Bortezomib, followed by R CHOP | No remission, died |
| Chen et al 22 | 58/F | 15.6 (Reference 8–10.4) |
Numerous | Normal | 1.1* | Cervical | Elevated PTHrP, Dkk-1, imbalance of RANKL/OPG | LN/BM biopsy DLBCL stage IVB | R CHOP | Remission |
| Covarrubias-Flores et al 23 | 56/M | 12.8* | Numerous | 2.5* | NA | No | Unknown | BM biopsy DLBCL germinal centre origin, |
NA | NA |
| Abdullah (this case) | 69/M | 14.2 (Reference 8.4–10.5) |
Numerous | 1.3 (Reference 0.72–1.2) |
1.01 (Reference 1.35-1.75) |
No | Unknown | DLBCL germinal centre origin, stage IV | R CHOP | In remission |
*Reference range not reported.
RANKL, receptor activator of nuclear factor kappa-Β ligand; R CHOP, R cyclophosphamide, doxorubicin, vincristine (oncovin).
Hypercalcaemia itself is a common presentation of malignancies. It is reported to occur around 20% of patients with malignancy at some point of time in their disease. However, it only occurs in 5% of patients with NHL and most of these occur later in the course of the disease.26 27 There are several reported mechanisms suggested for hypercalcaemia and malignancy in general, and lymphomas in particular. Seymour et al had reported three possible mechanisms, including mediated by PTH-related peptide (PTHrP), due to local osteolytic bone destruction and hypercalcaemia mediated by excessive production of calcitriol that is the active vitamin D metabolite.6 27 Esteve et al also added ectopic PTH secretion, though it is thought to be very rare.28 Lymphoma patients are generally thought to have hypercalcaemia from excessive calcitriol production, as opposed to local osteolytic-induced hypercalcaemia that is thought to be the primary mechanism in MM. The mechanism of hypercalcaemia in MMs is relatively well known and is thought to be mediated by the receptor activator of nuclear factor kappa-Β/r eceptor activator of nuclear factor kappa-Β ligand (RANK/RANKL) signalling pathway.28 RANKL, also known as tumour necrosis factor-related activation-induced cytokine is a member of the tumour necrosis family.29 It acts directly by increasing osteoclasts genesis and also inhibits the destruction of osteoclasts apoptosis, which is achieved by binding to its receptor that is present on immature and mature osteoclasts. RANKL is expressed by activated T lymphocytes and osteolytic cells and is released in response to cytokines and other substances including PTH, vitamin D, dexamethasone, interleukin (IL)-1, IL-11 and tumour necrosis factor.30 31 Shibata et al also demonstrated expression of RANKL in lymphomas, however, that was in primary BCL of the bone, which may be one of the mechanisms of hypercalcaemia in these patients too.32 Our patient also had osteolytic bone lesions like MM, which may explain the early occurrence of hypercalcaemia, as the presenting feature of DLBCL. PTHrP related hypercalcaemia is most commonly reported in solid organ tumours, however, it has also been reported in patients with NHL too on rare occasions.8 17 This was not the case in outpatient as PTHrP levels were low in our patient. In our case, the osteolytic bone lesions, along with excessive calcitriol production were most likely to have been the cause of the hypercalcaemia and may explain why our patient had it early in the course of his disease.
The diagnosis of DLBCL is based on pathology. This entails both morphological and immunophenotyping studies. The tumour cells in DLBCL usually express B cell antigens including CD19, CD20, CD22 and CD79a. This can be confirmed with immunohistochemistry or most of the tumours have some associated genetic abnormalities, though there is no single genetic abnormality that is diagnostic or typical of DLBCL. A large number of tumours also express surface monoclonal immunoglobulins, most commonly the IgM isotype. Some tumour cells can express CD30 which is associated with a better outcome, and in cases associated with the expression of CD5, they have a worse prognosis.33 BCL6 and t(14;18) translocation are some of the most frequently seen genetic mutations.34 35
The CRAB criteria, which stands for hypercalcaemia, renal dysfunction, anaemia and bone involvement is considered highly suggestive for MM.4 Though the CRAB features are far from ‘pathognomonic’ for MM, there is very little published literature available where CRAB features of other differentials have been explicitly quantified. This case report does hold a valuable reminder to the readers that the CRAB signs are not only <75% sensitive for MM, they are not too specific as well and thus must be used in context. For example, prior to 2014, the CRAB features were essentially used to differentiate symptomatic MM from smouldering myeloma, as the prior was usually treated and the latter not. However, it was discovered that some of the patients with smouldering myeloma were very high risk for progression to MM, and this led to a new group of patients described as SLiM myeloma described by Rajkumar et al in 2014.36 These are patients who have 60% or more clonal plasma cells in the bone marrow (S), have a light chain ratio of kappa-lambda or lambda–kappa of greater than 100 (Li) and have evidence of more than one focal bone lesion on MRI (M). After this update, patients who have the SLiM criteria qualify treatment even if they do not have the CRAB features.36 37 Also, prior to the development of safer treatment options for MM, a much more strict criteria was used for treating myeloma patients to avoid potential side effects in patients who were thought to be low risk. As such patients with all CRAB features were usually treated. However, as the treatment of MM evolved and newer safer options were discovered, the criteria was softened, and the trend evolved to treat patients with some of the CRAB features and not all. Essentially the treatment criteria evolved from patients with CRAB features combined with an ‘AND’ to CRAB features combined with an ‘OR’. As such, though the CRAB features were considered more of a constellation of symptoms that directed treatment, that is no longer true in a strict sense with the evolution of treatment.37
In the case of MM, the presence of the individual CRAB features including renal dysfunction, anaemia and bone lesions is quite common and can occur in around 74% of the patients, with some authors considering hypercalcaemia to be a subset of bone lesions.38 However, the combination of all these symptoms occur in only 30% of the patients with MM. This makes our case remarkable as it has all the features combined in a patient with DLBCL (though the kidney injury does not meet the criteria for CRAB). Our case and the similar cases described in the discussion highlights the fact that though the CRAB symptoms are not very sensitive for myeloma, they are not very specific either. As such CRAB includes isolated symptoms, and only have meaning when applied in the context of classifying already suspect myeloma-MGUS spectrum disease rather than using for diagnosis, due it its non-sensitivity and non-specificity.
As such our patient is a good case study in not taking a patient’s typical presentation for granted. There are always exceptions in medicine, and this should be taken into account for diagnosis. This is an important reminder that patient’s with a particular presentation may be misdiagnosed or mistreated or at least delayed diagnosis if we are not diligent enough, as previously highlighted by Mandal et al.21
Learning points.
The hypercalcaemia, renal dysfunction, anaemia and bone lesions (CRAB) criteria is the most common presentation of multiple myeloma (MM).
However, on other conditions like diffuse large B cell lymphoma (DLBCL) can also present with CRAB features.
The mechanism of hypercalcaemia is thought to be over activation of calcitriol in DLBCL and thgouht to be RANK/RANKL mediated in MM.
Footnotes
Contributors: HMAA, ME and QW were responsible for writing the summary and discussion. TO and AC were responsible for writing introduction and case presentation. AC also provided an expert read on the histology images. All of the authors were responsible for planning, conduct, reporting, conception and design, acquisition of data or analysis and interpretation of data.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol 2004;124:151–9. 10.1046/j.1365-2141.2003.04749.x [DOI] [PubMed] [Google Scholar]
- 2. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780–95. 10.1200/JCO.1998.16.8.2780 [DOI] [PubMed] [Google Scholar]
- 3. Anderson J, Armitage JO, Berger F, et al. A clinical evaluation of the International Lymphoma Study Group Classification of non-Hodgkin’s lymphoma: a report of the Non-Hodgkin’s Lymphoma Classification Project. Blood 1997. [PubMed] [Google Scholar]
- 4. Dimopoulos M, Kyle R, Fermand JP, et al. Guidelines for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011 Jan 1. Blood 2010. [DOI] [PubMed] [Google Scholar]
- 5. van Leeuwen MT, Turner JJ, Joske DJ, et al. Lymphoid neoplasm incidence by WHO subtype in Australia 1982-2006. Int J Cancer 2014;135:2146–56. 10.1002/ijc.28849 [DOI] [PubMed] [Google Scholar]
- 6. Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011;105:1684–92. 10.1038/bjc.2011.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsuhashi Y, Tasaka T, Uehara E, et al. Diffuse large B-cell lymphoma presenting with hypercalcemia and multiple osteolysis. Leuk Lymphoma 2004;45:397–400. 10.1080/10428190310001593139 [DOI] [PubMed] [Google Scholar]
- 8. Takasaki H, Kanamori H, Takabayashi M, et al. Non-Hodgkin’s lymphoma presenting as multiple bone lesions and hypercalcemia. Am J Hematol 2006;81:439–42. 10.1002/ajh.20559 [DOI] [PubMed] [Google Scholar]
- 9. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265–76. 10.1182/blood-2005-06-2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swerdlow SH. WHO classification of tumours of haematopoietic and lymphoid tissues. WHO classification of tumours 2008;22:439. [PubMed] [Google Scholar]
- 12. Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood 2011;117:5019–32. 10.1182/blood-2011-01-293050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldin LR, Landgren O, McMaster ML, et al. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev 2005;14:2402–6. 10.1158/1055-9965.EPI-05-0346 [DOI] [PubMed] [Google Scholar]
- 14. Goldin LR, Björkholm M, Kristinsson SY, et al. Highly increased familial risks for specific lymphoma subtypes. Br J Haematol 2009;146:91–4. 10.1111/j.1365-2141.2009.07721.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Avilés A, Neri N, Huerta-Guzmán J. Large bowel lymphoma: an analysis of prognostic factors and therapy in 53 patients. J Surg Oncol 2002;80:111–5. 10.1002/jso.10103 [DOI] [PubMed] [Google Scholar]
- 16. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7. 10.1200/JCO.2010.33.3419 [DOI] [PubMed] [Google Scholar]
- 17. Evron E, Goland S, Klepfish A, et al. Primary multifocal lymphoma of bone presenting as hypercalcemic crisis: report of a rare manifestation of extranodal lymphoma. Leuk Lymphoma 1999;34(1-2):197–200. 10.3109/10428199909083398 [DOI] [PubMed] [Google Scholar]
- 18. Rathmell AJ, Gospodarowicz MK, Sutcliffe SB, et al. Localised lymphoma of bone: prognostic factors and treatment recommendations. Br J Cancer 1992;66:603–6. 10.1038/bjc.1992.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horsman JM, Thomas J, Hough R, et al. Primary bone lymphoma: a retrospective analysis. Int J Oncol 2006;28:1571–5. 10.3892/ijo.28.6.1571 [DOI] [PubMed] [Google Scholar]
- 20. Fidias P, Spiro I, Sobczak ML, et al. Long-term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol Phys 1999;45:1213–8. 10.1016/S0360-3016(99)00305-3 [DOI] [PubMed] [Google Scholar]
- 21. Mandal SK, Ganguly J, Sil K, et al. Diagnostic dilemma in a case of osteolytic lesions. Case Rep Child Meml Hosp Chic 2014;2014:bcr2013201682 10.1136/bcr-2013-201682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen P, Li B, Zhuang W, et al. Multiple bone lesions and hypercalcemia presented in diffuse large B cell lymphoma: mimicking multiple myeloma? Int J Hematol 2010;91:716–22. 10.1007/s12185-010-0562-4 [DOI] [PubMed] [Google Scholar]
- 23. Covarrubias-Flores DL, García-Galaviz RA, Silva-Carmona A. Paraneoplastic hypercalcaemia and osteolytic lesions secondary to large B-cell lymphoma: Case report and literature review. Revista Médica del Hospital General de México 2018;81:28–32. 10.1016/j.hgmx.2016.05.014 [DOI] [Google Scholar]
- 24. Ruiz Argüelles GJ. Hieprcalcemia y lesiones osteoliticas asociadas a linfoma primario de medula osea de celulas pre-b. Informe de un caso. La Revista de Investigación Clínica 2008;42:226–30. [PubMed] [Google Scholar]
- 25. Iravani S, Singleton TP, Ross CW, et al. Precursor B lymphoblastic lymphoma presenting as lytic bone lesions. Am J Clin Pathol 1999;112:836–43. 10.1093/ajcp/112.6.836 [DOI] [PubMed] [Google Scholar]
- 26. Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004;350:1655–64. 10.1056/NEJMra030831 [DOI] [PubMed] [Google Scholar]
- 27. Seymour JF, Gagel RF. Calcitriol: the major humoral mediator of hypercalcemia in Hodgkin’s disease and non-Hodgkin’s lymphomas. Blood 1993;82:1383–94. [PubMed] [Google Scholar]
- 28. Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol 2007;20:613–24. 10.1016/j.beha.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 29. Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997;390:175–179. 10.1038/36593 [DOI] [PubMed] [Google Scholar]
- 30. Giuliani N, Colla S, Morandi F, et al. The RANK/RANK ligand system is involved in interleukin-6 and interleukin-11 up-regulation by human myeloma cells in the bone marrow microenvironment. Haematologica 2004;89:1118–23. [PubMed] [Google Scholar]
- 31. Tanaka S, Nakamura K, Takahasi N, et al. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev 2005;208:30–49. 10.1111/j.0105-2896.2005.00327.x [DOI] [PubMed] [Google Scholar]
- 32. Shibata H, Abe M, Hiura K, et al. Malignant B-lymphoid cells with bone lesions express receptor activator of nuclear factor-κB. Clin Cancer Res 2005;11:6109–15. [DOI] [PubMed] [Google Scholar]
- 33. Slack GW, Steidl C, Sehn LH, et al. CD30 expression in de novo diffuse large B-cell lymphoma: a population-based study from British Columbia. Br J Haematol 2014;167:608–17. 10.1111/bjh.13085 [DOI] [PubMed] [Google Scholar]
- 34. Bastard C, Deweindt C, Kerckaert JP, et al. LAZ3 rearrangements in non-Hodgkin’s lymphoma: correlation with histology, immunophenotype, karyotype, and clinical outcome in 217 patients. Blood 1994;83:2423–7. [PubMed] [Google Scholar]
- 35. Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997;90:244–51. [PubMed] [Google Scholar]
- 36. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–e548. 10.1016/S1470-2045(14)70442-5 [DOI] [PubMed] [Google Scholar]
- 37. Rajkumar SV, Fonseca R, San Miguel JF. Diagnosis and Staging of Multiple Myeloma and Related Disorders Multiple Myeloma and Other Plasma Cell Neoplasms: Springer, Cham, 2018:17–28. [Google Scholar]
- 38. Greenberg AJ, Rajkumar SV, Therneau TM, et al. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia 2014;28:398–403. 10.1038/leu.2013.258 [DOI] [PMC free article] [PubMed] [Google Scholar]