Abstract
Background
This study investigated the risk factors affecting development and prognosis of acute kidney injury (AKI) in patients with acute respiratory distress syndrome (ARDS).
Material/Methods
A total of 501 ARDS cases were retrospectively enrolled (296 males and 205 females) admitted to the First People’s Hospital of Lianyungang from Aug 2015 to Aug 2017. Multivariable logistic modeling was conducted to select significant variables, and the assigned integer score was proportional to the adjusted odds ratio (OR). Then, the sum of weighted variables was utilized to estimate the score in patients.
Results
Patients with ARDS who had unconsciousness (OR=2.778, 95% CI: 1.396–5.528), hypertension (OR=1.771, 95% CI: 1.089–2.881), ARDS (moderate–severe) (OR=1.630, 95% CI: 1.027–2.588), AST (OR=2.093, 95% CI: 1.251–3.499), and D-dimer (OR=2.372, 95% CI: 1.316–4.275) were more likely to also have AKI. The score was allocated in proportion to the corresponding adjusted OR, hypertension, ARDS (moderate–severe), aspartate aminotransferase (AST), D-dimer (2 points each), and unconsciousness (3 points). The incidences of AKI in group A (score 0–2, n=9), group B (score 3–4, n=16), group C (score 5–6, n=33), and group D (score ≥7, n=72) were 10.98%, 16.00%, 31.13%, and 49.66%, respectively (P<0.001). Higher scores were associated with higher prevalence of AKI, and the trend was statistically significant (P<0.001).
Conclusions
This scoring system may provide a risk-integrative evaluation for AKI in patients with ARDS.
MeSH Keywords: Acute Kidney Injury, Adult, Respiratory Distress Syndrome, Risk Factors
Background
Acute kidney injury (AKI) is a serious complication with high mortality and morbidity rates, and is characterized by sudden loss of kidney function [1–3]. Studies have demonstrated the application of specific therapies to alleviate AKI or accelerate rehabilitation [4–9], but the prognosis of patients with AKI is still poor due to various and complicated causes [10,11]. Therefore, it is necessary to establish a risk factor scoring system for AKI to improve the prognosis of patients with AKI.
Acute respiratory distress syndrome (ARDS), a severe form of acute respiratory failure, is characterized by disruption of the endothelial barrier of capillaries lining the alveoli with increased permeability, leading to an intense inflammatory insult, alveolar epithelial injury, and influx of protein-rich edema fluid into the alveoli, causing impaired gas exchange, decreased lung compliance, and increased work of breathing [12–14]. Previous studies have reported that the incidence of AKI is up to 35% among ARDS patients in the intensive care unit (ICU), revealing the relationship between patients with acute respiratory failure such as ARDS and the growing mortality rates in patients who developed AKI [15–17]. Multicenter studies show that the ARDS mortality rate in China is 68.5% in adult ICUs, and when AKI was complicated by acute lung injury (ALI), the mortality rate is as high as 80% [18]. To the best of our knowledge, however, the relationship of different levels of ARDS and AKI in patients with milder diseases have been rarely reported.
In this study we investigated the risk factors associated with development and prognosis of AKI in hospitalized ARDS patients, and established a scoring system based on these risks.
Material and Methods
Patients
A total of 501 patients with ARDS were (296 males and 205 females) admitted to the First People’s Hospital of Lianyungang from Aug 2015 to Aug 2017. This study was approved by the Institutional Review Board of the First People’s Hospital of Lianyungang (approval number LW20190125001).
Inclusion and exclusion criteria
Patients who met the following criteria were included: (1) met Berlin diagnostic criteria for ARDS, (2) at least 18 years old, and (3) admission to the respiratory intensive care unit or general ward.
Excluded criteria were: (1) tumors, (2) chronic kidney disease (CKD) stage 5 or undergoing renal replacement therapy (peritoneal dialysis or hemodialysis) before hospitalization, (3) incomplete clinical data and unclear basal serum creatinine, and (4) admission time ≤48 h.
Diagnosis criteria
The following Berlin diagnostic criteria for ARDS [13] were used: (1) the new or pejorative respiratory symptoms with clear clinical symptoms or injuries within 1 week, (2) infiltrates in both lungs cannot be completely exuded through the lungs, lung collapse, or pulmonary nodules, (3) respiratory failure cannot be fully explained via heart failure or excessive volume load, and (4) oxygenation index classified as (a) mild: 200 mmHg <PaO2/FiO2 ≤300 mmHg, and PEEP or CAPA ≥5 cmH2O; (b) moderate: 100 mmHg <PaO2/FiO2 ≤200 mmHg, and PEEP ≥5 cmH2O; and (c) severe: PaO2/FiO2 ≤100 mmHg, and PEEP ≥5 cmH2O.
Diagnostic criteria for patients with AKI conformed to the revised AKI diagnosis criteria of the 2012 Kidney Disease Improving Global Outcomes (KDIGO) [19], in which presence of any 1 of the following characteristics can lead to diagnosis of AKI: (a) suddenly decline in renal function within 48 h, (b) an increasing absolute value in serum creatinine not less than 0.3 mg/dL (≥26.4 mmol/L), (c) blood creatinine at least 50% above than the baseline value, and (d) decreased urine output (urinary volume <0.5 mL/kg.h) for more than 6 h. The base creatinine concentration was the 3-month creatinine level before admission or minimum creatinine after admission.
Clinical risk scoring system
Logistic regression analysis was used to establish a clinical risk scoring for AKI, which was refined by Takagi et al. [20]. The multivariable logistic model was constructed to select significant variables which were assigned scores proportional to the adjusted OR. The variables with no statistical significance were explicitly defined as 1 point. The predictors with statistical significance were assigned an integer fraction which was proportional to OR. Then, the sum of weighted variables was used to estimate a patient’s score. The 4 risk categories were determined based on quartile of total score: group A (score 0–2), group B (score 3–4), group C (score 5–6), and group D (score ≥7).
Statistical analysis
Statistical analysis was performed using SPSS 24.0 (SPSS, Inc., Chicago, IL). Count data were presented as n (%) with chi-square test or logistic regression. The risk factors were screened by multivariable logistic regression analysis. P<0.05 were considered to be a statistically significant difference.
Results
Study population
As shown in Table 1, the parameters of sex, age, smoke, respiratory failure type, blood glucose, pulmonary arterial (PA), and encephalopathy were no significantly different between the 2 groups (P>0.05).
Table 1.
Comparison for sociological characteristics between the 2 groups.
| Variable | Classification | Group n (%) | χ2 | P | |
|---|---|---|---|---|---|
| N-AKI (n=353) | AKI (n=148) | ||||
| Sex | Male | 203 (57.51) | 93 (62.84) | 1.226 | 0.268 |
| Female | 150 (42.49) | 55 (37.16) | |||
| Age | <60 | 50 (14.16) | 19 (12.84) | 1.233 | 0.540 |
| 60–75 | 148 (41.93) | 56 (37.84) | |||
| >75 | 155 (43.91) | 73 (49.32) | |||
| Smoke | No | 237 (67.14) | 110 (74.32) | 2.529 | 0.112 |
| Yes | 116 (32.86) | 38 (26.03) | |||
| Respiratory failure | I | 179 (50.71) | 76 (51.35) | 0.053 | 0.974 |
| II | 171 (48.44) | 71 (47.97) | |||
| Normal | 3 (0.85) | 1 (0.68) | |||
| Blood glucose | Anomaly | 202 (57.22) | 95 (64.19) | 2.096 | 0.148 |
| Normal | 151 (42.78) | 53 (35.81) | |||
| PA | Normal | 237 (69.10) | 105 (70.95) | 1.157 | 0.740 |
| Mild | 54 (15.74) | 20 (13.51) | |||
| Moderate | 33 (9.62) | 12 (8.11) | |||
| Severe | 19 (5.54) | 11 (7.43) | |||
| Encephalopathy | No | 277 (78.47) | 105 (70.95) | 3.260 | 0.071 |
| Yes | 76 (21.53) | 43 (29.05) | |||
N-AKI – non-acute kidney injury; AKI – acute kidney injury; PA – pulmonary arterial.
Factors analysis of AKI
The single and multiple factors analyses of AKI are presented in Tables 2 and 3, respectively. There were statistically obvious differences between the N-AKI and AKI groups in diabetics, consciousness, hypertension, ARDS, AF, pleural effusion, heart disease, aspartate aminotransferase (AST), and D-dimer (Table 2) (P<0.05). Patients with ARDS who had unconsciousness (OR=2.778, 95% CI: 1.396–5.528), hypertension (OR=1.771, 95% CI: 1.089–2.881), ARDS (moderate–severe) (OR=1.630, 95% CI: 1.027–2.588), AST (OR=2.093, 95% CI: 1.251–3.499), or D-dimer (OR=2.372, 95% CI: 1.316–4.275) were more likely to also have AKI (Table 3).
Table 2.
Single-factor analysis of AKI.
| Variable | Classification | Group n (%) | OR | 95% CI | P | |
|---|---|---|---|---|---|---|
| N-AKI (n=353) | AKI (n=148) | |||||
| Diagnosis | COPD | 188 (53.26) | 60 (40.54) | 1 | – | |
| Bronchial disease | 47 (13.31) | 11 (7.43) | 0.733 | 0.358–1.503 | 0.397 | |
| Pneumonia | 100 (28.33) | 68 (45.95) | 2.131 | 1.395–3.254 | 0.001 | |
| Others | 18 (5.10) | 9 (6.08) | 1.567 | 0.669–3.670 | 0.301 | |
| Diabetes | No | 309 (88.03) | 115 (77.70) | 1 | – | |
| Yes | 42 (11.97) | 33 (22.30) | 2.112 | 1.276–3.494 | 0.003 | |
| Miss | 2 | 0 | – | – | ||
| Consciousness | No | 23 (6.52) | 31 (20.95) | 1 | – | |
| Yes | 330 (93.48) | 117 (79.05) | 0.263 | 0.147–0.469 | <0.001 | |
| Hypertension | No | 239 (76.36) | 74 (23.64) | 1 | – | |
| Yes | 114 (60.64) | 74 (39.36) | 2.097 | 1.417–3.102 | <0.001 | |
| ARDS | Mild | 202 (57.22) | 57 (38.51) | 1 | – | |
| Moderate–severe | 151 (42.78) | 91 (61.49) | 2.121 | 1.420–3.170 | <0.001 | |
| AF | No | 327 (92.63) | 122 (82.43) | 1 | – | |
| Yes | 26 (7.37) | 26 (17.57) | 2.680 | 1.497–4.796 | 0.001 | |
| Pleural effusion | No | 259 (73.37) | 89 (60.14) | 1 | – | |
| Yes | 94 (26.63) | 59 (39.86) | 1.827 | 1.218–2.739 | 0.003 | |
| Heart disease | No | 215 (66.56) | 72 (53.33) | 1 | – | |
| Yes | 108 (33.44) | 63 (46.67) | 1.742 | 1.157–2.624 | 0.008 | |
| Miss | 30 | 13 | – | – | ||
| AST | Normal | 292 (82.72) | 92 (62.16) | 1 | – | |
| Abnormal | 61 (17.28) | 56 (37.84) | 2.914 | 1.892–4.487 | <0.001 | |
| D-dimer | Normal | 137 (40.90) | 23 (16.08) | 1 | – | |
| Abnormal | 198 (59.10) | 120 (83.92) | 3.610 | 2.197–5.931 | <0.001 | |
| Miss | 18 | 5 | – | – | ||
N-AKI – non-acute kidney injury; AKI – acute kidney injury; COPD – chronic obstructive pulmonary disease; ARDS – acute respiratory distress syndrome; AF – atrial fibrillation; AST – aspartate aminotransferase.
Table 3.
Multivariable logistic analysis of AKI.
| Variable | β | S.E. | Wald | P | OR | 95% CI | Assigned score | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Constant | −2.578 | 0.311 | 68.908 | <0.001 | – | – | – | – |
| Diagnosis (bronchial disease) | −0.100 | 0.421 | 0.057 | 0.812 | 0.905 | 0.397 | 2.063 | 1 |
| Diagnosis (pneumonia) | 0.156 | 0.266 | 0.342 | 0.558 | 1.168 | 0.694 | 1.968 | 1 |
| Diagnosis (others) | 0.156 | 0.521 | 0.089 | 0.765 | 1.168 | 0.421 | 3.244 | 1 |
| Diabetes (yes) | 0.359 | 0.314 | 1.301 | 0.254 | 1.431 | 0.773 | 2.651 | 1 |
| Consciousness (no) | 1.022 | 0.351 | 8.464 | 0.004 | 2.778 | 1.396 | 5.528 | 3 |
| Hypertension (yes) | 0.572 | 0.248 | 5.299 | 0.021 | 1.771 | 1.089 | 2.881 | 2 |
| ARDS (moderate–severe) | 0.489 | 0.236 | 4.292 | 0.038 | 1.630 | 1.027 | 2.588 | 2 |
| AF (yes) | 0.469 | 0.356 | 1.733 | 0.188 | 1.598 | 0.795 | 3.211 | 1 |
| Pleural effusion (yes) | 0.089 | 0.253 | 0.124 | 0.725 | 1.093 | 0.666 | 1.793 | 1 |
| Heart disease (yes) | 0.213 | 0.251 | 0.721 | 0.396 | 1.237 | 0.757 | 2.022 | 1 |
| AST (abnormal) | 0.738 | 0.262 | 7.925 | 0.005 | 2.093 | 1.251 | 3.499 | 2 |
| D-dimer (abnormal) | 0.864 | 0.301 | 8.258 | 0.004 | 2.372 | 1.316 | 4.275 | 2 |
ARDS – acute respiratory distress syndrome; AF – atrial fibrillation; AST – aspartate aminotransferase.
Model scoring
The assigned score of the correlative predictors are displayed in Table 3. Five significant predictors selected from the multivariable logistic model were assigned integer scores proportional to the OR. The variables with no significant differences (bronchial disease, diabetes, AF, pleural effusion, and heart disease) were assigned 1 point. Ultimately, these 12 predictors were integrated into the risk scoring system.
The distribution of the points and correlated prevalence of AKI is shown in Figure 1. The incidences of AKI were 10.71% (score 0), 10.53% (score 1), 11.43% (score 2), 10.71% (score 3), 22.73% (score 4), 35.42% (score 5), 27.59% (score 6), 37.50% (score 7), and 54.29% (score ≥8).
Figure 1.
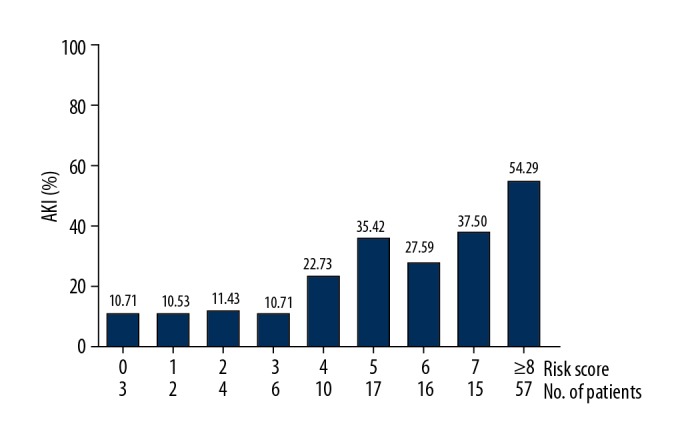
The distribution of the scoring points and associated prevalence of AKI.
Correlation analysis of different scoring groups
The prevalence of AKI was s significantly different in the different scoring groups (χ2=50.183, P<0.001). Higher scores were associated with higher prevalence of AKI (Ptrend <0.001) (Table 4).
Table 4.
The relationship between the 4 risk groups and the prevalence of AKI.
| Group | Group | χ2 | P | Ptrend | |
|---|---|---|---|---|---|
| N-AKI | AKI | ||||
| A | 73 (89.02) | 9 (10.98) | 50.183 | <0.001 | <0.001 |
| B | 84 (84.00) | 16 (16.00) | |||
| C | 73 (68.87) | 33 (31.13) | |||
| D | 73 (50.34) | 72 (49.66) | |||
443 cases were included in this scoring system based on the logistic regression analysis. N-AKI – non-acute kidney injury; AKI – acute kidney injury. A – score 0–2; B – score 3–4; C – score 5–6; D: score ≥7.
In this study, 4 risk categories were determined based on the scoring points: group A (score 0–2, n=9), group B (score 3–4, n=16), group C (score 5–6, n=33), and group D (score ≥7, n=72). As shown in Figure 2, the incidences of AKI in groups A, B, C, and D were 10.98%, 16.00%, 31.13%, and 49.66%, respectively (P<0.001).
Figure 2.
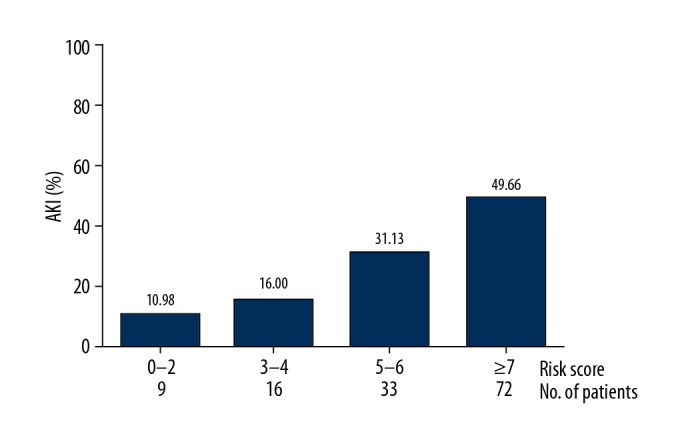
The 4 risk strata and corresponding prevalence of AKI.
The results of correlation analysis between the 3 risk groups and prognosis showed that less improvement was significantly associated with higher risk (χ2=23.057, P<0.001) (Table 5, Figure 3).
Table 5.
Correlation analysis of the 4 risk groups and prognosis.
| Group | Prognosis n (%) | χ2 | P | ||
|---|---|---|---|---|---|
| Improvement | Unapparent improvement | Abandon/hospital discharge/death | |||
| A | 74 (90.24) | 8 (9.76) | 0 (0.00) | 23.057 | <0.001 |
| B | 82 (82.00) | 10 (10.00) | 8 (8.00) | ||
| C | 83 (78.30) | 7 (6.60) | 16 (15.09) | ||
| D | 95 (65.22) | 18 (12.41) | 32 (22.07) | ||
443 cases were included in this scoring system based on the logistic regression analysis. A – score 0–2; B – score 3–4; C – score 5–6; D – score ≥7.
Figure 3.
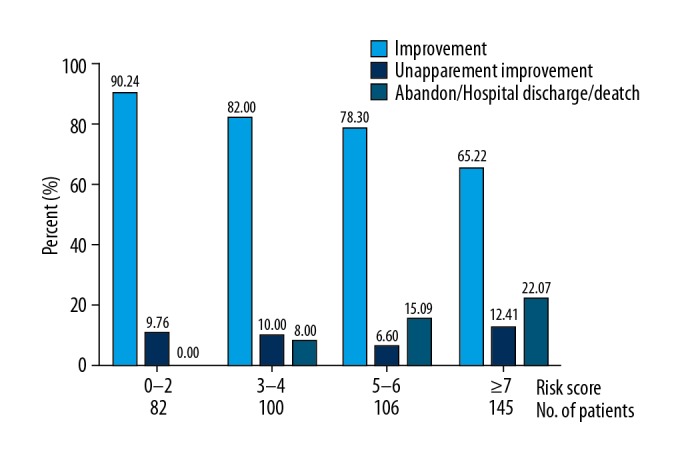
The prognosis in different scoring groups and associated prevalence of AKI.
Discussion
The present study established a risk scoring system for patients with ARDS who developed AKI. Our results determined that this AKI risk score, in which 5 predictive factors based on the multiple-factor logistic regression were analyzed, displayed an obvious correlation with the development and prognosis of AKI. According to the score, 4 risk levels were defined and used to develop explicit risk stratification of AKI, and risk prediction was performed using the risk scoring system in the internal validation. To the best of our knowledge, it is the first published clinical risk scoring system for AKI patients.
Ashbaugh et al. first performed risk scoring for ARDS in 1967 [21], which is characterized by diffuse lesions of pulmonary endothelial and alveolar epithelium cells, resulting in alveolar and interstitial tissue flooding and edema, reduced lung compliance, imbalanced lung ventilation flow ratio, decreased lung volume, and refractory dyspnea [22–24]. The literature shows that AKI can lead to the release of various inflammatory factors and cytokines in vivo and promote the expression of pro-inflammatory genes in healthy lungs [25]. AKI is an important cause of mortality and morbidity in critically ill patients in ICUs [26]. A study demonstrated that IL-6, soluble TNF receptors, and plasminogen activator inhibitor-1 are associated independently with AKI during mechanical ventilation [27]. Use of positive-pressure ventilation was reported to be associated with activation of the renin-angiotensin system, the sympathetic nervous system, and hemodynamic changes, which may reduce renal blood flow (RBF), glomerular filtration rate (GFR), and free water clearance [17]. It was reported that hypoxemia can affect renal vascular resistance and increase diuresis [17,28]. An experimental model of ARDS verified that renal end-organ injury can be induced through the high-volume ventilation [29,30]. Previous data analyses of ARDS co-occurring with AKI was mostly from ICU cases who had serious illness, many complications, and multiple-organ damage [17,31], and the survival rate was obviously decreased. In the present study, therefore, we developed and assessed a risk scoring system to predict development of AKI and prognosis for patients with ARDS.
In this work, a risk scoring system for AKI, which included multiple risk factors and assessed adverse events, was developed in AKI patients. The relevant clinical data required for this scoring system can be easily obtained, which would helpful predict prognosis of AKI patients. Furthermore, the prognostic stratification may inform individualized management involving assessment of the need for intensive medical therapy and close follow-up. Thus, it is of clinical significance to improve the compliance of high-risk patients by enhancing awareness using the risk scoring system.
A limitation of the present study is that the scoring system is only appropriate for AKI patients, and whether it would be applicable for other patients needs further study. The scoring system was developed and assessed at 2 hospitals, and whether it is applicable in other settings is unknown. As with all retrospective studies, there may have been some incompletely collected data and some missing values, and there was only 1 indicator of liver function. Thus, multicenter studies with larger samples and including multiple diseases and hemodynamic indicators are needed for further verification of this scoring system in clinical practice.
Conclusions
We analyzed risk factors in the development and prognosis of AKI in patients with ARDS, and developed a clinical risk prediction scoring system for AKI patients. The scoring system may provide risk-integrative evaluation and prognostic stratification for AKI patients.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (No. 81670634), the Natural Science Foundation of Jiangsu Province (BK20171485), and the 333 Project in Jiangsu Province
Conflicts of interest
None.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, et al. Epidemiology of acute kidney injury in critically Ill children and young adults. New Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuk A, Bonventre JV. Acute kidney injury. Annu Rev Med. 2016;67:293–307. doi: 10.1146/annurev-med-050214-013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015;87:62–73. doi: 10.1038/ki.2014.328. [DOI] [PubMed] [Google Scholar]
- 4.Qin N, Cai T, Ke QQ, et al. UCP2-dependent improvement of mitochondrial dynamics protects against acute kidney injury. J Pathol. 2019;247:392–405. doi: 10.1002/path.5198. [DOI] [PubMed] [Google Scholar]
- 5.Liang JY, Lin GF, Tian JW, et al. Measurement of urinary matrix metalloproteinase-7 for early diagnosis of acute kidney injury based on an ultrasensitive immunomagnetic microparticle-based time-resolved fluoroimmunoassay. Clin Chim Acta. 2019;490:55–62. doi: 10.1016/j.cca.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 6.Jamme M, Salem OB. Is fluid resuscitation the “Keyser Soze” of acute kidney injury in trauma patients? Crit Care. 2019;23:35–36. doi: 10.1186/s13054-019-2333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamada H, Doi K, Tsukamoto T, et al. Low-dose atrial natriuretic peptide for prevention or treatment of acute kidney injury: A systematic review andmeta-analysis. Crit Care. 2019;23:41–53. doi: 10.1186/s13054-019-2330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahu AK, Verma VK, Mutneja E, et al. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol Cell Biochem. 2019;452:141–52. doi: 10.1007/s11010-018-3420-y. [DOI] [PubMed] [Google Scholar]
- 9.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. New Engl J Med. 2018;378:1965–75. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA, Sileanu FE, Bihorac A, et al. Recovery after acute kidney injury. Am J Resp Crit Care. 2017;195:784–91. doi: 10.1164/rccm.201604-0799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugan R, Kellum JA. Acute kidney injury: What’s the prognosis? Nat Rev Nephrol. 2011;7:209–17. doi: 10.1038/nrneph.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome advances in diagnosis and treatment. Jama-J Am Med Assoc. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 13.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Fan E, Camporota L, et al. Erratum to: The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intens Care Med. 2012;38:1731–32. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu KD, Matthay MA. Advances in critical care for the nephrologist: Acute lung injury/ARDS. Clin J Am Soc Nephrol. 2008;3:578–86. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooke CR, Kahn JM, Caldwell E, et al. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit Care Med. 2008;36:1412–20. doi: 10.1097/CCM.0b013e318170a375. [DOI] [PubMed] [Google Scholar]
- 17.Darmon M, Clec’h C, Adrie C, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9:1347–53. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RL, Pascual MT, Gruta CG, et al. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–57. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 19.Fliser D, Laville M, Covic A, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27:4263–72. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi Y, Takahashi J, Yasuda S, et al. Prognostic stratification of patients with vasospastic angina: A comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J Am Coll Cardiol. 2013;62:1144–53. doi: 10.1016/j.jacc.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Ashbaugh D, Bigelow DB, Petty T, et al. ARDS acute respiratory distress in adults. Lancet. 1967;290:319–23. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 22.Mac Sweeney R, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–30. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laffey JG, Misak C, Kavanagh BP. Acute respiratory distress syndrome. BMJ. 2017;359:j5055. doi: 10.1136/bmj.j5055. [DOI] [PubMed] [Google Scholar]
- 24.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. New Engl J Med. 2017;377:562–72. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 25.Klein SJ, Brandtner AK, Lehner GF, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: A systematic review and meta-analysis. Intensive Care Med. 2018;44:323–36. doi: 10.1007/s00134-018-5126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intens Care Med. 2015;41:1411–23. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 27.Iglesias J, Marik PE, Levine JS. Elevated serum levels of the type I and type II receptors for tumor necrosis factor-alpha as predictive factors for ARF in patients with septic shock. Am J Kidney Dis. 2003;41:62–75. doi: 10.1053/ajkd.2003.50024. [DOI] [PubMed] [Google Scholar]
- 28.Darmon M, Schortgen F, Leon R, et al. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intens Care Med. 2009;35:1031–38. doi: 10.1007/s00134-008-1372-5. [DOI] [PubMed] [Google Scholar]
- 29.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–48. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 30.Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–12. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 31.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: Challenges and opportunities. Lancet Respir Med. 2017;5:524–34. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]


