Abstract
A 39-year-old woman with a history of Roux-en-Y gastric bypass (RYGB) surgery and alcohol use presented with a confluent erythematous rash involving the perineum spreading outward to the abdomen, thighs and lower back. She had angular cheilitis and glossitis. The rash was painful and blistering in scattered areas. She was hypotensive and appeared to be in septic or hypovolemic shock at presentation. Serum levels of zinc and vitamin B6 were critically low and biopsy of her rash returned suggestive of a nutritional deficiency as its source. The rash slowly improved over the following 2 weeks with oral zinc and vitamin B6 replacement. The body rash resembled that of infants born with inherited defects in zinc transporters, referred to as acrodermatitis enteropathica (AE). This case may represent an acquired case of AE in the setting of prior RYGB.
Keywords: nutrition, vitamins and supplements
Background
Bariatric surgeries can be broadly classified as restrictive or malabsorptive. Roux-en-Y gastric bypass (RYGB) surgery is both a restrictive and malabsorptive procedure. It involves creation of a bypass around the duodenum and most of the jejunum, sites where the majority of vitamin and mineral absorption occur.1 Following the procedure, patients are required to supplement vitamins and micronutrients for life. Even with supplementation, patients are at risk for deficiencies of vitamin B12, folate, iron, zinc and many of the B vitamins.2 Deficiencies of zinc, selenium, vitamin B2 and vitamin B6 are associated with skin rashes.1 3
Zinc deficiency can be either genetic or acquired. The genetic form of zinc deficiency is marked by the development of a large erythematous and eczematous rash involving the perineum, extremities and periorificial area within the first few days of life. The clinical syndrome is referred to as acrodermatitis enteropathica (AE).4 Acquired zinc deficiency occurs in the setting of malabsorption or with increased utilisation of zinc. Patients at risk for malabsorption include those with inflammatory bowel disease, celiac disease, chronic diarrhoea and those who have had surgical resection of the bowel or gastric bypass surgery.4 Wasting of zinc has been shown to occur in the setting of alcohol abuse, systemic infection, burns and neoplasms.1 4 Mahawar et al reported that up to 68% of gastric bypass surgery patients become deficient in zinc.3 The deficiency is asymptomatic in the majority of cases. In those who acquire symptomatic deficiency, skin changes and hair loss are common.
Deficiency of vitamin B6 rarely occurs in isolation and is often identified with other B vitamin deficiencies.5 Low vitamin B6 is associated with renal disease, malabsorption, medication side effects and alcohol use.5 6 This deficiency is associated with angular cheilitis, glossitis, cognitive symptoms and reduced immunity.6 There is also an established association between chronic alcohol abuse and vitamin B6 deficiency due to altered production by elevated acetaldehyde levels.
Case presentation
A 39-year-old woman presented with a painful skin rash. She had a past medical history of RYGB surgery in 2012 (6 years before presentation), chronic hepatitis B and hepatitis C infection and chronic pain. She had lost near 400 lbs following her surgery and reported herself to be compliant with her vitamin replacement therapy. Her social history was relevant for ongoing alcohol abuse and chronic daily opioid use of up to 90 mg of oxycodone daily. She had also been previously treated for a prior subdural haemorrhage with a ventriculoperitoneal (VP) shunt due to a mechanical fall 3 years prior to presentation. She had been admitted to a separate local hospital within the preceding month due to concern for malfunction of the shunt and discharged without surgical intervention. She denied new drug exposures during that hospital stay.
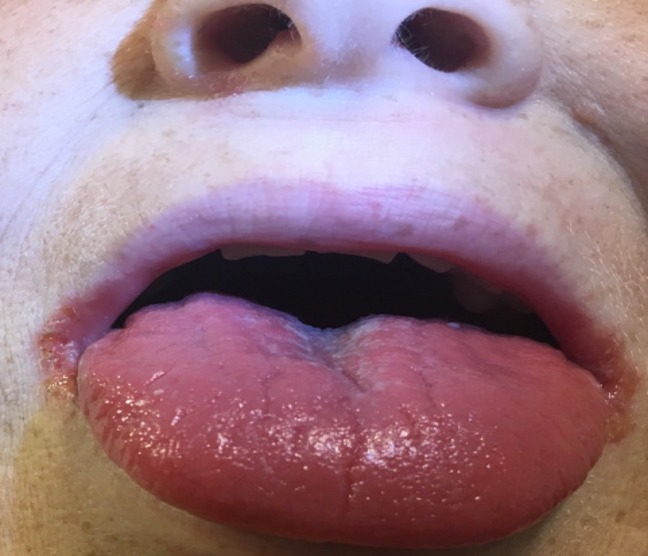
Two weeks later, she presented to our emergency department with 5 days of worsening red rash covering her abdomen, thighs and perineum. She was brought in by her mother due to increasing disorientation, hallucinations and multiple falls in the preceding 2 days. She reported worsening dizziness and progressive darkening of her urine as well. She described exquisite pain and tenderness at the sites where her rash was located. She also reported pain within her mouth and at the corners of her mouth. Her examination revealed angular cheilitis and redness of the tongue (figure 1). Her rash could be described as erythematous, confluent, maculopapular and blanchable (figure 2). It was present on her trunk, abdomen, genitalia, buttock, lower back and proximal thighs. The rash was inflamed and tender to the touch.
Figure 1.
angular chelitis and erythematous tongue.
Figure 2.
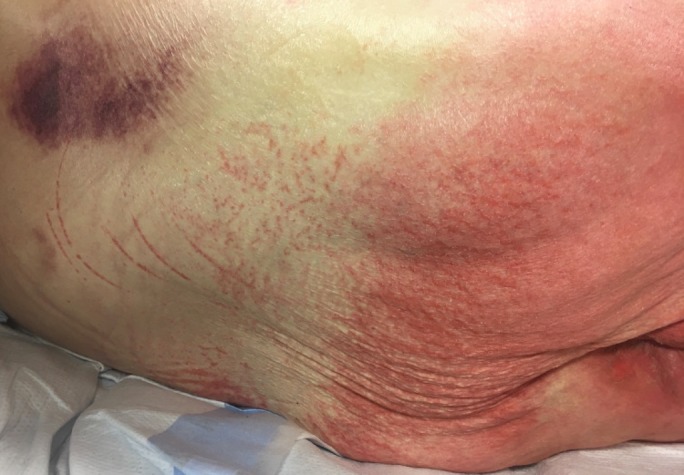
Diffuse erythematous rash involving abdomen, perineum and thighs.
She was hypotensive at presentation without tachycardia or fever and her blood pressure improved with fluid resuscitation. Her leucocyte count was within normal range. She had evidence of a consumptive coagulopathy based on thrombocytopenia (85000), low fibrinogen (120) and prolonged international normalized ratio (INR). She had low haptoglobin, elevated indirect bilirubin and elevated lactate dehydrogenase consistent with ongoing haemolysis and her peripheral smear revealed intermittent schistocytes. She had acute liver injury with AST 54, ALT 24, total bilirubin 6.4, direct bilirubin 3.2. Her lipase was 4 and amylase not measured. Vitamin levels were assessed on admission and were notable for low zinc at 24 (normal range 60–120), low vitamin B6 at <5 (normal range 20–125), low vitamin C at 19 (normal range 23–114), low vitamin A at <0.06 (normal range 0.3–1.2) and low vitamin B1 at 65 (normal range 70–180).
After initially receiving care at the general medicine ward, her hypotension worsened with simultaneous worsening encephalopathy and acute kidney injury. She only partially stabilised with fluids and required transfer to the medical intensive care unit to receive intravenous pressor support.
Investigations
After admission, dermatology and infectious disease specialists were consulted. Both consultants initially thought her presentation was consistent with toxic shock syndrome. Antibiotics were initiated empirically. Dermatology performed a punch biopsy of her rash but the results did not return for several days. Transthoracic echocardiogram did not show evidence of a vegetation and her cardiac function appeared normal. Blood cultures were obtained on admission and at the time of worsening hypotension and did not identify a clear microbial pathogen. Review of a peripheral blood smear confirmed the presence of schistocytes but haematology felt a primary hematologic process was unlikely.
Differential diagnosis
Based on her initial presentation with a large, red rash in conjunction with multiorgan failure and hypotension, there was concern for toxic shock syndrome. Factors arguing against this diagnosis were the absence of an indwelling nidus for infection or recent surgical procedure to serve as a source for pathogen entry. Sepsis was considered and empirically treated but no clear source for infection was identified. Her VP shunt was felt to be in good condition and both infectious disease and neurosurgical consultants did not suspect a hardware infection based on her clinical presentation.
For her rash, we initially considered diffuse causes of rash and volume loss such as Steven-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and the toxic shock syndrome as mentioned above. She had no new drug exposures to raise suspicion for SJS or TEN. We initially considered vitamin deficiency from her gastric bypass surgery as a cause for the rash. However, her surgery was several years prior and she had no history of a similar rash, leaving question as to why she would develop an acute presentation due to a vitamin deficiency at the present time.
Autoimmune causes of red rash and systemic illness such as vasculitis or cryoglobulinemia were considered in the setting of untreated hepatitis C. However, the clinical presentation and biopsy findings were inconsistent with a vasculitis picture. Her serum cryoglobulins were negative as well.
Treatment
She was started on an intravenous multivitamin, thiamine and folate. Dermatology advised empiric replacement of zinc and vitamin B6 based on the appearance of the rash. Zinc was provided orally at 220 mg daily and vitamin B6 at 25 mg daily orally.
Outcome and follow-up
About a week after her initial admission, the results of her skin biopsy returned without evidence of toxic shock syndrome. The pathology report described confluent hyperkeratosis, hypogranulosis, severe epidermal atrophy, mild spongiosis and superficial perivascular lymphocytic infiltrate. No bacterial or fungal organisms were seen. The findings were reportedly not specific but could be associated with a vitamin deficiency. In conjunction with her vitamin deficiencies found on admission, a combined deficiency of zinc and vitamin B6 were suspected to be the cause.
After about 14 days her rash began to dissipate and she clinically stabilised. By the time of discharge, the areas of redness had receded significantly, and the remaining rash was significantly less erythematous. She was discharged to a subacute rehab and subsequently deceased due to unknown causes. Records were not available to confirm cause of death.
Discussion
We believe many of the features in this presentation were suggestive of a combined vitamin deficiency of B6 and micronutrient deficiency of zinc. The large rash on the perineum and trunk resembled the presentation described in infants with congenital zinc deficiency referred to as AE. The rash appeared to spread outward per her history and on examination from the perineum to the abdomen and thighs with some areas appearing seborrheic and others showing signs of desquamation. Since her zinc deficiency developed in the context of a prior RYGB procedure, this may represent an example of acquired AE. In our review, we identified just three similar cases. One group described acquired AE in a teenager with anorexia and zinc deficiency.7 Another reported acquired AE in a vegan patient with pancreatic insufficiency presenting with a full body rash.8 Lastly, Mankaney et al published a case very similar to ours.9 Theirs was a patient who had undergone previous bariatric surgery and presented with a rash on the trunk. In contrast to our case, the patient they describe was much less acutely ill and had been dealing with the rash at home for several months. The patient in our case was critically ill. Her presentation led the primary team and multiple specialists to suspect an infectious or autoimmune process as the driver of her condition. We cannot rule out additional contributing factors to her shock-like presentation. Her multiorgan dysfunction and acute encephalopathy are also frequently seen in patients with systemic infection. However, after biopsy results returned, there did not appear to be a more likely cause for the skin manifestations.
After undergoing RYGB, patients are provided dietary instructions and also advised to supplement iron, vitamin B12 and B-complex vitamins. Cambi et al recently published a Plate Model Template to be used by patients after bariatric surgery. Their review summarises the sources for micronutrients and protein and describes individual roles for each micronutrient within the body.10 In addition to malabsorption as a cause for micronutrient deficiencies, post RGBY patients have been shown to consume inadequate amounts of many micronutrients following surgery.11 Gesquiere recently prospectively followed the intake of commonly deficient micronutrients in this group and showed that with supplementation most patients consume adequate intake by 1 year after surgery. However, they noted that intake of zinc did not normalise by 1 year even with supplementation. Zinc levels may be low in up to 68% of RYGB patients but it is rare for patients to be symptomatic.3 In a meta-analysis, Mawahar et al reviewed more than 1000 cases and only identified 6 cases of symptomatic deficiency. Among those who were symptomatic, all displayed findings related to the skin. Their review also included the time frame to presentation following bypass surgery, and interestingly, the average presentation was 6 years after surgery, with the longest interval to presentation being 10 years. Most patients reportedly improved quickly after providing oral zinc, but one patient required intravenous therapy to recover. In the case we presented, the presentation approximately 5 years after bypass surgery is consistent with this data but the recovery of her rash was delayed, taking more than 2 weeks to show significant signs of improvement. Another factor which likely contributed to zinc deficiency in our case was ongoing alcohol abuse prior to admission. Alcohol abuse is known to contribute to acquired zinc deficiency through increased wasting of zinc in urine.1 12 In addition to zinc, other features of her presentation are seen with vitamin B6 deficiency.
The most noteworthy risk for B6 deficiency in her case was chronic and possibly heavy alcohol use. Her angular cheilitis and glossitis are changes described in B6 deficiency.6 Interestingly, vitamin B6 has an association with cognitive symptoms such as depression and even with psychotic features such as hallucinations, which our patient exhibited.13 14 Her altered mentation may have also been multifactorial and worsened by ongoing alcohol and narcotic use prior to admission.
When evaluating a patient with prior RYBG with a rash, clinicians should have a low threshold to measure levels of zinc and vitamin B6. Positive screening for alcohol use in this population should further prompt clinicians to evaluate for deficiencies. The combination of significant alcohol use, poor oral nutrition and suspected inconsistent compliance with vitamin replacement were likely contributors to this presentation. Moving into the future, bariatric surgery will continue to be a competing intervention for management of obesity. Physicians will benefit from recognising this population as being at risk for a variety of vitamin and nutrient deficiencies by virtue of both reduced consumption and malabsorption when pertinent to the specific procedure.
Learning points.
Zinc deficiency may present in an adult with an inflammatory rash starting in the genital region and spreading to the abdomen and proximal legs.
Symptomatic zinc deficiency is rare and may occur years following gastric bypass surgery without clear provoking factors.
Alcohol use may worsen both vitamin B6 and zinc deficiencies.
Clinicians should consider measurement of zinc and vitamin B6 levels in patients presenting with body rash, angular cheilitis and cognitive symptoms.
Footnotes
Contributors: JF authored the primary version of the paper and performed a literature review to gather sources and confirm our findings. PB contributed edits to the primary version, participated in literature review and prepared the bibliography, reviewed the medical record and compiled clinical information relevant to the case. RC is the senior author who guided preparation of the initial draft, editing of the manuscript and then finalised the manuscript in the form which is being submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Manzoni AP, Weber MB. Skin changes after bariatric surgery. An Bras Dermatol 2015;90:157–66. 10.1590/abd1806-4841.20153139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clements RH, Katasani VG, Palepu R, et al. Incidence of vitamin deficiency after laparoscopic Roux-en-Y gastric bypass in a university hospital setting. Am Surg 2006;72:1196–202. [DOI] [PubMed] [Google Scholar]
- 3. Mahawar KK, Bhasker AG, Bindal V, et al. Zinc deficiency after gastric bypass for morbid obesity: a systematic review. Obes Surg 2017;27:522–9. 10.1007/s11695-016-2474-8 [DOI] [PubMed] [Google Scholar]
- 4. Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007;56:116–24. 10.1016/j.jaad.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 5. McCormick D. Vitamin B6 : Bowman B, Russell R, Present knowledge in nutrition. 9th edn Washington, DC: International Life Sciences Institute, 2006. [Google Scholar]
- 6. Institute of Medicine. Food and nutrition board. dietary reference intakes: thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC: National Academy Press, 1998. [PubMed] [Google Scholar]
- 7. Kelly S, Stelzer JW, Esplin N, et al. Acquired acrodermatitis enteropathica: a case study. Cureus 2017;9:e1667 10.7759/cureus.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol 2010;37:726–9. 10.1111/j.1346-8138.2010.00835.x [DOI] [PubMed] [Google Scholar]
- 9. Mankaney GN, Vipperla K. Acquired acrodermatitis enteropathica. N Engl J Med Overseas Ed 2014;371:67 10.1056/NEJMicm1312911 [DOI] [PubMed] [Google Scholar]
- 10. Cambi MPC, Baretta GAP. Bariatric diet guide: plate model template for bariatric surgery patients. Arq Bras Cir Dig 2018;31:e1375 10.1590/0102-672020180001e1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gesquiere I, Foulon V, Augustijns P, et al. Micronutrient intake, from diet and supplements, and association with status markers in pre- and post-RYGB patients. Clin Nutr 2017;36:1175–81. 10.1016/j.clnu.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 12. Jen M, Yan AC. Syndromes associated with nutritional deficiency and excess. Clin Dermatol 2010;28:669–85. 10.1016/j.clindermatol.2010.03.029 [DOI] [PubMed] [Google Scholar]
- 13. Petrilli MA, Kranz TM, Kleinhaus K, et al. The emerging role for zinc in depression and psychosis. Front Pharmacol 2017;8:414 10.3389/fphar.2017.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerner V, Miodownik C, Kaptsan A, et al. Vitamin B6 as add-on treatment in chronic schizophrenic and schizoaffective patients: a double-blind, placebo-controlled study. J Clin Psychiatry 2002;63:54–8. [DOI] [PubMed] [Google Scholar]