Abstract
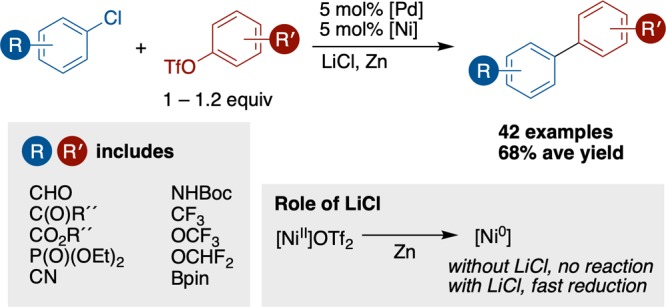
While the synthesis of biaryls has advanced rapidly in the past decades, cross-Ullman couplings of aryl chlorides, the most abundant aryl electrophiles, have remained elusive. Reported here is the first general cross-Ullman coupling of aryl chlorides with aryl triflates. The selectivity challenge associated with coupling an inert electrophile with a reactive one is overcome using a multimetallic strategy with the appropriate choice of additive. Studies demonstrate that LiCl is essential for effective cross-coupling by accelerating the reduction of Ni(II) to Ni(0) and counteracting autoinhibition of reduction at Zn(0) by Zn(II) salts. The modified conditions tolerate a variety of functional groups on either coupling partner (42 examples), and examples include a three-step synthesis of flurbiprofen.
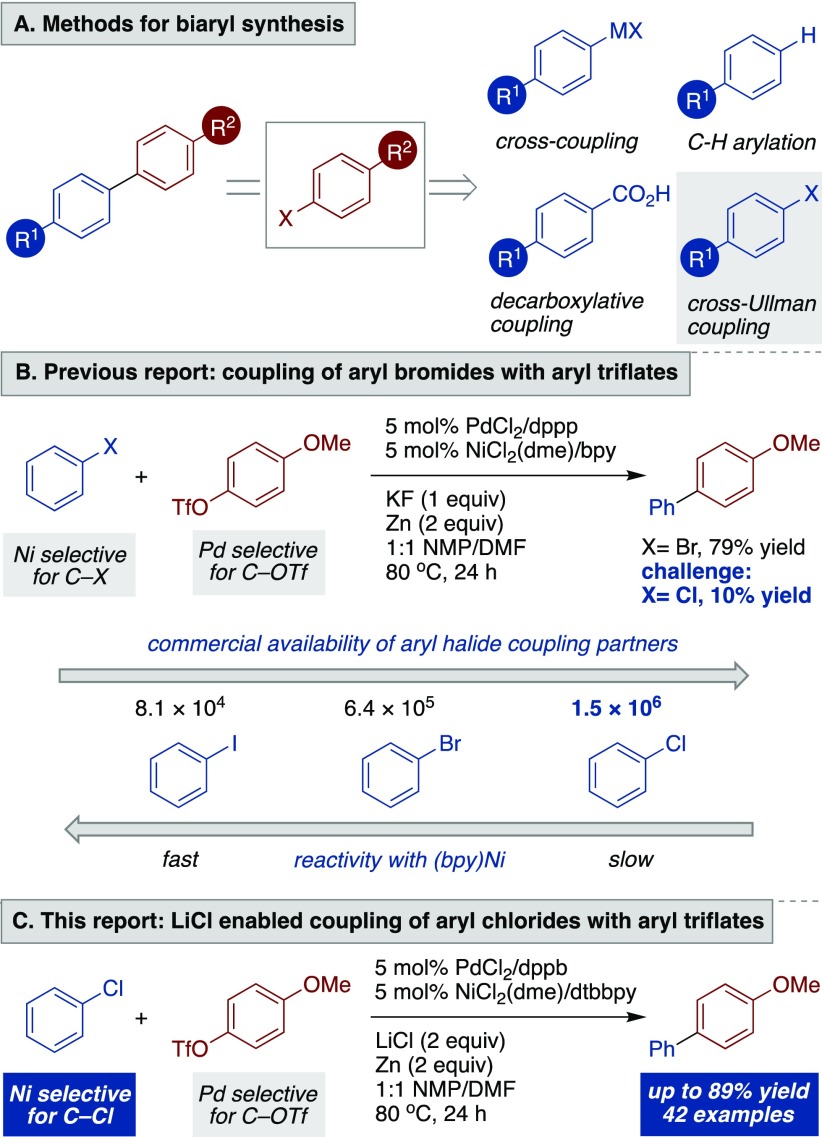
The synthesis of biaryls has become one of the most commonly used reactions in pharmaceutical, agrochemical, and materials science industries,1 yet access to arylmetal reagents remains limiting. The low commercial availability of arylmetal reagents has inspired a number of active areas of research (Scheme 1A), including improved methods for arylmetal synthesis,2 C–H arylation,3 and decarboxylative cross-coupling.4
Scheme 1. Cross-Ullman Reaction in Biaryl Synthesis.
The relative abundance of aryl electrophiles (Scheme 1B5) would make the cross-Ullman reaction6,7 an attractive approach, but our recently reported catalytic nickel and palladium method was not broadly effective with the most abundant and versatile aryl electrophiles, aryl chlorides.8 In addition to opening up more chemical space, aryl chlorides are often lower in cost, and their lower reactivity would allow for sequential coupling in fragment-based drug discovery9 or late-stage coupling on complex molecules.10
Although significant advances in the use of aryl chlorides in cross-coupling have been made recently,7c,11−14there are no general methods for the direct cross-coupling of electron-neutral or electron-rich aryl chlorides with other aryl electrophiles.15,16 In our prior report, we established that in order to promote a successful cross-Ullman reaction, the electrophiles employed had to be orthogonally paired in reactivity: the Ni catalyst activated aryl bromides at a higher rate than aryl triflates; the Pd catalyst activated aryl triflates at a higher rate than aryl bromides. When sufficiently electron-deficient aryl chlorides were substituted for aryl bromides, they were still activated enough to maintain catalyst selectivity. However, when less activated aryl chlorides were used, poor results were obtained. Preliminary studies attempting to couple more electron-rich chlorides with aryl triflates led to production of the triflate-derived dimer and incomplete conversion of both the aryl chloride and the aryl triflate. Herein we report a general multimetallic solution that achieves the selective coupling of a variety of aryl chlorides with aryl triflates (Scheme 1C).
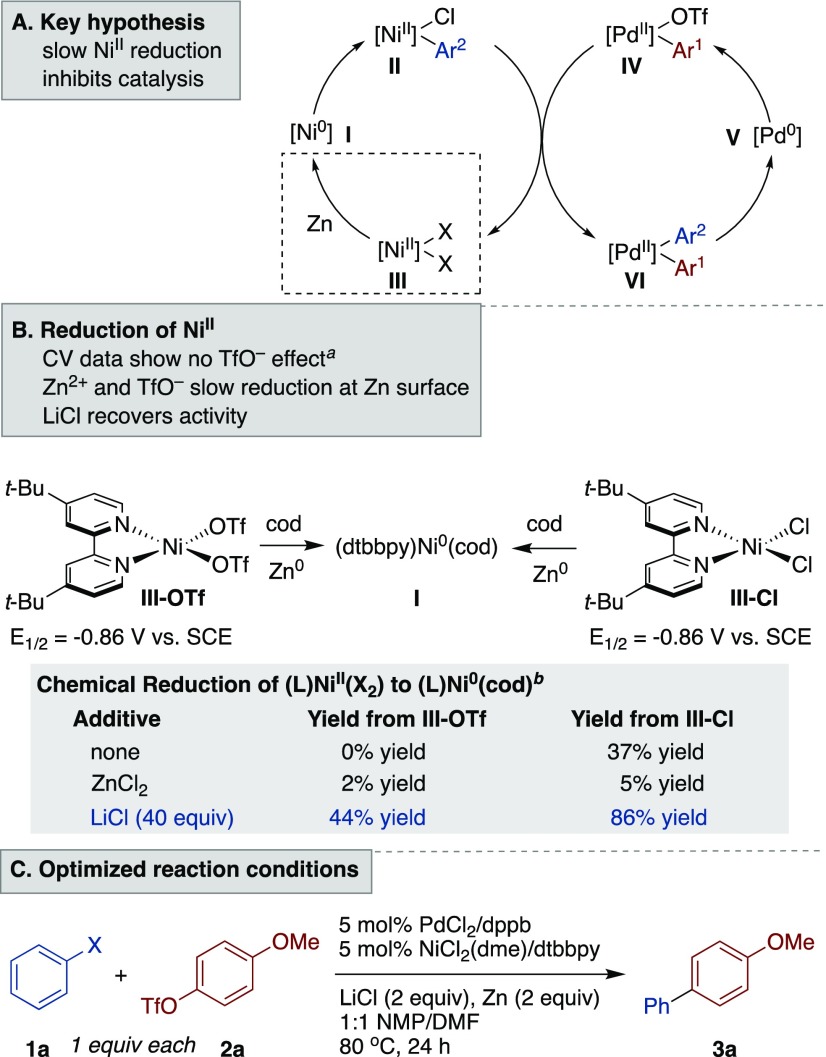
On the basis of the mechanism proposed in our earlier studies with nickel and palladium multimetallic catalysis (Table 1A),8 the slow consumption of the aryl chloride and aryl triflate suggested that arylnickel (II) formation was being inhibited (Table 1A). Arylpalladium (IV) will not consume aryl triflate without arylnickel (II) present. The inhibition could arise from slow oxidative addition (I to II),17 slow reduction (III to I), or an off-cycle loss of nickel catalyst.
Table 1. Mechanistic Study and Optimization of the Ar–Cl Cross-Ullman Reaction.
| entry | change from the optimized conditionsc | 3a (%)d |
|---|---|---|
| 1 | none | 84 |
| 2 | NaCl instead of LiCl | 62 |
| 3 | LiBr instead of LiCl | 59 |
| 4 | Bu4NCl instead of LiCl | 53 |
| 5 | TMSCl instead of LiCl | 16 |
| 6 | no LiCl | <10 |
| 7 | Mn instead of Zn | 62 |
| 8 | Mn instead of Zn, LiBr instead of LiCl | 77 |
| 9 | without PdCl2 and dppb | 44 |
| 10 | without NiCl2(dme) and dtbbpy | <5 |
| 11 | reaction set up on the benchtope | 80 |
| 12 | 1.2 equiv of 2a | 90 (89f) |
In DMF. See the Supporting Information for details on electrochemical studies.
Reduction of III was conducted in DMF at a concentration of 0.025 M with Zn powder (40 equiv). Cyclooctadiene (0.125 M) was added to stabilize the product. Salts (1–40 equiv) were added in some cases. See the Supporting Information for additional results and experimental details.
Reactions were run on a 0.5 mmol scale in 2 mL of solvent. NMP = N-methyl-2-pyrrolidinone.
GC yield vs dodecane as an internal standard.
The reaction was set up under air with dry solvent.
Isolated yield.
Reduction of the (dtbbpy)NiIIX2 complexes III-Cl and III-OTf was studied by both electrochemical and chemical methods (Table 1B). Cyclic voltammetry (CV) studies, which are commonly used to assess the ease of reduction of metal complexes,18 showed no difference between III-Cl and III-OTf (Table 1B and Figures S7 and S8 in the Supporting Information). While CV provides information on the thermodynamic driving force for a reduction, it does not account for the complex kinetic picture of reduction at a metal surface.19,20 Indeed, the reduction of complexes III-OTf and III-Cl over zinc flakes in the presence or absence of additives showed that III-OTf is not reduced unless chloride salts are present (Table 1B,C, Figures S8 and S9, and Table S2). There is also a cation effect: while LiCl enhances the rate of reduction of both nickel complexes III-OTf and III-Cl, ZnCl2 does not. In fact, zinc chloride and zinc triflate, salts formed during the reaction, inhibit reduction of (dtbbpy)NiIICl2 (37% yield with no salt, 2–5% yield with 1 equiv of ZnCl2 or Zn(OTf)2). Lithium chloride21 can overcome zinc inhibition and was generally the most useful additive studied (Table 1, entries 1–6, and Table S2).22,23 While we found that reduction of octyl bromide to octylzinc bromide was also inhibited by zinc salts,21d,24 reduction of palladium(II) phosphine complexes to palladium(0) was fast with or without added LiCl or Zn (Figures S11–S16).25,26
These studies show that the low reactivity observed for the coupling of aryl chlorides with aryl triflates (Scheme 1B) is due to autoinhibition: the zinc salts (ZnCl2 and Zn(OTf)2) formed in the reduction of III to I inhibit subsequent reductions of III. While it had previously been noted that halide anions accelerate reduction of NiX2 intermediates at zinc surfaces,27,28 the inhibitory effect of less-coordinating anions (OTf–, BF4–, PF6–)29 and zinc salts has not been previously reported. This result has broad implications for cross-electrophile coupling reactions that rely upon heterogeneous metallic reductants.
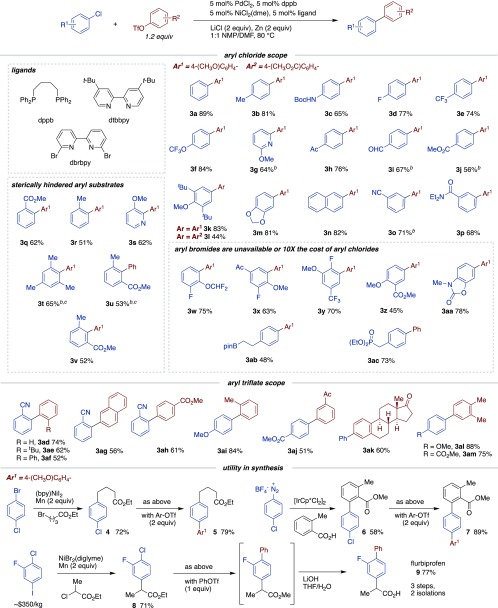
The catalytic reaction behaved as expected from the stoichiometric studies: the addition of LiCl enabled turnover (Table 1C, entries 1–6, and Figures S1 and S2).30 Consistent with previous reports,8 these reactions were still promoted by the cooperativity between the two metal catalysts: reactions without palladium were poorly selective, and reactions without nickel did not consume starting materials (Table 1, entries 9 and 10). Similar to other cross-electrophile coupling reactions,28a the reaction was tolerant of adventitious O2, allowing reactions to be set up on the benchtop (Table 1, entry 11), although O2 in the reaction headspace resulted in an induction period (Figure S6).31 Both Zn and Mn could be utilized as reductants. As in our previous report, LiBr was superior to LiCl with Mn (Table 1, entries 7 and 8, and Figure S5).20a Finally, while dtbbpy and dppb were generally the best pair of ligands for this coupling, PCy3 was also effective (Figures S2 and S3). While 6,6′-dibromo-2,2′-bipyridine was not an effective ligand for the model reaction, it was effective for couplings of electron-poor aryl chlorides (Scheme 2).
Scheme 2. Reaction Scope and Applications.
Reactions were run on a 0.5 mmol scale in 2 mL of solvent for 2–24 h.
Using 5 mol % 6,6′-dibromo-2,2′-bipyridine instead of dtbbpy.
The aryl bromide was used instead of the aryl chloride.
With these modified reaction conditions and an effective way to promote aryl chloride reactivity, we examined the couplings of a variety of aryl chlorides and triflates containing an array of functional groups and steric environments (Scheme 2). Electron-poor fluorine-containing substrates as well as electron-neutral and electron-rich substrates were well-tolerated, including sensitive functionalities such as a Boc-protected amine (3c), an aldehyde (3i), an alkyl Bpin ester (3ab), and a phosphonate ester (3ac). More reactive aryl halides, such as aryl chlorides bearing strongly electron-withdrawing groups, heteroaryl halides, or aryl bromides, could be selectively coupled with an aryl triflate by the use of the hindered, electron-poor ligand 6,6′-dibromo-2,2′-bipyridine (3g, 3i, 3j, 3o, 3t, and 3u). Under these reaction conditions, ortho-substituted (3q–s) and 2,6-disubstituted aryl bromides and chlorides (3t–v) were also coupled efficiently. In contrast, steric hindrance was not as well tolerated in our previous report.32,33 The ability to couple unactivated aryl chlorides can be beneficial in synthesis when the corresponding aryl bromide is either more expensive or not commercially available (3w–ac). The most challenging combination was electron-rich aryl chlorides with electron-poor aryl triflates (3l), which suffered from lower selectivity (about 2.5:1 biaryl to product).
The scope of the aryl triflate was also examined (Scheme 2), demonstrating good yields with both electron-donating and electron-withdrawing substituents (3ad–am). The lower yields observed for the coupling of electron-poor aryl triflates with electron-poor aryl chlorides (3ah and 3aj) were due to competing homodimer formation. In these cases, the use of 6,6′-dibromo-2,2′-bipyridine as the ligand did not improve the yield. The couplings with 2-cyano-1-chlorobenzene form biaryls that could be useful for the synthesis of angiotensin II receptor antagonists (3ad–ah).34
Besides their improved availability and lower cost, an additional benefit of using aryl chlorides is that their lower reactivity facilitates multistep synthesis (Scheme 2). For example, cross-electrophile coupling with an alkyl bromide (5), C–H arylation (7), and reductive α-arylation (9) can all be conducted while preserving the C–Cl bond.35 As an example of how this can be applied in synthesis, a concise three-step synthesis of flurbiprofen (9) was demonstrated that would be amenable to rapid analogue synthesis.36
This report shows how the nickel and palladium system can be rationally modulated to couple less reactive substrates: an unselective multimetallic reaction was made selective with the use of an additive, LiCl, that facilitates the reduction of the nickel catalyst at the zinc surface. Combined with our previous reports, these results suggest that the Ni/Pd system is general and that multimetallic catalysis may have broad generality. Finally, this work demonstrates how reactivity in cross-electrophile coupling reactions can be influenced by the reductant choice as much as the ligand choice: salts formed in the reaction may be autoinhibitory, and new reductant combinations can unlock new reactivity.
Acknowledgments
The authors gratefully acknowledge funding from the NIH NIGMS (R01GM097243 to D.J.W.), the NSF (NSF DGE-1419118 to A.M.P. and L.K.G.A.), and SIOC (fellowship to K.K.). D.J.W. is a Camille Dreyfus Teacher-Scholar. Additional funding from Novartis and Boehringer Ingelheim is also gratefully acknowledged. We thank Amanda Spiewak (University of Wisconsin) for assistance in the Pd complex reduction study, Yixing Guo and Prof. Kara Bren (University of Rochester) for assistance in obtaining CV data, and Prof. Alison R. Fout for a helpful discussion on the mechanism of action for LiCl.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b05461.
Materials, methods, compound characterization, and supplementary figures, schemes, and tables (PDF)
Author Present Address
§ L.H.: Department of Chemistry, South China University of Technology, Guangzhou, Guangdong 510641, China.
Author Present Address
∥ L.K.G.A.: Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- a Magano J.; Dunetz J. R. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. 10.1021/cr100346g. [DOI] [PubMed] [Google Scholar]; b Busacca C. A.; Fandrick D. R.; Song J. J.; Senanayake C. H. The Growing Impact of Catalysis in the Pharmaceutical Industry. Adv. Synth. Catal. 2011, 353, 1825–1864. 10.1002/adsc.201100488. [DOI] [Google Scholar]; c Roughley S. D.; Jordan A. M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- a Knochel P.Handbook of Functionalized Organometallics: Applications in Synthesis; Wiley-VCH: Weinheim, Germany, 2005; p 653. [Google Scholar]; b Mkhalid I. A. I.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. C–H Activation for the Construction of C–B Bonds. Chem. Rev. 2010, 110, 890–931. 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]; c Cheng C.; Hartwig J. F. Catalytic Silylation of Unactivated C–H Bonds. Chem. Rev. 2015, 115, 8946–8975. 10.1021/cr5006414. [DOI] [PubMed] [Google Scholar]
- a Ackermann L.Modern Arylation Methods; Wiley-VCH: Weinheim, Germany, 2009; p 543. [Google Scholar]; b Ackermann L.; Vicente R.; Kapdi A. R. Transition-Metal-Catalyzed Direct Arylation of (Hetero)Arenes by C–H Bond Cleavage. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. 10.1002/anie.200902996. [DOI] [PubMed] [Google Scholar]; c McGlacken G. P.; Bateman L. M. Recent advances in aryl–aryl bond formation by direct arylation. Chem. Soc. Rev. 2009, 38, 2447–2464. 10.1039/b805701j. [DOI] [PubMed] [Google Scholar]; d Alberico D.; Scott M. E.; Lautens M. Aryl–Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev. 2007, 107, 174–238. 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- a Goossen L. J.; Deng G.; Levy L. M. Synthesis of Biaryls via Catalytic Decarboxylative Coupling. Science 2006, 313, 662–664. 10.1126/science.1128684. [DOI] [PubMed] [Google Scholar]; b Dzik W. I.; Lange P. P.; Goossen L. J. Carboxylates as sources of carbon nucleophiles and electrophiles: comparison of decarboxylative and decarbonylative pathways. Chem. Sci. 2012, 3, 2671–2678. 10.1039/c2sc20312j. [DOI] [Google Scholar]
- Commercial availability of different arene sources: ArB(OH)2 (8110); ArI (64 259); ArCO2H (198 638); ArOH (279 487), ArBr (541 596); ArCl (1 392 569). Source: eMolecules database, accessed March 17, 2017 using Elsevier REAXYS.
- a Ullmann F.; Bielecki J. Ueber Synthesen in der Biphenylreihe. Ber. Dtsch. Chem. Ges. 1901, 34, 2174–2185. 10.1002/cber.190103402141. [DOI] [Google Scholar]; b Fanta P. E. The Ullmann Synthesis of Biaryls, 1945–1963. Chem. Rev. 1964, 64, 613–632. 10.1021/cr60232a002. [DOI] [PubMed] [Google Scholar]; c Hassan J.; Sévignon M.; Gozzi C.; Schulz E.; Lemaire M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; d Nelson T. D.; Crouch R. D. Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction. Org. React. 2004, 63, 265–555. 10.1002/0471264180.or063.03. [DOI] [Google Scholar]
- a Wang L.; Zhang Y.; Liu L.; Wang Y. Palladium-Catalyzed Homocoupling and Cross-Coupling Reactions of Aryl Halides in Poly(ethylene glycol). J. Org. Chem. 2006, 71, 1284–1287. 10.1021/jo052300a. [DOI] [PubMed] [Google Scholar]; b Sengmany S.; Léonel E.; Polissaint F.; Nédélec J.-Y.; Pipelier M.; Thobie-Gautier C.; Dubreuil D. Preparation of Functionalized Aryl- and Heteroarylpyridazines by Nickel-Catalyzed Electrochemical Cross-Coupling Reactions. J. Org. Chem. 2007, 72, 5631–5636. 10.1021/jo070429+. [DOI] [PubMed] [Google Scholar]; c Amatore M.; Gosmini C. Efficient Cobalt-Catalyzed Formation of Unsymmetrical Biaryl Compounds and Its Application in the Synthesis of a Sartan Intermediate. Angew. Chem., Int. Ed. 2008, 47, 2089–2092. 10.1002/anie.200704402. [DOI] [PubMed] [Google Scholar]; d Qian Q.; Zang Z.; Wang S.; Chen Y.; Lin K.; Gong H. Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides. Synlett 2013, 24, 619–624. 10.1055/s-0032-1318237. [DOI] [Google Scholar]; e Sengmany S.; Vitu-Thiebaud A.; Le Gall E.; Condon S.; Léonel E.; Thobie-Gautier C.; Pipelier M.; Lebreton J.; Dubreuil D. An Electrochemical Nickel-Catalyzed Arylation of 3-Amino-6-Chloropyridazines. J. Org. Chem. 2013, 78, 370–379. 10.1021/jo3022428. [DOI] [PubMed] [Google Scholar]; f Sengmany S.; Vasseur S.; Lajnef A.; Le Gall E.; Léonel E. Beneficial Effects of Electrochemistry in Cross-Coupling Reactions: Electroreductive Synthesis of 4-Aryl- or 4-Heteroaryl-6-pyrrolylpyrimidines. Eur. J. Org. Chem. 2016, 2016, 4865–4871. 10.1002/ejoc.201600790. [DOI] [Google Scholar]
- a Ackerman L. K. G.; Lovell M. M.; Weix D. J. Multimetallic Catalysis Enabled Cross-Coupling of Aryl Bromides with Aryl Triflates. Nature 2015, 524, 454–457. 10.1038/nature14676. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Olivares A. M.; Weix D. J. Multimetallic Ni- and Pd-Catalyzed Cross-Electrophile Coupling To Form Highly Substituted 1,3-Dienes. J. Am. Chem. Soc. 2018, 140, 2446–2449. 10.1021/jacs.7b13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. E.; Coyne A. G.; Hudson S. A.; Abell C. Fragment-Based Approaches in Drug Discovery and Chemical Biology. Biochemistry 2012, 51, 4990–500. 10.1021/bi3005126. [DOI] [PubMed] [Google Scholar]
- Blakemore D. C.; Castro L.; Churcher I.; Rees D. C.; Thomas A. W.; Wilson D. M.; Wood A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. 10.1038/s41557-018-0021-z. [DOI] [PubMed] [Google Scholar]
- For heteroaryl chlorides, see:; a Gosmini C.; Lasry S.; Nedelec J.-Y.; Perichon J. Electrochemical cross-coupling between 2-halopyridines and aryl or heteroaryl halides catalysed by nickel-2,2′-bipyridine complexes. Tetrahedron 1998, 54, 1289–1298. 10.1016/S0040-4020(97)10225-3. [DOI] [Google Scholar]; b Gosmini C.; Nédélec J. Y.; Périchon J. Electrochemical cross-coupling between functionalized aryl halides and 2-chloropyrimidine or 2-chloropyrazine catalyzed by nickel 2,2’-bipyridine complex. Tetrahedron Lett. 2000, 41, 201–203. 10.1016/S0040-4039(99)02037-7. [DOI] [Google Scholar]; c Gosmini C.; Nédélec J. Y.; Périchon J. Electrosynthesis of functionalized 2-arylpyridines from functionalized aryl and pyridine halides catalyzed by nickel bromide 2,2’-bipyridine complex. Tetrahedron Lett. 2000, 41, 5039–5042. 10.1016/S0040-4039(00)00760-7. [DOI] [Google Scholar]; d Gosmini C.; Bassene-Ernst C.; Durandetti M. Synthesis of functionalized 2-arylpyridines from 2-halopyridines and various aryl halides via a nickel catalysis. Tetrahedron 2009, 65, 6141–6146. 10.1016/j.tet.2009.05.044. [DOI] [Google Scholar]; e Liao L.-Y.; Kong X.-R.; Duan X.-F. Reductive Couplings of 2-Halopyridines without External Ligand: Phosphine-Free Nickel-Catalyzed Synthesis of Symmetrical and Unsymmetrical 2,2′-Bipyridines. J. Org. Chem. 2014, 79, 777–782. 10.1021/jo402084m. [DOI] [PubMed] [Google Scholar]
- a Grushin V. V.; Alper H. Transformations of Chloroarenes, Catalyzed by Transition-Metal Complexes. Chem. Rev. 1994, 94, 1047. 10.1021/cr00028a008. [DOI] [Google Scholar]; b Littke A. F.; Fu G. C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem., Int. Ed. 2002, 41, 4176–4211. . [DOI] [PubMed] [Google Scholar]
- For decarboxylative cross-coupling with aryl chlorides, see:; Tang J.; Biafora A.; Goossen L. J. Catalytic Decarboxylative Cross-Coupling of Aryl Chlorides and Benzoates without Activating ortho Substituents. Angew. Chem., Int. Ed. 2015, 54, 13130–13133. 10.1002/anie.201505843. [DOI] [PubMed] [Google Scholar]
- For C–H arylation with aryl chlorides, see:; a Ackermann L. Phosphine Oxides as Preligands in Ruthenium-Catalyzed Arylations via C–H Bond Functionalization Using Aryl Chlorides. Org. Lett. 2005, 7, 3123–3125. 10.1021/ol051216e. [DOI] [PubMed] [Google Scholar]; b Gürbüz N.; Özdemir I.; Çetinkaya B. Selective palladium-catalyzed arylation(s) of benzaldehyde derivatives by N-heterocarbene ligands. Tetrahedron Lett. 2005, 46, 2273–2277. 10.1016/j.tetlet.2005.02.023. [DOI] [Google Scholar]; c Chiong H. A.; Pham Q.-N.; Daugulis O. Two Methods for Direct ortho-Arylation of Benzoic Acids. J. Am. Chem. Soc. 2007, 129, 9879–9884. 10.1021/ja071845e. [DOI] [PubMed] [Google Scholar]; d Gao K.; Lee P.-S.; Long C.; Yoshikai N. Cobalt-Catalyzed Ortho-Arylation of Aromatic Imines with Aryl Chlorides. Org. Lett. 2012, 14, 4234–4237. 10.1021/ol301934y. [DOI] [PubMed] [Google Scholar]; e Zha G.-F; Qin H.-L; Kantchev E. A. B. Ruthenium-catalyzed direct arylations with aryl chlorides. RSC Adv. 2016, 6, 30875–30885. 10.1039/C6RA02742C. [DOI] [Google Scholar]
- The best current alternative is probably a one-pot borylation/cross-coupling strategy. See:; a Baudoin O.; Guénard D.; Guéritte F. Palladium-Catalyzed Borylation of Ortho-Substituted Phenyl Halides and Application to the One-Pot Synthesis of 2,2‘-Disubstituted Biphenyls. J. Org. Chem. 2000, 65, 9268–9271. 10.1021/jo005663d. [DOI] [PubMed] [Google Scholar]; b Billingsley K. L.; Barder T. E.; Buchwald S. L. Palladium-Catalyzed Borylation of Aryl Chlorides: Scope, Applications, and Computational Studies. Angew. Chem., Int. Ed. 2007, 46, 5359–5363. 10.1002/anie.200701551. [DOI] [PubMed] [Google Scholar]; c Molander G. A.; Trice S. L. J.; Dreher S. D. Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives. J. Am. Chem. Soc. 2010, 132, 17701–17703. 10.1021/ja1089759. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Molander G. A.; Trice S. L. J.; Tschaen B. A modified procedure for the palladium catalyzed borylation/Suzuki-Miyaura cross-coupling of aryl and heteroaryl halides utilizing bis-boronic acid. Tetrahedron 2015, 71, 5758–5764. 10.1016/j.tet.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian reported one example of the coupling of 3,4,5-trimethoxybromobenzene with 4-chloroanisole in 58% yield.7d It is not clear whether this approach is general, and no further reports on this monometallic strategy have appeared.
- Our own studies and those of others have shown that aryl chlorides react rapidly with (N–N)Ni0 complexes of the type used in this study. For example, carboxylation of aryl chlorides using similar catalysts and reductants is known. For two examples, see:; a Fujihara T.; Nogi K.; Xu T.; Terao J.; Tsuji Y. Nickel-Catalyzed Carboxylation of Aryl and Vinyl Chlorides Employing Carbon Dioxide. J. Am. Chem. Soc. 2012, 134, 9106–9109. 10.1021/ja303514b. [DOI] [PubMed] [Google Scholar]; b Charboneau D. J.; Brudvig G. W.; Hazari N.; Lant H. M. C.; Saydjari A. K. Development of an Improved System for the Carboxylation of Aryl Halides through Mechanistic Studies. ACS Catal. 2019, 9, 3228–3241. 10.1021/acscatal.9b00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyclic voltammetry studies of the reduction of III showed only an apparent two-electron reduction at −0.86 V vs SCE (Figure S7). The observed reduction potential did not depend upon the counterion of nickel (Cl– or OTf–) or the electrolyte (Bu4NPF6 or Bu4NCl), suggesting that all of these species were ionized in solution. These results match those previously reported for related catalysts:; a Durandetti M.; Devaud M.; Périchon J. Investigation of the reductive coupling of aryl halides and/or ethylchloroacetate electrocatalyzed by the precursor NiX2(bpy) with X– = Cl–, Br– or MeSO3– and bpy = 2,2′-dipyridyl. New J. Chem. 1996, 20, 659–667. [Google Scholar]; b Mikhaylov D.; Gryaznova T.; Dudkina Y.; Khrizanphorov M.; Latypov S.; Kataeva O.; Vicic D. A.; Sinyashin O. G.; Budnikova Y. Electrochemical nickel-induced fluoroalkylation: synthetic, structural and mechanistic study. Dalton Trans. 2012, 41, 165–172. 10.1039/C1DT11299F. [DOI] [PubMed] [Google Scholar]
- This strategy for evaluating the reduction of nickel(II) to nickel(0) grew out of our studies of nickel(0) complexes and their reactivity. We previously reported this approach (with LiBr over Mn surfaces).20a
- a Huang L.; Olivares A. M.; Weix D. J. Reductive Decarboxylative Alkynylation of N-Hydroxyphthalimide Esters with Bromoalkynes. Angew. Chem., Int. Ed. 2017, 56, 11901–11905. 10.1002/anie.201706781. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ni S.; Muñoz Padial N.; Kingston C.; Vantourout J. C.; Schmitt D. C.; Edwards J. T.; Kruszyk M.; Merchant R. R.; Mykhailiuk P. K.; Sanchez B.; Yang S.; Perry M.; Gallego G. M.; Mousseau J. J.; Collins M. R.; Cherney R. J.; Lebed P. S.; Chen J. S.; Qin T.; Baran P. S. A Radical Approach to Anionic Chemistry: Synthesis of Ketones, Alcohols, and Amines. J. Am. Chem. Soc. 2019, 141, 6726–6739. 10.1021/jacs.9b02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The effect of LiCl on reduction of organic halides has been studied in some detail. See:; a Krasovskiy A.; Malakhov V.; Gavryushin A.; Knochel P. Efficient Synthesis of Functionalized Organozinc Compounds by the Direct Insertion of Zinc into Organic Iodides and Bromides. Angew. Chem., Int. Ed. 2006, 45, 6040–6044. 10.1002/anie.200601450. [DOI] [PubMed] [Google Scholar]; b Koszinowski K.; Böhrer P. Formation of Organozincate Anions in LiCl-Mediated Zinc Insertion Reactions. Organometallics 2009, 28, 771–779. 10.1021/om800947t. [DOI] [Google Scholar]; c Feng C.; Cunningham D. W.; Easter Q. T.; Blum S. A. Role of LiCl in Generating Soluble Organozinc Reagents. J. Am. Chem. Soc. 2016, 138, 11156–11159. 10.1021/jacs.6b08465. [DOI] [PubMed] [Google Scholar]; d Jess K.; Kitagawa K.; Tagawa T. K. S.; Blum S. A. Microscopy Reveals: Impact of Lithium Salts on Elementary Steps Predicts Organozinc Reagent Synthesis and Structure. J. Am. Chem. Soc. 2019, 141, 9879–9884. 10.1021/jacs.9b02639. [DOI] [PubMed] [Google Scholar]
- These results do not conclusively show that nickel(0) is the operative oxidation state of the nickel catalyst. Love and Schaefer have recently shown that alkene ligands can influence the relative stability of nickel(I) and nickel(II/0) (see ref (23)).
- Beattie D. D.; Lascoumettes G.; Kennepohl P.; Love J. A.; Schafer L. L. Disproportionation Reactions of an Organometallic Ni(I) Amidate Complex: Scope and Mechanistic Investigations. Organometallics 2018, 37, 1392–1399. 10.1021/acs.organomet.8b00074. [DOI] [Google Scholar]
- Huo S. Highly Efficient, General Procedure for the Preparation of Alkylzinc Reagents from Unactivated Alkyl Bromides and Chlorides. Org. Lett. 2003, 5, 423–425. 10.1021/ol0272693. [DOI] [PubMed] [Google Scholar]
- As determined by 31P NMR spectroscopy versus an internal standard. The DMF/THF solvent mixture was used because the palladium complexes were poorly soluble in pure DMF. The catalytic reaction works well in DMF/THF mixtures. For detailed conditions, see the Figures S9–S12.
- Another alternative hypothesis is insertion of zinc into Ar–Cl or Ar–OTf bonds to form arylzinc reagents. This does not happen under our conditions, consistent with the literature. See:; a Jin M.-Y.; Yoshikai N. Cobalt–Xantphos-Catalyzed, LiCl-Mediated Preparation of Arylzinc Reagents from Aryl Iodides, Bromides, and Chlorides. J. Org. Chem. 2011, 76, 1972–1978. 10.1021/jo102417x. [DOI] [PubMed] [Google Scholar]; b Fillon H.; Gosmini C.; Périchon J. New chemical synthesis of functionalized arylzinc compounds from aromatic or thienyl bromides under mild conditions using a simple cobalt catalyst and zinc dust. J. Am. Chem. Soc. 2003, 125, 3867–3870. 10.1021/ja0289494. [DOI] [PubMed] [Google Scholar]; c Klatt T.; Markiewicz J. T.; Sämann C.; Knochel P. Strategies To Prepare and Use Functionalized Organometallic Reagents. J. Org. Chem. 2014, 79, 4253–4269. 10.1021/jo500297r. [DOI] [PubMed] [Google Scholar]; d Haas D.; Hammann J. M.; Greiner R.; Knochel P. Recent Developments in Negishi Cross-Coupling Reactions. ACS Catal. 2016, 6, 1540–1552. 10.1021/acscatal.5b02718. [DOI] [Google Scholar]
- For a thorough discussion of iodide additives in nickel-catalyzed cross-electrophile coupling, see ref (28a) and references cited therein. For the effect of MgCl2 and LiBr on the reduction of nickel complexes at metal surfaces, see refs (28b) and (20), respectively.
- a Everson D. A.; Jones B. A.; Weix D. J. Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides. J. Am. Chem. Soc. 2012, 134, 6146–6159. 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wang X.; Ma G.; Peng Y.; Pitsch C. E.; Moll B. J.; Ly T. D.; Wang X.; Gong H. Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides. J. Am. Chem. Soc. 2018, 140, 14490–14497. 10.1021/jacs.8b09473. [DOI] [PubMed] [Google Scholar]
- Other noncoordinating anions, such as BF4 and PF6, also appeared to be inhibitory. While zinc appears to be inhibitory (ZnCl2 slowed the reduction), both LiCl and Bu4NCl accelerated the reduction, suggesting that there is not a special lithium effect but rather a special zinc effect. See Table S2 for additional data.
- A mixture of solvents was used to help with solubility of precatalyst solutions. DMF provided the same yields and selectivities as the mixture. See Table S1.
- While most isolated reactions were run for 24 h for convenience, the reaction in Table 1 was 90% complete in 2 h and finished in less than 8 h when run under nitrogen.
- The homodimerization of hindered aryl bromides with nickel has been reported. See the following reference and references cited therein:; Hong R.; Hoen R.; Zhang J.; Lin G.-Q. Nickel-catalyzed Ullmann-type Coupling Reaction to Prepare Tetra-ortho-substituted Biaryls. Synlett 2001, 2001, 1527–1530. 10.1055/s-2001-17488. [DOI] [Google Scholar]
- Biaryls with four different ortho substituents are still not coupled in high yield. For these types of substrates, cross-coupling with organometallic reagents is the best approach. See:; a Altenhoff G.; Goddard R.; Lehmann C. W.; Glorius F. An N-Heterocyclic Carbene Ligand with Flexible Steric Bulk Allows Suzuki Cross-Coupling of Sterically Hindered Aryl Chlorides at Room Temperature. Angew. Chem., Int. Ed. 2003, 42, 3690–3693. 10.1002/anie.200351325. [DOI] [PubMed] [Google Scholar]; b Valente C.; Çalimsiz S.; Hoi K. H.; Mallik D.; Sayah M.; Organ M. G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem., Int. Ed. 2012, 51, 3314–3332. 10.1002/anie.201106131. [DOI] [PubMed] [Google Scholar]
- a Carini D. J.; Duncia J. V.; Aldrich P. E.; Chiu A. T.; Johnson A. L.; Pierce M. E.; Price W. A.; Santella J. B.; Wells G. J. Nonpeptide angiotensin II receptor antagonists: the discovery of a series of N-(biphenylylmethyl)imidazoles as potent, orally active antihypertensives. J. Med. Chem. 1991, 34, 2525–2547. 10.1021/jm00112a031. [DOI] [PubMed] [Google Scholar]; b Kubo K.; Kohara Y.; Yoshimura Y.; Inada Y.; Shibouta Y.; Furukawa Y.; Kato T.; Nishikawa K.; Naka T. Nonpeptide angiotensin II receptor antagonists. Synthesis and biological activity of potential prodrugs of benzimidazole-7-carboxylic acids. J. Med. Chem. 1993, 36, 2343–2349. 10.1021/jm00068a011. [DOI] [PubMed] [Google Scholar]; c Wexler R. R.; Greenlee W. J.; Irvin J. D.; Goldberg M. R.; Prendergast K.; Smith R. D.; Timmermans P. B. M. W. M. Nonpeptide Angiotensin II Receptor Antagonists: The Next Generation in Antihypertensive Therapy. J. Med. Chem. 1996, 39, 625–656. 10.1021/jm9504722. [DOI] [PubMed] [Google Scholar]
- a Johnson K. A.; Biswas S.; Weix D. J. Cross-Electrophile Coupling of Vinyl Halides with Alkyl Halides. Chem. - Eur. J. 2016, 22, 7399–7402. 10.1002/chem.201601320. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang L.; Hackenberger D.; Gooßen L. J. Iridium-Catalyzed ortho-Arylation of Benzoic Acids with Arenediazonium Salts. Angew. Chem., Int. Ed. 2015, 54, 12607–12611. 10.1002/anie.201505769. [DOI] [PubMed] [Google Scholar]; c Durandetti M.; Gosmini C.; Périchon J. Ni-catalyzed activation of α-chloroesters: a simple method for the synthesis of α-arylesters and β-hydroxyesters. Tetrahedron 2007, 63, 1146–1153. 10.1016/j.tet.2006.11.055. [DOI] [Google Scholar]
- a Peretto I.; Radaelli S.; Parini C.; Zandi M.; Raveglia L. F.; Dondio G.; Fontanella L.; Misiano P.; Bigogno C.; Rizzi A.; Riccardi B.; Biscaioli M.; Marchetti S.; Puccini P.; Catinella S.; Rondelli I.; Cenacchi V.; Bolzoni P. T.; Caruso P.; Villetti G.; Facchinetti F.; Del Giudice E.; Moretto N.; Imbimbo B. P. Synthesis and Biological Activity of Flurbiprofen Analogues as Selective Inhibitors of β-Amyloid1–42 Secretion. J. Med. Chem. 2005, 48, 5705–5720. 10.1021/jm0502541. [DOI] [PubMed] [Google Scholar]; b Quasdorf K. W.; Riener M.; Petrova K. V.; Garg N. K. Suzuki–Miyaura Coupling of Aryl Carbamates, Carbonates, and Sulfamates. J. Am. Chem. Soc. 2009, 131, 17748–17749. 10.1021/ja906477r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.