Abstract
Objective—
A total of 30% to 50% of patients with bicuspid aortic valve (BAV) require surgery for aortic valve replacement (AVR), ascending aortic replacement (AA), or both. To prevent adverse aortic events, they are risk stratified using imperfect criteria based on imaging modalities. As a result, a significant number of dissections occur outside of the parameters suggested by the guidelines. Advanced glycation end products (AGEs) are associated with valve and vascular remodeling and trigger the release of a soluble receptor (soluble receptor for advanced glycation end product [sRAGE]). This study aims to characterize sRAGE as a diagnostic and risk-stratification tool for patients with BAV referred for surgery.
Approach and Results—
sRAGE was measured in 135 patients (BAV, n=74; tricuspid aortic valve, n=61) meeting inclusion criteria from 338 enrolled patients undergoing AVR and AA. Univariate and multivariate analyses were performed. sRAGE level was significantly associated with the presence of BAV, independent of age, sex, and common risk factors for vascular disease (P<0.001). Within the BAV cohort, patients referred for AA and AVR had higher sRAGE values than patients undergoing AVR only (P=0.002). Patients with BAV <60 years of age, presenting with both valve and aortic diseases (fast progressors), had higher sRAGE than older patients who only needed AVR (slow progressors). Histological analysis showed that sRAGE correlates with dysfunctional aortic microstructure and does not correlate with aortic diameter (R2=0.007; P=0.51) or diameter/body surface area (R2=0.011; P=0.42).
Conclusions—
These results show that elevated level of circulating sRAGE is associated with the presence of BAV and associated aortopathies, independent of aortic diameter.
Keywords: advanced glyosylation end-product receptor; aortic aneurysm, thoracic; bicuspid aortic valve
It is estimated that ≈1 in 50 people is born with a bicuspid aortic valve (BAV), making BAV the most common congenital heart defect in the United States.1–16 The presence of a BAV is associated with frequent and premature valve dysfunction, such as aortic stenosis (AS) and aortic insufficiency (AI).1–16 In patients <50 years of age having aortic valve replacement (AVR) for severe AS, virtually all of them have BAV. Until the age of 70 years, patients with BAV outnumber those with tricuspid aortic valve (TAV) having AVR for AS and not until the age of >80 years do patients with TAV predominate.1,2,4–8,10,16
In addition, the presence of a bicuspid valve is a strong indicator of adverse aortic events as these patients have higher occurrence of life-threatening cardiac events, such as ascending aortic aneurysm (thoracic aortic aneurysm) and dissection (thoracic aortic dissection). The current American College of Cardiology/American Heart Association guidelines recommend replacing the ascending aorta in patients with both BAV and TAV undergoing AVR if the ascending aortic diameter is >4.5 cm. However, the optimal timing of aortic surgery in thoracic aortic aneurysm patients remains uncertain for patients with both BAV and TAV.1,2,4–8,10,12–14,16 In a recent study, we showed that >60% of patients with acute aortic dissection presented with aortic diameters smaller than those indicated for surgery.1,2,5–8,10,12–14,16–19 Therefore, the current indications for elective surgical intervention are imperfect predictors of aortic dissection and rupture, especially for patients with BAV.1,2,4–8,10,12,16,20 Also, of concern is the common development of subsequent ascending aortopathies in patients who have had previous AVR for BAV in the near or remote past. The early and accurate diagnosis of a BAV has therefore important implications for both clinical care and surgical intervention.
A congenital BAV is diagnosed accurately when short axis 2- or 3-dimensional images show 2 leaflets in systole; diastolic images are unreliable as a bicuspid valve with raphe in 1 leaflet may be mistaken for a TAV and vice versa. When images are adequate echocardiography can reach 92% sensitivity and 96% specificity.1,2,5–8,10,12–14,16–19 In the presence of AV calcification, sensitivity and specificity could drop to 67% and 69%, respectively.1,2,4,5,8,10,12,20 Higher diagnostic accuracy has been reported in a relatively small sample population using color Doppler transesophageal echocardiography, a method that may be poorly tolerated in frail patients. Because of these limitations, cardiac MRI and computed tomography need to be implemented to improve the diagnostic process.1,2,5–8,10,16,21–27 Because of the natural history of BAV leading to premature calcified AV, the use of echocardiography can be limited in patients referred to surgery. BAV is therefore identified via routine echocardiography only in a subgroup of patients with BAV: in addition, echocardio-graphic identification can be challenging after cusp fusion secondary to inflammation.1,5,12,28 Moreover, common imaging techniques do not generally provide information on the risk of aortopathies.
Recent studies have also demonstrated that aneurysms associated with BAV exhibit cellular and extracellular matrix differences when compared with TAV aneurysms.21–27,29,30 These considerations suggest a differential regulation vascular smooth muscle cell (VSMC) phenotype in BAV and TAV aortas, as well as difference response to oxidative damage, which could affect the microregional structure of the proximal aorta. An association between valve and vascular dysfunctions and advanced glycation end products (AGEs) has been described recently. These products seem to play an important role for the development and progression of cardiovascular disease mainly through induction of oxidative stress and inflammation.1,2,7,8,10,28,31 AGEs are a heterogenous group of molecules formed by the nonenzymatic reaction of reducing sugars with amino acids of proteins, lipids, and nucleic acids. The receptor for AGEs (RAGEs) is a multiligand member of the immunoglobulin superfamily of cell surface molecules.1,2,7,8,10,15,29,30 RAGE has been described as a receptor for AGEs, the products of nonenzymatic glycation and oxidation of proteins/lipids. Later studies indicated that RAGE was also a signal transduction receptor for proinflammatory S100/calgranulins and high mobility group box 1 (HMGB-1)/amphoterin and amyloid-β-peptide and β-sheet fibrils.1,2,6–8,10,31,32 If coupled with failure of regenerative mechanisms, the AGE–RAGE axis may lead to irreversible tissue injury.1,2,7,8,10,15 Recent studies have shown that s100A12, a proinflammatory protein and a ligand of RAGE, is highly expressed in thoracic aortic aneurysm and dissection.1,2,6–8,32 Based on these data, we hypothesized a direct correlation between circulating soluble RAGE (sRAGE) level and the presence of BAV and BAV-associated aortopathies. A predictive, plasma-based, low cost tool for the identification of patients with BAV who are at high risk for aortic complications would therefore improve management, surgical planning, and long-term results.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
Circulating Plasma Levels of sRAGE Identify Patients With BAV in a Surgical Patient Population Independently of Age, Sex, and Common Cardiovascular Comorbidities
A total of 135 surgical patients (Tables 1 and 2) matching the inclusion criteria were analyzed (Figure 1). We first examined plasma levels of sRAGE in all patients with BAV (n=74) and TAV (n=61). As shown in Figure 2A, sRAGE mean values are significantly higher in patients with BAV (1765±142.9 pg/mL) than in patients with TAV (733.9±49.26 pg/mL; P<0.0001). Receiver operator characteristic curve was performed to determine whether plasma levels of sRAGE could discriminate patients with BAV and TAV. As shown in (Figure 2B and 2C), a cutoff of sRAGE plasma level equal to 996 pg/mL maximized area under the curve values to 0.80 and identifies BAV with 63.5% sensitivity and 79% specificity (Figure 2C). These patients have concomitant occurrence of aortic valve stenosis/insufficiency and ascending aortic dilatation: because the current American College of Cardiology/American Heart Association guidelines suggest ascending aortic replacement (AA) for patients with BAV and TAV undergoing AVR if the aortic diameter is >4.5 cm, we measured plasma level of sRAGE in the BAV and TAV subgroups of patients with diameter lower than the parameter suggested by the guidelines. In this subanalysis of patients with 27 TAV and 40 BAV, a cutoff of 766 pg/mL maximized area under the curve values to 0.81 and identified BAV with 81.5% sensitivity and 72.5% specificity (likelihood ratio of 3.9; Figure 1C–1E).
Table 1.
Patient Enrollment
| Enrollment (n=338) | TAV (178) | BAV (160) |
|---|---|---|
| Exclusion criteria | ||
| Syndromic TAA/connective tissue diseases | 7 (3.9%) | 0 |
| Other heart defect | 0 | 4 (2.5%) |
| Thoracic aortic dissection | 10 (5.6%) | 0 |
| Endocarditis | 0 | 6 (3.75) |
| Chronic disease | 25 (14.0%) | 14 (8.75%) |
| Inflammatory disease | 19 (10.7%) | 12 (7.5%) |
| Previous MI | 13 (7.3%) | 11 (6.9%) |
| Heart failure (NYHA III+/IV) | 18 (10.1%) | 14 (8.8%) |
| History of cancer | 34 (19.1%) | 25 (15.6%) |
| Included (n=135) | 61 (34.3%) | 74 (46.3%) |
BAV indicates bicuspid aortic valve; MI, myocardial infarction; NYHA, New York Heart Association; TAA, thoracic aortic aneurysm; and TAV, tricuspid aortic valve.
Table 2.
Patient Demographics
| Demographics | TAV (n=61) | BAV (n=74) | P Value |
|---|---|---|---|
| Age, y | 64.4±11 | 55.5±13 | <0.001 |
| Male subjects | 71% | 64.4% | 0.164 |
| Smokers | 19.4% | 43% | 0.004 |
| Diabetes mellitus | 13.2% | 5.3% | 0.312 |
| Hypertension | 43.40% | 31.6% | 0.024 |
| Coronary artery disease | 22.6% | 5.4% | 0.003 |
| Hyperlipidemia | 43.4% | 27.7% | 0.024 |
| Baseline sRAGE | 733.9±49.3 | 1765±142.9 | <0.0001 |
| Diagnosis and type of surgery | |||
| Aortic valve insufficiency | 68.9% | 70.3% | 0.351 |
| Aortic valve stenosis | 34.4% | 74.3% | 0.002 |
| Aortic valve repair/replacement | 55.73% | 43.24% | 0.025 |
| AVR and AA repair/replacement | 44.3% | 56.8% | 0.080 |
Demographic and clinical details: total n=135 surgical patients. AA indicates ascending aortic replacement; AVR, aortic valve replacement; BAV, bicuspid aortic valve; sRAGE, soluble receptor for advanced glycation end product; and TAV, tricuspid aortic valve.
Figure 1.
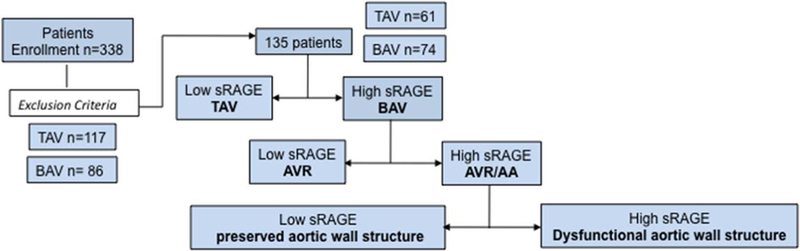
Study workflow AA indicates ascending aortic replacement; AVR, aortic valve replacement; BAV, bicuspid aortic valve; and TAV, tricuspid aortic valve.
Figure 2.
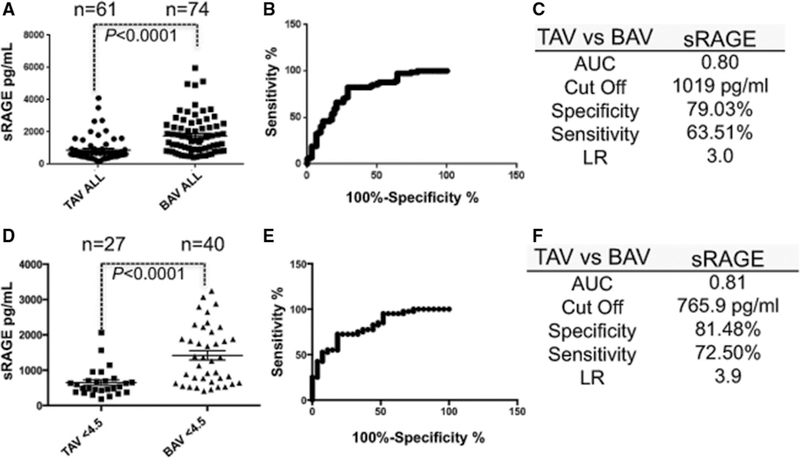
Circulating plasma levels of soluble receptor for advanced glycation end product (sRAGE) identify patients with bicuspid aortic valve (BAV) in a surgical patient population. A, sRAGE quantification of tricuspid aortic valve (TAV; n=61) and BAV (n=74) patients. Dots represent values (pg/mL) from each patient±SEM. B, sRAGE quantification receiver operator characteristic curve (ROC) curve. C, Sensitivity, specificity, and likelihood ratio (LR) for a cutoff of sRAGE concentration of 1019 pg/mL. D, sRAGE values in TAV (n=27) and BAV (n=40) patients meeting the criteria for surgical intervention (ascending aortic diameter <4.5 cm). E, sRAGE ROC curve relative to D. F, Sensitivity, specificity, and LR for a cutoff of sRAGE concentration of 766 pg/mL. AUC indicates area under the curve.
As shown in Tables 3 and 4, multivariate general linear model ANOVA was performed to evaluate whether sRAGE was a significant and independent predictor of BAV in the data set. In addition to the sRAGE values, factors entered into the model were age, sex, coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, history of smoking/tobacco use, body surface area (BSA), AI, and AS. The model for the overall group showed that the presence of BAV is significantly and independently related to higher levels of sRAGE. Table 4 demonstrates that this relationship is even more pronounced in patients who have an ascending aortic artery measurement of <4.5 cm. In addition, regression analysis was used to test whether BAV status is predicted by level of sRAGE. A univariate regression model demonstrated sRAGE level was a predictor of BAV status with R2=0.151, F(1, 134)=23.9, P<0.0001. Multivariate regression was used to evaluate this relationship considering the effect of age, BSA, AA diameter, sex, presence of coronary artery disease, smoking, hypertension, diabetes mellitus, hypercholesterolemia, and presence of aortic valve stenosis of insufficiency. The R2 for the overall model was 0.21, F(1, 123)=8.4, P=0.002. It was found that sRAGE significantly predicted BAV status (β=0.309; P=0.005; unstandardized β =692.8 with 95% confidence interval, 218.1–1167.5). No other factors were significant in the model. A multiple regression model was also created for patients with AA diameter <4.5 cm. The R2 for the overall model was 0.42, F(1, 53)=8.9, P=0.004. It was again found that sRAGE was the only factor that significantly predicted BAV status (β =0.453; P=0.004; unstandardized β =747.2 with 95% confidence interval, 243.9–1250.5).
Table 3.
Multivariate General Linear Model ANOVA Analyzing the Relationship Between Soluble Receptor for Advanced Glycation End Product Level and Study Variables in Patients With 134 BAV and TAV
| Factors in the Model | df | F-Statistic | P Value |
|---|---|---|---|
| Covariates | |||
| Age, y | 1 | 1.941 | 0.166 |
| AA diameter | 1 | 2.822 | 0.096 |
| Body surface area | 1 | 2.355 | 0.128 |
| Combined covariates | 3 | 2.028 | 0.114 |
| Main effects | |||
| BAV vs TAV group | 1* | 7.330* | 0.008* |
| AVS | 1 | 1.295 | 0.257 |
| Sex | 1 | 0.065 | 0.800 |
| Coronary artery disease | 1 | 0.009 | 0.080 |
| Diabetes mellitus | 1 | 0.664 | 0.417 |
| Smoking | 1 | 0.093 | 0.761 |
| Hyperlipidemia | 1 | 0.364 | 0.548 |
| Hypertension | 1 | 0.488 | 0.486 |
| AVI | 4 | 0.471 | 0.757 |
| Combined model | 15 | 2.254 | 0.008* |
AA indicates ascending aortic replacement; AVI, aortic valve insufficiency; AVS, aortic valve stenosis; BAV, bicuspid aortic valve; and TAV, tricuspid aortic valve.
indicates significance.
Table 4.
Multivariate General Linear Model ANOVA Analyzing the Relationship Between Soluble Receptor for Advanced Glycation End Product Level and Study Variables in 66 Patients With BAV and TAV With AA Diameter <4.5 cm
| Factors in the Model | Df | F-Statistic | P Value |
|---|---|---|---|
| Covariates | |||
| Age, y | 1 | 0.249 | 0.620 |
| AA diameter | 1 | 3.526 | 0.066 |
| Body surface area | 1 | 3.879 | 0.055 |
| Combined covariates | 3 | 2.817 | 0.049* |
| Main effects | |||
| BAV vs TAV group | 1* | 7.750* | 0.008* |
| AVS | 3 | 0.527 | 0.666 |
| Sex | 1 | 1.156 | 0.288 |
| Coronary artery disease | 1 | 0.451 | 0.505 |
| Diabetes mellitus | 1 | 0.004 | 0.947 |
| Smoking | 1 | 2.125 | 0.151 |
| Hyperlipidemia | 1 | 0.386 | 0.537 |
| Hypertension | 1 | 0.810 | 0.372 |
| AVI | 3 | 0.593 | 0.623 |
| Combined model | 16 | 2.307 | 0.013* |
AA indicates ascending aortic diameter; AVI, aortic valve insufficiency; AVS, aortic valve stenosis; BAV, bicuspid aortic valve; and TAV, tricuspid aortic valve.
indicates significance.
Patients Requiring AA Have Higher sRAGE Level Than Patients Requiring AVR Only
Patients with BAV were then divided in 2 subgroups: those who underwent only aortic valve repair/replacement (AVR) because of severe AS or AI (n=30) but no ascending aortic dilatation (diameter <4.5 cm) and those who presented ascending aortic dilatation (ascending aneurysm >4.5 cm) concomitantly to AS or AI (n=30). As shown in Figure 3A, patients with BAV with only valve pathology (mean ascending aortic diameter, 3.7 cm) presented sRAGE plasma values of 1365±150.4 pg/mL, whereas patients who presented aortic valve pathology and aortic dilatation (mean ascending aortic diameter, 4.9 cm) show sRAGE levels equal to 2209±278.8 pg/mL (P=0.02). Logistic regression was then performed to test whether sRAGE values were correlated with ascending aortic diameter or the ratio between ascending aortic diameter and BSA. As shown in Figure 3B there is no correlation between sRAGE levels and ascending aortic diameter (R2=0.007; P=0.51) or the ratio between ascending aortic diameter/BSA (R2=0.011; P=0.42).
Figure 3.
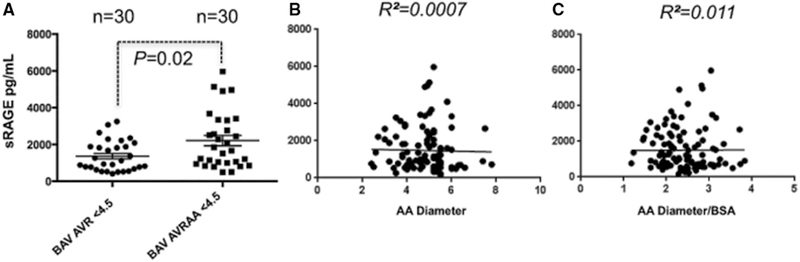
Patients requiring ascending aortic replacement (AA) have higher soluble receptor for advanced glycation end product (sRAGE) level than patients requiring aortic valve replacement (AVR) only. A, sRAGE quantification in plasma samples of patients with BAV undergoing AVR/repair only (BAV AVR, n=30) and patients with BAV undergoing AVR combined with an ascending aortic procedure (aortoplasty or replacement, BAVAVRAA, n=30). B and C, Linear correlation between sRAGE plasma quantification and patient’s ascending aortic diameter (P=0.51; R2=0.007) or ratio between ascending aortic diameter/BSA (P=0.42; R2=0.011).
Furthermore, we divided our patients with BAV population into 4 groups using the age of 60 years as an arbitrary cut-off: those aged <60 years undergoing AVR only, those aged <60 years requiring both AVR and AA, those aged >60 years undergoing AVR only, and those aged >60 years requiring both AVR and AA (Figure 4). In the setting of AA development, we considered those patients who required AVR and AA before the age of 60 as fast progressors, whereas those requiring AA and AVR or just AVR above the age of 60 years as slow progressors (P<0.05; Figure 4A). Interestingly, plasma levels of sRAGE in the fast progressors group were significantly higher when compared with any of the other group, independently of aortic diameter (Figure 4B).
Figure 4.
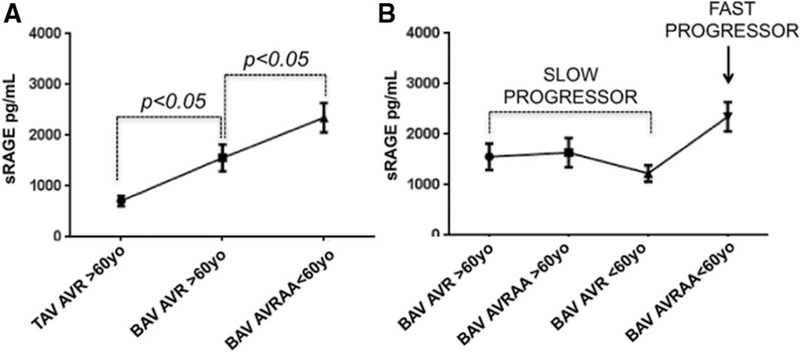
Soluble receptor for advanced glycation end product (sRAGE) level distribution in slow and fast progressors. A, sRAGE distribution according to the morphology of the valve (tricuspid aortic valve [TAV], bicuspid aortic valve [BAV]), the type of surgery performed (aortic valve replacement [AVR] or AVR/ascending aortic replacement [AA]), and the patient’s age (< or >60, <60, and >60 years). B, Patients with BAV discrimination in slow progressors vs fast progressors based on sRAGE plasma values distribution.
Plasma Levels of sRAGE Ligands, s100A12 and HMGB-1, Are Not Significantly Different in Patients With BAV When Compared With TAV
It is known that one of the mechanisms associated with the release of sRAGE in the circulation is related to the shedding of cellular RAGE on activation from its ligands. A subgroup of patients with BAV and TAV was then analyzed for the presence of s100A12 and HMGB-1 in the circulation. s100A12 was measured in a total of 30 patients (TAV, 10; BAV, 20) and HMGB-1 in 38 patients (TAV, 18; BAV, 20). As shown in Figure IA in the online-only Data Supplement plasma s100A12 was not significantly different in patients with BAV when compared with those with TAV (P=0.078) and was not significantly different in patients with BAV and TAV with ascending aortic diameter <4.5 cm (P=0.9828; Figure IB in the online-only Data Supplement). Similarly, the analysis of circulating levels of HMGB-1 shows no differences in patients with BAV versus TAV (P=0.8218; P=0.4982; Figure IIA and IIB in the online-only Data Supplement). Linear regression was performed to test whether s100A12 or HMGB-1 values would correlate with ascending aortic diameter. As shown in Figures IC and IIC in the online-only Data Supplement, neither s100A12 nor HMGB-1 values correlate with ascending aortic diameter. In addition, no significant correlation was found between these markers and circulating sRAGE values (Figure IIIA and IIIB in the online-only Data Supplement). Furthermore, none of these circulating markers are significantly different in fast and slow progressor patients with BAV (Figure IIIC and IIID).
Plasma Levels of sRAGE Correlate With Altered Ascending Aortic Microstructures
Ascending aortic tissues were then analyzed for patients with BAV using sRAGE values. A subgroup of 6 patients with BAV expressing either high or low sRAGE levels were selected and analyzed by a pathologist based on modified Movat Pentachrome staining. Age, maximum aortic diameter, and sRAGE are indicated in Figure 5A. Quantification of elastin degradation and proteoglycans deposition show that sRAGE correlate with dysfunctional ascending aortic microstructures (Figure 5B and 5C). Control TAV level shows, as expected, low level of sRAGE, normal elastin fiber alignment, and limited proteoglycan deposition. Patients with BAV with low levels of sRAGE show, despite great variation in the individual ascending aortic diameters, similar elastin and proteoglycan value comparable with the TAV reference. Interestingly, patients with BAV with high level of soluble sRAGE show dysfunctional aortic microstructure with elastin fragmentation and proteoglycan deposition regardless of the aortic diameter.
Figure 5.
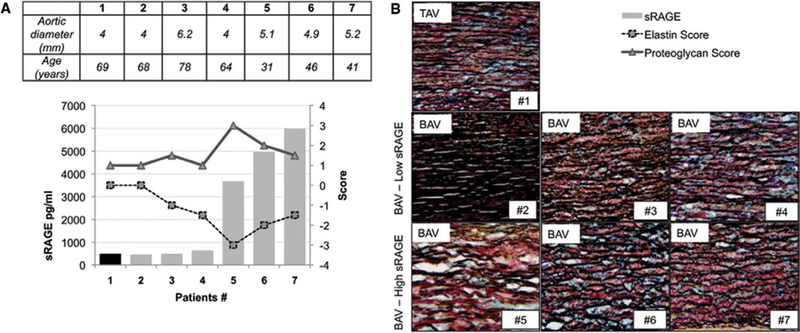
Plasma levels of soluble receptor for advanced glycation end product (sRAGE) correlate with altered ascending aortic microstructures. A, Ascending aortic diameter measurement by computed tomographic scan (cm), age, and sRAGE concentration (pg/mL) detected in the plasma of the patient nos. 1 to 7. B, Graph representing sRAGE values (bars), proteoglycan deposition (scored from 0 to +3; triangles), and elastin fragmentation (scored from 0 to −3; squares). C, Representative images of modified Movat’s pentachrome staining performed on optimal cutting temperature compound section of ascending aortic tissues excised from patient nos. 1 to 7. Media layer (magnification, ×40). AA indicates ascending aortic replacement.
RAGE Expression in the Ascending Aorta Linearly Correlates With Circulating sRAGE in Patients With BAV
The expression of RAGE and its ligands (HMGB-1 and s100A12) was tested by Western blotting using whole tissue extracts of ascending aortic tissues excised from n=7 patients already tested for sRAGE (Figure 5). As shown in Figure 6A to 6C, both RAGE and HMGB-1 expression is increased in aortic tissue obtained from patients with high levels of sRAGE and dysfunctional aortic structure. Densitometry analysis and linear regression revealed that RAGE expression (but not HMGB-1) linearly correlates with circulating sRAGE levels (R2=0.7229 and R2=0.2594 respectively; Figure 6B and 6C). s100A12 expression was not detected by Western blotting. Immunohistochemistry and immunofluorescence were performed to test which cell types in the aorta are expressing RAGE and its ligands. As shown in Figure 5D, RAGE and HMGB-1 expression is significantly increased in dysfunctional aortic tissue of patients with BAV with high levels of circulating sRAGE when compared with those with a conserved aortic structure and low levels of sRAGE. Both RAGE and HMGB-1 expression is evenly distributed through all the aortic layers, suggesting that resident endothelial cells as well as VSMCs and fibroblasts express these proteins. Similar results have been obtained by immunofluorescence analysis (Figure IV in the online-only Data Supplement). No significant inflammation has been detected in patients with low or high levels of sRAGE (Figure V in the online-only Data Supplement). Morphological analysis revealed few HMGB-1 and RAGE-positive inflammatory cells (mostly lymphocytes) in the adventitial layer. On the contrary, s100A12 was not detected in the aorta of patients with low levels of sRAGE and was minimally detected in adventitial inflammatory infiltrates in the aorta of patients with high levels of circulating sRAGE (Figure 6).
Figure 6.
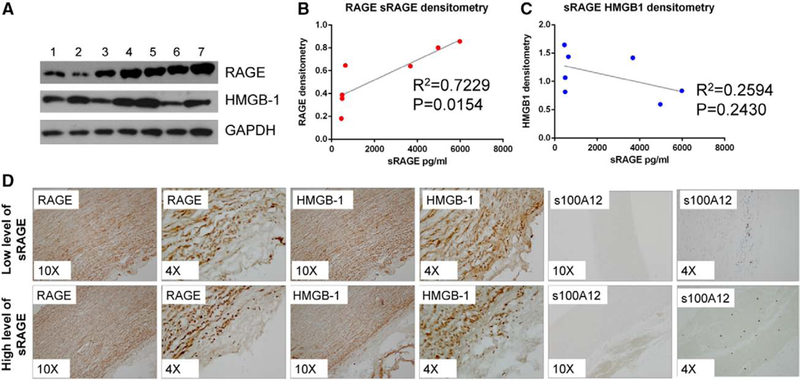
Receptor for advanced glycation end product (RAGE) expression in the ascending aorta linearly correlates with circulating sRAGE in patients with bicuspid aortic valve. A, RAGE and high mobility group box 1 (HMGB-1) expression tested by Western blotting analysis of whole aortic tissue extract. GAPDH was used as loading control. B and C, Graph representing the linear correlation between RAGE or HMGB-1 expression in the aortic tissue (quantified by densitometry analysis) and soluble RAGE (sRAGE) plasma levels in respective patients. C, Representative images of HMGB-1, RAGE, and s100A12 staining performed on optimal cutting temperature compound section of ascending aortic tissues excised from patients with low or high levels of circulating sRAGE (magnification, ×10 and ×4).
Discussion
Patients with BAV have frequent and premature occurrence of valvular diseases, as well as ascending thoracic aortopathies. Although aortic diameter, expansion rate, and ratio of aortic area/diameter to body weight/surface are the current indications for elective surgical intervention of the proximal aorta, they are imperfect predictors of adverse aortic events. Current guidelines for ascending aortic aneurysm repair would fail to prevent the majority of acute aortic dissections even with the more aggressive guidelines adopted for patients with BAV.1–3,6–12,15 Therefore, the identification of patients with BAV and the ability to risk stratify those at high risk of aortopathies, independently of aortic diameters, is of paramount importance both clinically and socially. Given the role of AGE and its soluble receptor in controlling tissue homeostasis and vascular remodeling, we aimed to characterize sRAGE as a diagnostic and risk-stratification tool for patients with BAV referred for surgery. Several important innovative concepts for the diagnosis and risk stratification of patients with BAV are reported in this article.
Our results show that sRAGE levels are significantly higher in patients with BAV compared with TAV, and that a cutoff equal to 766 pg/mL is able to identify patients with BAV with 82% sensitivity and 69% specificity. The presence of BAV is identified via routine echocardiography only in a subgroup of patients: echocardiographic identification can be challenging in severe stenosis and after cusp fusion secondary to inflammation.1,2,4–14,16 Because of these limitations, cardiac MRI and computed tomography need to be used to augment the diagnostic process. Both computed tomography angiography and MR angiography also have relatively high associated costs as well as potential renal side effects related to the administration of contrast dye. In addition, although this is currently the gold standard, routine computed tomography angiography scanning is not recommended in women of child-bearing years because of the radiation exposure. Finally, in the pediatric or frail population, the stress associated with conventional screening may also be a deterrent. Here, it is concluded that circulating levels of sRAGE can identify patients with BAV with higher sensitivity and specificity and independently from the condition of the valve (insufficient, stenotic or heavily calcified; Figures 1–3).
Furthermore, sRAGE ability to identify patients with BAV and BAV-associated aortopathies is not affected by the presence of coronary artery disease and diabetes mellitus or by common risk factor for cardiovascular diseases (Tables 3 and 4). Regression analyses indicate that increasing levels of sRAGE are predictive of BAV status. The relationship of sRAGE levels was confirmed in both a single variable regression model and a model that included all of the patient characteristics available for analysis, reflecting the independent and unique relationship of sRAGE level to BAV status. In addition, this relationship was demonstrated to be even more pronounced for patients with AA diameter <4.5 cm.
These results could have significant diagnostic implications for patients with BAV. The availability of a blood-based test to identify BAV disease creates the possibility of routine screens for patients and family members, thus allowing early identification and surveillance with anticipated better compliance.
It should also be noted that commonly used imaging techniques to assess the presence of a BAV do not generally provide information on the risk of aortopathies (either proximal or distal). We reported that higher levels of sRAGE were detected in patients with BAV with aortic valve pathology and ascending aortopathies when compared with patients with BAV with only valvular pathologies. No linear correlation has been found between high levels of sRAGE and aortic diameter or aortic diameter/BSA in patients with BAV with ascending aneurysm. These results suggest that higher levels of sRAGE may be a marker for the diagnosis of aortic complications in patients with BAV with no linear correlation with aortic diameter. In addition, we reported that patients requiring both AVR and AA <60 years of age (fast progressors) have higher sRAGE levels than any other groups in our surgical population (slow progressors; Figure 4).
On the contrary, circulating levels of 2 known sRAGE ligands, HMGB-1 and s100A12, are not significantly different in patients with BAV when compared with patients with TAV and did not show any correlation with sRAGE concentration or ascending aortic diameter (Figures I–III in the online-only Data Supplement).
Finally, we show in a subpopulation of patients with BAV that sRAGE level directly correlated with altered ascending aortic microstructures. Although patients with BAV with low sRAGE show an overall organized aortic microstructure, higher levels of sRAGE are associated with elastin degradation and proteoglycan deposition (Figure 5). RAGE and HMGB-1 are known to be expressed in endothelial cells, VSMCs, as well as inflammatory cells (monocytes and macrophages). s100A12 is mostly released by neutrophils and it is expressed in VSMC within atherosclerotic plaques or after endothelial cell wire injury. More recently, s100A12 has been found in VSMC of human aneurysmal and dissected aorta with variable degrees of expression. Interestingly we found that RAGE tissue expression is increased in the aorta of patients with BAV with high levels of circulating sRAGE (Figure 6). Furthermore, the expression of RAGE in the tissue linearly correlates with the levels of sRAGE in the plasma of the same patients suggesting that RAGE activation in the tissue may be responsible for the shedding of the receptor and consequent release of sRAGE in the circulation. HMGB-1 expression is generally increased in patients with dysfunctional aortic structure and high level of circulating sRAGE but its expression in the tissue did not show a linear correlation with sRAGE levels (Figure 6). Immunohistochemistry analysis suggested that both RAGE and HMGB-1 expression is evenly distributed through all the aortic layers suggesting that resident endothelial cells as well as VSMCs and myofibroblasts express these proteins. Similar results have been obtained by immunofluorescence analysis (Figure IV in the online-only Data Supplement). It has been previously shown that the ascending aorta of aneurysmal patients with BAV do not present high levels of inflammatory cells as opposed to aneurysms of patients with TAV.22,33 Few inflammatory infiltrates, mostly present in the adventitial layer (Figure V in the online-only Data Supplement), were found in the aortic tissue of patients with low and high sRAGE levels and they also express both RAGE and HMGB-1. s100A12 was undetected in the ascending aortic tissue by Western blotting analysis and minimally detected by immunohistochemistry in few adventitial inflammatory infiltrates. These findings are suggesting that HMGB-1 but not s100A12 may contribute to the activation of tissue RAGE that ultimately results in circulating sRAGE accumulation. Our results are in accordance with the 2-hit model for vascular perturbation mediated by RAGE and its ligands postulated by Schmidt et al34. The 2-hit model hypothesizes that the first hit is increased expression of RAGE and its ligands within the vasculature. Various forms of stress (ischemic stress, immune/inflammatory stimuli, physical/hemodynamic stress, or modified lipoproteins) represent the second hit, which leads to exaggerated cellular response promoting development of vascular lesions.
Although larger group size and further prospective studies are needed, our results provide evidence that testing sRAGE in the plasma may be an important step in the development of a blood-based tool for the diagnosis of BAV and BAV-associated aortopathies. The availability of such a diagnostic tool would have important outcomes for public health: first, it would provide a low cost tool for the identification of patients with BAV in addition to the current imaging techniques. Second, it would open the possibility to routinely screen BAV family members allowing an early identification and surveillance of this population. In addition, our study suggests that plasma levels of sRAGE could be used for the risk stratification of patients with BAV for adverse aortic events. Low-risk patients (low sRAGE plasma level) could be spared costly follow-up or surgical intervention, whereas high-risk patients (high sRAGE plasma level) could be followed up closely both clinically and surgically. This study opens a new direction in the risk stratification of BAV and aortic aneurysm patients; although aortic diameter and expansion rate remain key factors in the diagnosis and prognosis of adverse aortic event, our study highlights the need to link aortic wall microstructure—determined by the impact of AGEs on VSMCs and extracellular matrix components—to the risk of dissection/rupture independent of metric measurements.
Supplementary Material
Significance.
Bicuspid aortic valve disease is the most common congenital heart defect occurring in 2% of the general population. Fifty percent of patients with bicuspid aortic valve require surgery for either aortic valve calcification or ascending aortic aneurysm and dissection. The presence of a bicuspid aortic valve is currently diagnosed using imaging techniques, and surgical intervention is based on ascending aortic measurements. However, the majority of adverse aortic events occur outside of the suggested parameters of surgical intervention. This study opens a new direction in the risk stratification of bicuspid aortic valve and aortic aneurysm patients: although aortic diameter and expansion rate remain key factors in the diagnosis and prognosis of adverse aortic event, our study highlights the need to provide a link between aortic wall microstructure—determined by the impact of advanced glycation end products on vascular smooth muscle cells and extracellular matrix components—and the risk of dissection/rupture independent of metric measurements.
Acknowledgments
We thank Dr Robert J. Levy (Children’s Hospital of Philadelphia) for his critical reading of the article and Dr Paolo Cotzia (Thomas Jefferson University Hospital, Philadelphia) for his contribution with histological and immunohistochemical studies. This work is dedicated to Mario A. Ferrari (1948–2011) and Emma R. Branchetti (1914–1987).
Sources of Founding
This project was supported by the Harrison Memorial Fund of the University of Pennsylvania, (GF) and by the Marjorie G Bunnell Charitable Fund of The Valley Hospital Foundation (Drs Grau and Ferrari).
Nonstandard Abbreviations and Acronyms
- AA
ascending aortic replacement
- AGE
advanced glycation end products
- AI
aortic insufficiency
- AS
aortic stenosis
- AVR
aortic valve replacement
- BAV
bicuspid aortic valve
- BSA
body surface area
- HMGB-1
high mobility group box 1
- RAGE
receptor for advanced glycation end product
- sRAGE
soluble receptor for advanced glycation end product
- TAD
thoracic aortic dissection
- TAV
tricuspid aortic valve
- VSMC
vascular smooth muscle cell
Footnotes
Disclosures
None.
References
- 1.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55:2789–2800. doi: 10.1016/j.jacc.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 2.Evangelista A Bicuspid aortic valve and aortic root disease. Curr Cardiol Rep. 2011;13:234–241. doi: 10.1007/s11886-011-0175-4. [DOI] [PubMed] [Google Scholar]
- 3.Wittwer C, Lehner J, Fersching D, Siegele B, Stoetzer OJ, Holdenrieder S. Methodological and preanalytical evaluation of a RAGE immunoassay. Anticancer Res. 2012;32:2075–2078. [PubMed] [Google Scholar]
- 4.Roberts WC, Ko JM, Hamilton C. Comparison of valve structure, valve weight, and severity of the valve obstruction in 1849 patients having isolated aortic valve replacement for aortic valve stenosis (with or without associated aortic regurgitation) studied at 3 different medical centers in 2 different time periods. Circulation. 2005;112:3919–3929. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka R, Yoshioka K, Niinuma H, Ohsawa S, Okabayashi H, Ehara S. Diagnostic value of cardiac CT in the evaluation of bicuspid aortic stenosis: comparison with echocardiography and operative findings. AJR Am J Roentgenol. 2010;195:895–899. [DOI] [PubMed] [Google Scholar]
- 6.Parish LM, Gorman JH 3rd, Kahn S, Plappert T, St John-Sutton MG, Bavaria JE, Gorman RC. Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35:941–945; discussion 945. [DOI] [PubMed] [Google Scholar]
- 7.Friedman T, Mani A, Elefteriades JA. Bicuspid aortic valve: clinical approach and scientific review of a common clinical entity. Expert Rev Cardiovasc Ther. 2008;6:235–248. [DOI] [PubMed] [Google Scholar]
- 8.Lewin MB, Otto CM. The bicuspid aortic valve: adverse outcomes from infancy to old age. Circulation. 2005;111:832–834. [DOI] [PubMed] [Google Scholar]
- 9.Rajamannan NM. Bicuspid aortic valve disease: the role of oxidative stress in Lrp5 bone formation. Cardiovasc Pathol. 2011;20:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fedak PWM. Clinical and Pathophysiological Implications of a Bicuspid Aortic Valve. Circulation. 2002;106:900–904. [DOI] [PubMed] [Google Scholar]
- 11.Zegdi R, Ciobotaru V, Huerre C, Allam B, Bouabdallaoui N, Berrebi A, Florens E, Fabiani JN. Detecting aortic valve bicuspidy in patients with severe aortic valve stenosis: high diagnostic accuracy of colour Doppler transoesophageal echocardiography. Interact Cardiovasc Thorac Surg. 2013;16:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiratzka LF, Bakris GL, Beckman JA, et al. American College of Cardiology Foundation, Guidelines AHATFOP, Surgery AAFT, Radiology ACO, Association AS, Anesthesiologists SOC, Interventions SFCAA, Radiology SOI, Surgeons SOT, Medicine SFV, Imaging NASFC. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. JAC. 2010;55:e27–e129. [DOI] [PubMed] [Google Scholar]
- 13.Pacini D, Leone O, Turci S, Camurri N, Giunchi F, Martinelli GN, Di Bartolomeo R. Incidence, etiology, histologic findings, and course of thoracic inflammatory aortopathies. Ann Thorac Surg. 2008;86:1518–1523. [DOI] [PubMed] [Google Scholar]
- 14.Ikonomidis JS, Ruddy JM, Benton SM Jr, Arroyo J, Brinsa TA, Stroud RE, Zeeshan A, Bavaria JE, Gorman JH 3rd, Gorman RC, Spinale FG, Jones JA. Aortic dilatation with bicuspid aortic valves: cusp fusion correlates to matrix metalloproteinases and inhibitors. Ann Thorac Surg. 2012;93:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan SF. Glycation, Inflammation, and RAGE: A Scaffold for the Macrovascular Complications of Diabetes and Beyond. Circ Res. 2003;93:1159–1169. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mautner GC, Mautner SL, Cannon RO 3rd, Hunsberger SA, Roberts WC. Clinical factors useful in predicting aortic valve structure in patients > 40 years of age with isolated valvular aortic stenosis. Am J Cardiol. 1993;72:194–198. [DOI] [PubMed] [Google Scholar]
- 18.Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, Eidem B, Edwards WD, Sundt TM 3rd, Enriquez-Sarano M. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–1112. [DOI] [PubMed] [Google Scholar]
- 19.Parolari A, Tremoli E, Songia P, Pilozzi A, Di Bartolomeo R, Alamanni F, Mestres CA, Pacini D. Biological features of thoracic aortic diseases. Where are we now, where are we heading to: established and emerging biomarkers and molecular pathways. Eur J Cardiothorac Surg. 2013;44:9–23. [DOI] [PubMed] [Google Scholar]
- 20.Ayad RF, Grayburn PA, Ko JM, Filardo G, Roberts WC. Accuracy of two-dimensional echocardiography in determining aortic valve structure in patients >50 years of age having aortic valve replacement for aortic stenosis. Am J Cardiol. 2011;108:1589–1599. [DOI] [PubMed] [Google Scholar]
- 21.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. miR-143 and miR-145: molecular keys to switch the phenotype of vascular smooth muscle cells. Circ Cardiovasc Genet. 2011;4:197–205. [DOI] [PubMed] [Google Scholar]
- 22.LeMaire SA, Wang X, Wilks JA, Carter SA, Wen S, Won T, Leonardelli D, Anand G, Conklin LD, Wang XL, Thompson RW, Coselli JS. Matrix metalloproteinases in ascending aortic aneurysms: bicuspid versus trileaflet aortic valves. J Surg Res. 2005;123:40–48. [DOI] [PubMed] [Google Scholar]
- 23.Kang H, Hata A. MicroRNA regulation of smooth muscle gene expression and phenotype. Curr Opin Hematol. 2012;19:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano Amarelli C, Scardone M, Di Meglio F, Guerra G, Scarano M, Vitale S, Castaldo C, Montagnani S. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130:504–511. [DOI] [PubMed] [Google Scholar]
- 25.Davis-Dusenbery BN, Chan MC, Reno KE, Weisman AS, Layne MD, Lagna G, Hata A. down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J Biol Chem. 2011;286:28097–28110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM Jr, Elefteriades JA, Coady MA. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg. 2007;83:1338–1344. [DOI] [PubMed] [Google Scholar]
- 27.Fedak PW, de Sa MP, Verma S, Nili N, Kazemian P, Butany J, Strauss BH, Weisel RD, David TE. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003;126:797–806. [DOI] [PubMed] [Google Scholar]
- 28.Elefteriades JA. Reasons to Investigate the Soluble Receptor for Advanced Glycation End-Product (sRAGE) Pathway in Aortic Disease. aorta. 2013:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barlovic DP, Thomas MC, Jandeleit-Dahm K. Cardiovascular disease: what’s all the AGE/RAGE about? Cardiovasc Hematol Disord Drug Targets. 2010;10:7–15. [DOI] [PubMed] [Google Scholar]
- 30.Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KAM. RAGE biology, atherosclerosis and diabetes. Clin Sci. 2011;121:43–55. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. [DOI] [PubMed] [Google Scholar]
- 32.Das D, Gawdzik J, Dellefave-Castillo L, McNally EM, Husain A, Raman J, Hofmann Bowman MA. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol. 2012;60:775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid FX, Bielenberg K, Schneider A, Haussler A, Keyser A, Birnbaum D. Ascending aortic aneurysm associated with bicuspid and tricuspid aortic valve: involvement and clinical relevance of smooth muscle cell apoptosis and expression of cell death-initiating proteins. Eur J Cardiothorac Surg. 2003;23:537–543. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


