Abstract
Background:
Glucose-stimulated insulin secretion (GSIS) from the pancreatic β-cell involves several intracellular metabolic events which lead to the translocation of insulin granules towards the membrane for fusion and release. It is well established that loss of β-cell function and decreased GSIS underlie the pathogenesis of diabetes. Evidence from several laboratories, including our own, demonstrated requisite roles of Rac1 and phagocyte-like NADPH oxidase (Nox2)-derived reactive oxygen species (ROS) in optimal function of the pancreatic β-cell, including GSIS. However, it is becoming increasingly clear that prolonged exposure of β-cells to hyperglycemic conditions, leads to sustained activation of Rac1-Nox2 signaling axis culminating in excessive generation of intracellular ROS (oxidative stress) and β-cell dysregulation and demise. Such “cytotoxic” effects of ROS appear to be mediated via the stress-activated protein kinases/mitogen-activated protein kinases (SAPK/MAPK) signaling pathways.
Objective:
This review discusses our current understanding of regulation and functions of the conventional MAPKs, namely, ERK1/2, JNK1/2 and p38MAPK.
Conclusion:
The MAPK pathways are activated in the presence of various stress stimuli including intracellular ROS, via distinct signaling cascades. Once activated, MAPKs participate in specific intracellular signaling processes via interaction with several downstream kinases including the MAPK-activated protein kinases (MAPKAPKs) and transcription factors including c-jun and p53. We have provided an overview of existing evidence in the islet β-cell on the regulatory roles of these MAPKs in mediating cellular responses to alterations in intracellularly generated ROS, which is mediated by the Rac1-Nox2 signaling module. Additionally, we enlisted recent patents developed to improve β-cell function in diabetes and novel pharmacological agents that target oxidative stress and MAPK pathways.
Keywords: Glucotoxicity, MAPK signaling, NADPH oxidase-2, oxidative stress, pancreatic β-cell, Rac1
INTRODUCTION
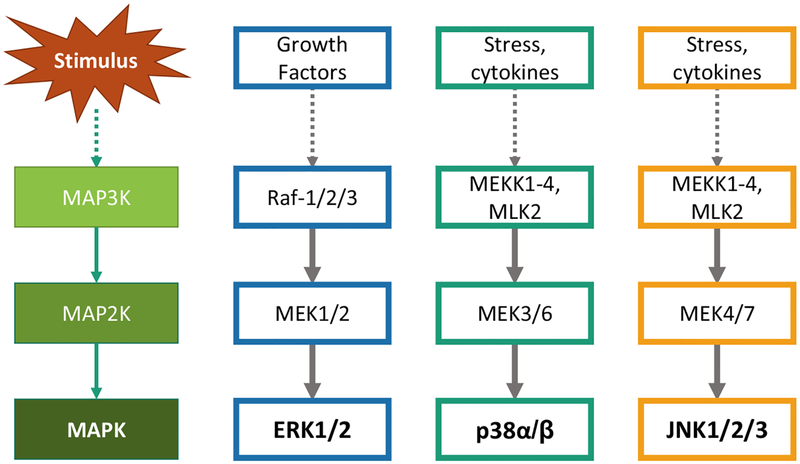
Mitogen-Activated Protein Kinases are evolutionary conserved serine-threonine protein kinases that are activated in response to extracellular stress stimuli. In mammals, these MAPKs can be categorized into conventional and atypical MAPKs. As depicted in Fig. (1), the conventional MAPKs include ERK1/2, JNK1/2, p38MAPK and ERK5 isoforms, which are activated by the classical three-tier kinase cascade that is comprised of upstream MAPK kinase kinase (MAP3Ks) and MAPK kinase (MAP2Ks) [1–3]. The activation mechanisms of atypical MAPKs, including ERK3/ERK4, NLK and ERK7 remain to be further studied [4]. Once activated, these ubiquitously expressed MAPKs phosphorylate a wide range of downstream substrate proteins at their serine/threonine residues [3]. These substrate proteins include transcription factors such as Elk-1, c-jun, ATF, STAT3, p53 and downstream kinases including the MAPK-activated kinases (MAPKAPKs). Such interactions are mediated by docking interactions and scaffolding proteins which ensure optimal enzyme-substrate interaction and specificity [5]. Therefore, the MAPK pathways mediate signal integration and induce various cellular responses including proliferation, differentiation, cell cycle arrest and apoptosis [2, 6, 7]. The regulation and functional role of conventional MAPKs have been extensively studied. In this review, we will discuss our current understanding of ERK1/2, JNK1/2 and p38MAPK isoforms, focusing on their structure, regulation and function.
Fig. (1).
Activation of MAPK signaling pathway: The conventional MAPKs ERK1/2, JNK1/2 and p38MAPK are activated in the presence of specific stress stimuli, mediated by their respective upstream MAP3Ks which activate MAP2Ks that in turn phosphorylate and activate their substrate MAPKs. [Modified from Cargnello M, et al. Ref. 3]. (The color version of the figure is available in the electronic copy of the article).
THE CONVENTIONAL MAPK PATHWAYS
The ERK1/2 Module
ERK 1 and 2 isoforms share near 84% homology in their amino acid sequence and are commonly referred to as ERK1/2 [3, 8]. These ubiquitously expressed protein kinases are activated by phosphorylation at two phospho-acceptor sites, tyrosine and threonine, which are separated by a glutamate residue, in their activation loop (the TEY motif). Although numerous studies have suggested that ERK1 and ERK2 share many similar functional roles, evidence from genetic ablation studies have indicated differential roles of these two isoforms in cellular functions. For example, studies by Vantaggiato et al. have demonstrated that siRNA-mediated knockdown of ERK1 promotes Ras-ERK2-dependent cell proliferation in mouse embryonic fibroblasts, while ectopic expression of ERK1 ablated Ras-dependent tumor formation [9]. However, Lefloch et al. have suggested that all known stimuli activate both ERK1 and ERK2 in parallel, and their functional role in cell proliferation is dependent on total ERK activity [10].
Mechanisms of Activation of ERK1/2
ERK1/2 are mostly activated by growth factors, which is mediated by the ERK1/2 module consisting of MAP3Ks (Raf isoforms) and MAP2Ks (MEK1 and 2) [11, 12]. Upon ligand binding, these surface receptors are activated, which results in receptor dimerization and auto-phosphorylation of Tyr residues of their intracellular domains. This results in the recruitment of proteins that possess a SH2 or phospho-tyrosine-binding domains, such as Grb2. This, in turn, leads to recruitment of SOS, a guanine nucleotide exchange factor (GEF) from the cytosol, whereby, it catalyzes the GDP/GTP exchange of the small G-protein, Ras. Activated Ras (GTP-bound) interacts with Raf isoforms (A-Raf, B-Raf and C-Raf), the initiating MAP3K of the ERK1/2 module [3]. This results in the activation of Raf isoforms by an intricate process involving phosphorylation at multiple residues and dimerization [13]. Raf kinases have limited substrate specificity and activate MEK 1 and 2. Studies by Zheng et al. have shown that phosphorylation of serine-218 and serine-222 in the activation segments of MEK1 are required for their functional activation [14]. The corresponding phosphorylation sites on MEK2 are serine-222 and serine-226. As recently reviewed [15], the only known downstream substrates for MEK1/2 are ERK1 or ERK2. Activated MEK1/2 activate ERK1/2 by phosphorylation of the threonine and tyrosine residues in the TEY motif.
Downstream Substrates/Biological Functions
Activated ERK1/2 are known to interact substrate proteins involved in cell growth, proliferation, migration, differentiation and cell survival [3, 12]. Recent studies in cancer cell lines have also reported apoptotic functions of the MEK-ERK pathway [2]. These substrate proteins are known to be localized both in the cytosolic and nuclear fractions. The cytoplasmic substrates include ribosomal S6 kinase (RSK) family of proteins, which are the major downstream targets of the ERK module, and are involved in cell proliferation, growth and survival [16]. These family of kinases are known to inactivate apoptotic proteins, while promoting cell proliferation by upregulating several substrates involved in gene transcription and protein synthesis. ERK1/2 are also known to mediate cell motility by interacting with and phosphorylating actin-binding proteins involved in cytoskeletal remodeling [17]. In addition to the cytoplasmic functions, multiple studies have reported nuclear translocation of ERK1/2 upon activation, whereby, they interact with transcription factors. Several active and passive mechanisms have been proposed to be involved in nuclear accumulation and detachment of cytoplasmic anchors of ERKs [18]. Recent studies have also identified a nuclear localization sequence, which is phosphorylated upon stimulation. In the nucleus, ERK1/2 phosphorylate nuclear target proteins which include transcription factors and their regulators. Elk-1 is one of the major nuclear substrate for ERK1/2, which mediates the transcription of c-Fos. C-Fos, along with c-jun, makes up the AP-1 transcription factor that is involved in multiple cellular processes following extra-cellular stimulation [19, 20]. ERK1/2 are also known to stabilize c-Fos by phosphorylation at serine-374 thereby preventing its degradation [21]. Additionally, ERK1/2 has also implicated in actin polymerization via its interaction with actin regulatory proteins including synapsin I, FAK and myosin light-chain kinase [22].
The JNK1/2 Module
JNK1, JKN2 and JNK3, which are ~85% identical in their amino acid sequence, are activated by dual phosphorylation at tyrosine and threonine residues. These two phosphorylation sites are separated by a proline residue (TPY] in the activation loop [3, 8]. They are encoded by three genes JNK1, JNK2 and JNK3 which can be alternatively spliced giving rise to more than 10 splice variants. Although JNK1/2 are ubiquitously expressed in several tissues and mostly have overlapping cellular functions, JNK3 has been shown to be expressed mostly in neurons and cardiac myocytes [23–25]. Therefore, JNK3 is relatively tissue-specific and known to be involved in functions distinct from JNK1/2. As recently reviewed by Antoniou and associates, multiple studies have reported the role of JNK3 in neurodegeneration [25]. The authors suggest that increased expression of JNK3 in the nervous system and its contributory role in neurotoxicity, make it a potential molecular target in the development of therapeutic strategies.
Mechanisms of Activation of JNK1/2
JNK1/2 are activated by phosphorylation under the regulatory control of MAP3Ks and MAP2Ks involved in the JNK signaling module. This signaling cascade is initiated in the presence of a stress stimuli including inflammatory cytokines, oxidative stress, hypoxia and ionizing radiations. One of the MAP3Ks involved in the activation of JNKs is the apoptosis signal-regulating kinase-1/2 (ASK-1/2), which are activated in response to oxidative stress and TNF-receptor activation. It has been demonstrated that in response to inflammatory TNF and oxidative stress, TRAF2 and TRAF6 are recruited which interact with ASK-1/2, thereby inducing its auto-phosphorylation and functional activation [26, 27]. In addition, as recently reviewed by Craige et al., mixed lineage kinases (MLK) also seem to play a major role in the activation of JNK signaling module [28]. A recent study by Lee and associates demonstrated that MLK3 functions similar to ASK-1 as a MAP3K and is directly involved in the activation of JNKs induced by increased ROS concentrations [29]. They further demonstrated that genetic ablation of MLK3 or in the presence of a MLK3 inhibitor, K252A, resulted in increased phosphorylation levels of ERKs and decreased phosphorylated JNKs, and conferred resistance to cell death in the presence of oxidative stress. In the presence of stimulation, MLK3 is activated by TRAF-mediated ubiquitination and autophosphorylation [30]. Notably, studies by Sharma et al. have suggested roles of small G-proteins, Cdc42 and Rac1 in the MLK3-dependent activation of JNK1/2 in response to saturated fatty acids in hepatocytes [31]. Activation of these MAP3Ks results in the phosphorylation of downstream dual-specificity kinases MKK4 and MKK7. These MAP2K, although similar to MEK1/2, specifically activate JNK1/2 but do not interact with ERKs [2]. It has also been suggested that both MKK4 and MKK7 are requisite for activating JNK1/2 by phosphorylation of threonine and tyrosine at TPY motifs [32].
Downstream Substrates/Biological Functions
The major functional role of activated JNKs is the phosphorylation of c-jun, a member of AP-1 transcription factor family [3]. Phosphorylation at Serine-63 and Serine-73 have been reported to be requisite for the transcriptional activation of c-jun [33]. Therefore, JNK1/2 mediates AP-1 complex formation through c-jun phosphorylation, and thereby, mediates the transcription of AP-1 target genes involved in cell cycle and apoptosis. Indeed, genetic depletion of JNK isoforms conferred resistance to chemotherapy and UV radiation in mice [2]. JNK1/2 have also known to interact with other nuclear substrate proteins including ATF-1, Elk-1, STAT3, c-myc and p53 [34]. In addition, earlier studies have also suggested that JNKs induce apoptosis by interacting with its substrate proteins of the outer mitochondrial membrane. Studies by Aoki et al. have reported JNK-dependent release of cytochrome C and activation of caspases in response to oxidative stress, without new protein synthesis [35]. Furthermore, they demonstrated accumulation of JNK in the mitochondrial fraction upon activation, and that active JNK was sufficient to cause cytochrome C release from isolated mitochondria. These observations suggest the involvement of non-nuclear mechanisms in JNK-induced apoptotic machinery [36]. Additionally, recent findings have also implicated JNK1/2 in the development of insulin resistance and defective insulin receptor signaling. Phosphorylation of insulin receptor substrate (IRS-1) at serine-307 by JNK1/2 has been suggested to be detrimental to the interaction of IRS-1 and insulin receptor, thereby blocking insulin signaling [37]. Since, JNKs are known to be activated by TNFα, which is secreted by adipocytes, it has been suggested that JNK1/2-induced phosphorylation of IRS-1 could represent a mechanism involved in obesity-related insulin resistance [38]. Additional substrate proteins of JNK1/2 including adaptor proteins and cell motility proteins have also been reported [34].
The p38 Module
The p38 family of MAPKs include four isoforms namely p38α, p38β, p38ɣ and p38δ which share about 60% homology in their amino acid sequence [8]. These isoforms are encoded by distinct genes, with p38α and p38β ubiquitously expressed in multiple tissues, while p38ɣ and p38δ have more restricted tissue-expression profiles. Activation of p38MAPKs are induced by phosphorylation at threonine and tyrosine residues which are separated by a glycine residue in their activation loop (TGY motifs). Studies involving genetic depletion of specific p38 isoforms have suggested overlapping functions of these proteins [39]. However, p38α and p38β possess substrate binding motifs distinct from p38ɣ and p38δ. Therefore, these kinases, although perform overlapping functions, exhibit differential substrate selectivity [40].
Mechanisms of Activation
Similar to the JNKs, p38MAPKs are activated in the presence of inflammatory cytokines, oxidative stress, UV radiation and hypoxia [3]. This is mediated by p38MAPK phosphorylation via the p38MAPK signaling module. Inflammatory cytokines have been shown to activate JNK and p38MAPKs by recruiting TRAF E3 ubiquitin ligases. The ubiquitin ligase activity of TRAF proteins induce activation of several MAP3Ks involved in p38MAPK activation including ASK1, MLK-3 and TAK-1 [41]. These MAP3Ks have also been shown to be activated in presence of oxidative stress [42]. Once activated, MAP3Ks further phosphorylate the major MAP2Ks responsible for phosphorylating p38MAPKs, namely MKK3 and MKK6. It is noteworthy that Rac1 and Cdc42 have been shown to induce MLK3-dependent activation of MKK4/7-JNK1/2 signaling axis [43]. However, MLK3 is also known to phosphorylate MKK3/6, implicating its involvement in activating p38MAPK [44]. In this context, using pharmacological inhibitors of Rac1 function, we have demonstrated its role in the activation of p38MAPK in pancreatic β-cells [45]. Previous observations from our laboratory have also implicated the role of Rac1 in JNK1/2 activation. Therefore, as evidenced by multiple observations, JNKs and p38MAPKs are activated under similar stimulatory conditions, and also share many MAP3Ks in their activation module [46].
Downstream Substrates/Biological Functions
Activated p38MAPK interacts with several substrate proteins localized in both the nucleus and cytoplasm [47]. These include several kinases belonging to the MAPK-activated protein kinases (MAPKAPK) including MSKs, MAPKAPK-2/3 and MNK1/2. These kinases act as signal amplifiers and are involved in the activation of several target genes involved in multiple biological functions including cell differentiation, cytokine production, cell cycle regulation and apoptosis [48]. Furthermore, p38MAPK is also known to phosphorylate transcription factors including ATF, Elk-1 and p53 [49]. Studies by Teodoro et al. have indicated that ATF6 is activated by proteolytic cleavage in response to ER stress, which in turn, translocates to the nucleus and upregulates the expression of ER chaperone protein grp78 [50]. Indeed, earlier studies by Luo et al. have reported that p38MAPK-induced phosphorylation of ATF6 promotes its nuclear translocation and upregulation of grp78 [51]. Together, these findings suggest roles of p38MAPK-ATF signaling in ER stress response. Several lines of evidence have also demonstrated the role of p38MAPK in the functional activation of p53 tumor suppressor. Studies by Bulavin et al. have reported the requisite role of p38MAPK in phosphorylating p53 at several sites in the transactivation domain [52]. This phosphorylation mediates functional activation of p53 by upregulating interaction with positive modulators including p300, stabilizes p53 and prevents its MDM2-induced proteosomal degradation, thereby resulting in nuclear accumulation and transcription activation of apoptotic p53 target genes [53, 54]. In addition to pro-apoptotic functions, p38MAPK has also been shown to be involved in inflammatory responses, whereby it interacts with nuclear transcription factors such as ATF-2, NF-κB resulting in the expression of inflammatory cytokines [48, 55, 56]. p38MAPK has also been reported to be involved in other cellular functions including cell cycle check points and cell survival [48].
MAPKs IN MODELS OF PANCREATIC β-CELL DYSFUNCTION AND DIABETES
Diabetes mellitus is a complex metabolic disorder caused by insufficient insulin action in the peripheral tissues and defective insulin secretion from the pancreatic β-cells. It is becoming increasingly clear that decreased insulin secretory response and loss of β-cell mass precedes the onset of diabetes [57, 58]. Evidence from multiple studies including from own laboratory, have demonstrated that chronic exposure of pancreatic β-cells to elevated levels of glucose, free fatty acids and inflammatory cytokines results in impaired insulin secretion and β-cell death [59, 60]. Studies from our own laboratory have reported the role of the small G-protein, Rac1, and phagocyte-like NADPH oxidase (Nox2) in causing oxidative stress, resulting in β-cell dysfunction, following exposure to glucolipotoxic conditions and inflammatory cytokines [45, 61, 62]. Although previous studies have reported that Rac1 function and physiological levels of Rac1-Nox2-derived reactive oxygen species (ROS) are requisite for glucose-stimulated insulin secretion [63–65], recent evidence suggest that sustained activation of Rac1-Nox2 enzyme and excess accumulation of ROS underlie β-cell dysfunction under diabetic conditions [66, 67]. However, understanding the downstream roles of MAPK pathways in models of diabetes would provide crucial insights into the mechanisms involved in β-cell dysfunction.
Several lines of evidence have suggested a positive regulatory role of the ERK1/2 signaling module in glucose-stimulated insulin secretion and β-cell survival. Earlier studies have reported activation of ERK1/2 signaling by glucose under physiological conditions [22, 68–70]. Benes et al. have suggested that glucose-mediated activation of ERK1/2 is requisite for insulin gene transcription [71]. They reported significant reduction in glucose-induced rat insulin I gene promoter activity and insulin mRNA levels in MIN6 β-cells co-incubated with PD98059, an ERK1/2 inhibitor. Furthermore, studies by Longuet et al. have demonstrated a cytoplasmic role of ERK1/2 in mediating insulin release from MIN6 β-cells and rodent pancreatic islets [72]. The ERK1/2 signaling module is typically activated by an upstream small G-protein activation (Ras), which in turn activates Raf (MAP3K) and MEK1/2 (MAP2K). Indeed studies by Arnette et al. have reported inhibition of glucose-induced ERK1/2 activation in INS-1 cells expressing kinase-negative mutants of Ras and Raf kinases [73]. However, studies by Trumper et al. have reported the role of Rap1, another small GTPase, in the activation of Raf-MEK-ERK signaling axis in human pancreatic β-cells upon stimulation with glucose and GLP-1 [74]. These differential regulation by small GTPases may be attributed to the different beta-cell line used in these studies. In addition to its roles in physiological β-cell functions, ERK1/2 signaling module has also been implicated in the activation of pro-survival and proliferative pathways. For example, Wijesekara and associates have reported protective functions of Adiponectin, a hormone secreted by adipocytes, in pancreatic β-cells [75]. The presence of a MEK1 inhibitor blocked the actions of adiponectin, suggesting the role of ERK1/2 in adiponectin signaling. Together, these findings implicate pro-survival function of ERK1/2 module in pancreatic β-cells. Interestingly, a recent study by Yeo and associates reported defects in cytoskeletal organization and cell death in ERoSHK6 β-cells exposed to chronic hyperglycemic conditions [76]. They observed aberrant morphological changes, cell-cell adhesion under hyperglycemic conditions, which was prevented in presence of ERK1/2 inhibitor. However, the presence of ERK1/2 inhibitor did not prevent, but rather promoted β-cell death induced by glucotoxic conditions. The authors concluded that ERK1/2 module could be mediating diverse cellular functions in the β-cell depending on the stimulatory conditions and the cell type used. Further studies focusing on identifying the specific interacting substrates of ERK1/2 are required to understand the role of this MAPK pathway in the β-cell.
In the context of β-cell dysfunction, it is well established that inflammatory cytokines induce cytotoxic effects in Type1 diabetes by activating apoptotic JNK1/2 [77]. Interestingly, it is also becoming increasingly clear that, viral infection of β-cells is one of the major risk factor of T1DM. Several enteroviruses, including Coxsackievirus B, have been demonstrated to induce loss of β-cell mass, mainly by mediating release of inflammatory cytokines and inducing an inflammatory response [78, 79]. It is noteworthy, however, that studies in other cell types have shown increased MAPK-dependent synthesis of inflammatory cytokines, following exposure to enteroviruses [80, 81]. In addition, studies by Peng et al. suggest a requisite role of p38MAPK and JNK1/2 in viral replication [80]. However, the role of MAPKs in β-cell dysfunction induced by viral infection remains to be further explored.
Previous studies in our own laboratory have demonstrated the role of Rac1-Nox2 enzyme complex in beta-cell dysfunction induced by inflammatory cytokines in models of Type1 diabetes [61]. Furthermore, studies by Syed et al. have demonstrated abnormal activation of Rac1 and increased ROS generation in the ZDF rat, a model for type 2 diabetes and islets from human T2DM patients [82]. Furthermore, islets from the ZDF rat showed decreased phosphorylation of pro-survival ERK1/2. In addition, apoptotic JNK1/2 signaling was significantly increased in INS-1 832/13 cells under glucolipotoxic conditions, diabetic ZDF and human islets from T2DM patients [62, 82]. Indeed, earlier studies by Kawamori et al. have reported that JNK1/2 mediates cytoplasmic translocation of PDX-1 resulting in suppression of insulin gene expression, under glucotoxic and oxidative stress conditions [83]. Recent studies in our laboratory have also demonstrated activation of apoptotic p38MAPK in clonal INS-1 832/13 β-cells, rodent [45], ZDF and human pancreatic islets [unpublished] exposed to glucotoxic conditions. Using pharmacological inhibitors of Rac1-Nox2 enzyme complex, we reported the involvement of this holoenzyme and associated oxidative stress in the activation of p38MAPK. These findings suggest the role of Rac1-Nox2-derived oxidative stress in the activation of apoptotic MAPK pathways in the β-cell under glucolipotoxic conditions [60]. Previous reports by Kyaw and associates have examined the role of NADPH oxidase-derived ROS in Angiotensin-induced vascular smooth muscle hypertrophy [84]. Their observations have demonstrated that anti-oxidant treatment confers protection to VSMCs against Angiotensin-induced oxidative stress, mostly by inhibiting JNK1/2 and p38MAPK activation [85].
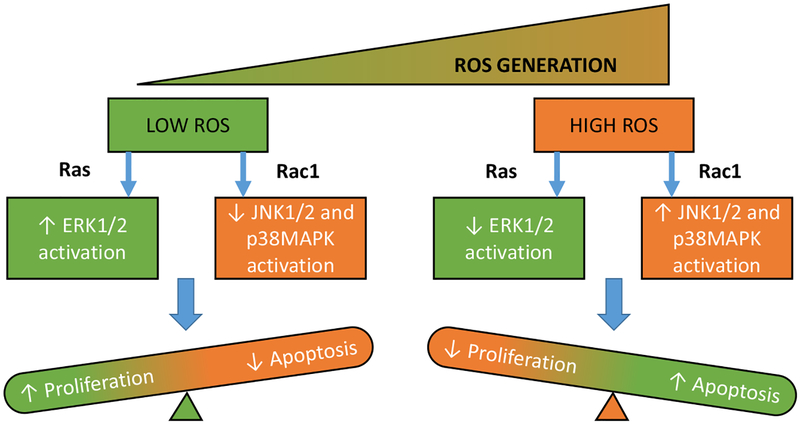
Several recent investigations have implicated the role of MLK3, a MAP3K involved in the regulation of MAPK pathways, as a potential trigger for the pathological roles of MAPKs [28]. Earlier studies have demonstrated that MLK3 is activated by directly binding to Cdc42 and Rac1, which in turn activates the JNK1/2 signaling axis [43]. These observations are further supported by Sharma and associates who reported the involvement of Cdc42 and Rac1-induced activation of MLK3-JNK1/2 signaling in hepatocytes exposed to saturated fatty acids [31]. In addition, studies by Lee et al. have indicated apoptotic functions of MLK3 in response to oxidative stress [29]. They have shown that low physiological levels of ROS stimulate proliferative ERK1/2 activity, and such an increase was inversely related to increasing ROS concentrations. Furthermore, they observed increased apoptotic MLK3-dependent JNK1/2 activity as a function of increasing ROS levels, suggesting the role of MLK3-JNK1/2 activation in oxidative damage. In pancreatic β-cells, Humphrey and associates have demonstrated the role of MLK3 in cytokine-induced pancreatic β-cell dysfunction [30]. They reported increased activation of MLK3 in pancreatic islets of non-obese Type1 diabetic mice, following leukocyte infiltration. These findings, together suggest the upstream regulatory role of MLK3 in the activation of apoptotic MAPKs and represents a potential therapeutic target for modulating cellular responses to stress stimuli [28]. However, the role of MLK-3 in β-cell dysfunction under diabetic conditions remains to be further elucidated.
CURRENT & FUTURE DEVELOPMENTS
Evidence from previous and recent studies have dissected the roles of MAPK signaling pathways in several cellular responses to extraneous stimulation [3, 8]. These kinases play a major role as signal integrators and are involved in several cellular processes including proliferation and apoptosis. Previous experiments in the β-cell have shown that physiological levels of ROS are requisite for normal cell functioning. However, excess generation and accumulation of ROS leads to oxidative damage and results in β-cell demise. As discussed above, several studies have implicated the role of ERK1/2 in insulin secretion and β-cell survival. Furthermore, recent evidence from our laboratory have demonstrated the role of Rac1-Nox2-induced oxidative stress in activating apoptotic p38MAPK and JNK1/2 signaling modules under the duress of glucolipotoxicity and inflammatory cytokines (Fig. 2). However, several knowledge gaps still exist with regard to the upstream regulators of MAPKs under specific stress stimuli. Indeed, emerging evidence implicate MLK3, as a crucial switch, regulating proliferative and apoptotic MAPK functions in response to varying concentrations of ROS [29]. Furthermore, the downstream substrate proteins of JNK1/2 and p38MAPK including the transcription factors p53, ATF, STAT also need to be further examined in the β-cell. In this context, Cnop and associates overviewed potential mechanisms underlying pancreatic β-cell dysfunction and demise in Type 1 and Type 2 diabetes [86]. Proinflammatory cytokine-induced islet death involves activation of gene networks that are regulated precisely by transcription factors such as NF-κB and STAT-1. In contrast, high glucose- or free fatty acid-mediated β-cell dysregulation appears not to involve NF-κB and nitric oxide-sensitive mechanisms [86]. Therefore, additional investigations are necessary to identify the candidate signaling mechanisms that require MAP Kinase-mediated regulation of various transcription factors in the islet β-cell.
Fig. (2).
Proposed model for ROS-dependent activation of MAPKs: Low-levels of ROS activate proliferative ERK signaling under the regulatory control of Ras, while increased levels of ROS lead to Rac1-induced activation of apoptotic JNK1/2 and p38MAPK pathways. [Modified from Lee HS, et al. Ref. 29]. (The color version of the figure is available in the electronic copy of the article).
Recent patents on pharmacological agents designed to target MAPKs in the treatment of various diseases is provided in Table 1 [87–95]. Peptide-based inhibitors of JNK signaling pathway are currently under development for the treatment of diseases caused by altered JNK signaling [87, 88]. Furthermore, inhibition of p38α MAPK with derivatives of thiophene, is being examined as a treatment modality for various diseases including inflammation [89]. As discussed in this review, modulating the activity of upstream MAP3Ks and MAP2Ks can be used as a tool to regulate MAPK activity. Imidazopyridine compounds have been reported to block MLK, in particular MLK3 activity, and are currently being examined for their therapeutic effects [90]. In addition, inhibitors of TAK-1, a common MAP3K for JNK1/2 and p38MAPK, have been reported to confer glycemic control in mammals [91]. As evidenced by several studies in multiple cell types, excess ROS generation and oxidative stress results in the activation of apoptotic p38MAPK and JNK1/2 signaling. A recent invention by Ranayhossaini and associates relates to a method of treating disorders associated with oxidative stress using inhibitors of NADPH oxidase 1 isoform (Nox1) [92]. In the context of β-cell, a recent patent discloses the use of Nox1 inhibitors to protect β-cells in the treatment of diabetes [93]. Additional patents on recent methods developed to prevent pancreatic β-cell dysfunction are also provided in Table 1 [94, 95].
Table 1.
Summary of Recent Patents Developed for Modulating MAPK Function.
| Inventors | Patent Number | Description |
|---|---|---|
| Combette, J.M. [87] | WO2016055160 | New use of cell-permeable peptide inhibitors of the JNK signal transduction pathway for the treatment of various diseases |
| Bonny, C. [88] | EP1911458 | Cell-permeable peptide inhibitors of the JNK signal transduction pathway |
| Wang, B.H. [89] | WO2016029263 | Inhibitors of MAPK |
| Goodfellow, V.S. [90] | US20140256733 | Mixed lineage kinase inhibitors and method of treatments |
| Oommen, A.M. [91] | US20140154263 | Use of TAK-1 inhibitor to achieve glycemic control in mammals |
| Ranayhossaini, D.J. [92] | US20160089414 | Novel inhibitors of Nox1 |
| Taylor-Fishwick, D. [93] | WO2014153227 | Methods of preserving and protecting pancreatic beta cells and treating or preventing diabetes by inhibiting NOX-1 |
| Kim, D.J. [94] | W02014017787 | Pharmaceutical composition having antidiabetic and antiobesity activities |
| Antinozzi, P.A. [95] | WO2014117080 | Compositions and methods for maintaining and improving pancreatic islet cell function and stability |
In conclusion, studies focusing on the regulation and functions of MAPK signaling would provide valuable insights into their contributory role(s) in pancreatic β-cell dysfunction and diabetes, and are critical for the development of novel therapeutics to modulate their function and prevent β-cell death.
ACKNOWLEDGMENTS
This work was supported by grants to Anjaneyulu Kowluru from the Department of Veterans Affairs (Merit Review Program; IBX002801A) and National Institutes of Health, and a Research Stimulation Award from Wayne State University. A.K is the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs (13SRCS-006). Vaibhav Sidarala received a Pre-Doctoral Fellowship from Graduate School at Wayne State University.
LIST OF ABBREVIATIONS
- ERK1/2
Extracellular Signal-Regulated Kinases
- GSIS
Glucose-Stimulated Insulin Secretion
- JNK1/2
c-jun N-Terminal Kinases
- MAPK
Mitogen-Activated Protein Kinase
- MAPK2K
Mitogen-Activated Protein Kinase Kinase
- MAPK3K
Mitogen-Activated Protein Kinase Kinase Kinase
- MAPKAPK
MAPK-Activated Protein Kinase
- MLK
Mixed Lineage Kinase
- Nox2
NADPH Oxidase 2
- p38MAPK
p38-Mitogen Activated Protein Kinase
- Rac1
Ras-related C3 Botulinum Toxin Substrate 1
- ROS
Reactive Oxygen Species
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, et al. MAP kinases. Chem Rev 2001; 101(8): 2449–76. [DOI] [PubMed] [Google Scholar]
- [2].Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004; 23(16): 2838–49. [DOI] [PubMed] [Google Scholar]
- [3].Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev 2011; 75(1): 50–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coulombe P, Meloche S. Atypical mitogen-activated protein kinases: Structure, regulation and functions. Biochim Biophys Acta 2007; 1773(8): 1376–87. [DOI] [PubMed] [Google Scholar]
- [5].Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem 2008; 283(28): 19511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene 2007; 26(22): 3100–12. [DOI] [PubMed] [Google Scholar]
- [7].Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res 2002; 12(1): 9–18. [DOI] [PubMed] [Google Scholar]
- [8].Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 2001; 22(2): 153–83. [DOI] [PubMed] [Google Scholar]
- [9].Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol 2006; 5(5): 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lefloch R, Pouyssegur J, Lenormand P. Total ERK1/2 activity regulates cell proliferation. Cell Cycle 2009; 8(5): 705–11. [DOI] [PubMed] [Google Scholar]
- [11].Roskoski R, Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res 2012; 66(2): 105–43. [DOI] [PubMed] [Google Scholar]
- [12].Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, et al. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem 1993; 268(7): 5097–106. [PubMed] [Google Scholar]
- [13].Roskoski R Jr. RAF protein-serine/threonine kinases: Structure and regulation. Biochem Biophys Res Commun 2010; 399(3): 313–7. [DOI] [PubMed] [Google Scholar]
- [14].Zheng CF, Guan KL. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J 1994; 13(5): 1123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Roskoski R, Jr. MEK1/2 dual-specificity protein kinases: Structure and regulation. Biochem Biophys Res Commun 2012; 417(1): 5–10. [DOI] [PubMed] [Google Scholar]
- [16].Carriere A, Ray H, Blenis J, Roux PP. The RSK factors of activating the Ras/MAPK signaling cascade. Front Biosci 2008; 13: 4258–75. [DOI] [PubMed] [Google Scholar]
- [17].Viala E, Pouyssegur J. Regulation of tumor cell motility by ERK mitogen-activated protein kinases. Ann NY Acad Sci 2004; 1030: 208–18. [DOI] [PubMed] [Google Scholar]
- [18].Wolf I, Rubinfeld H, Yoon S, Marmor G, Hanoch T, Seger R. Involvement of the activation loop of ERK in the detachment from cytosolic anchoring. J Biol Chem 2001; 276(27): 24490–7. [DOI] [PubMed] [Google Scholar]
- [19].Babu GJ, Lalli MJ, Sussman MA, Sadoshima J, Periasamy M. Phosphorylation of ELK-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J Mol Cell Cardiol 2000; 32(8): 1447–57. [DOI] [PubMed] [Google Scholar]
- [20].Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta 1991; 1072(2–3): 129–57. [DOI] [PubMed] [Google Scholar]
- [21].Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta 2007; 1773(8): 1285–98. [DOI] [PubMed] [Google Scholar]
- [22].Kalwat MA, Thurmond DC. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet beta cells. Exp Mol Med 2013; 45: e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci USA 2003; 100(25): 15184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev 2010; 90(4): 1507–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Antoniou X, Falconi M, Di Marino D, Borsello T. JNK3 as a therapeutic target for neurodegenerative diseases. J Alzheimers Dis 2011; 24(4): 633–42. [DOI] [PubMed] [Google Scholar]
- [26].Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol Rev 2012; 92(2): 689–737. [DOI] [PubMed] [Google Scholar]
- [27].Iriyama T, Takeda K, Nakamura H, Morimoto Y, Kuroiwa T, Mizukami J, et al. ASK1 and ASK2 differentially regulate the counteracting roles of apoptosis and inflammation in tumorigenesis. EMBO J 2009; 28(7): 843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Craige SM, Reif MM, Kant S. Mixed - Lineage Protein Kinases (MLKs) in inflammation, metabolism, and other disease states. Biochim Biophys Acta 2016; 1862(9): 1581–6. [DOI] [PubMed] [Google Scholar]
- [29].Lee HS, Hwang CY, Shin SY, Kwon KS, Cho KH. MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species. Sci Signal 2014; 7(328): ra52. [DOI] [PubMed] [Google Scholar]
- [30].Humphrey RK, Ray A, Gonuguntla S, Hao E, Jhala US. Loss of TRB3 alters dynamics of MLK3-JNK signaling and inhibits cytokine-activated pancreatic beta cell death. J Biol Chem 2014; 289(43): 29994–30004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol 2012; 56(1): 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J 2000; 352(Pt 1): 145–54. [PMC free article] [PubMed] [Google Scholar]
- [33].Li L, Feng Z, Porter AG. JNK-dependent phosphorylation of c-Jun on serine 63 mediates nitric oxide-induced apoptosis of neuroblastoma cells. J Biol Chem 2004; 279(6): 4058–65. [DOI] [PubMed] [Google Scholar]
- [34].Bogoyevitch MA, Kobe B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev 2006; 70(4): 1061–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem 2002; 277(12): 10244–50. [DOI] [PubMed] [Google Scholar]
- [36].Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene 2008; 27(48): 6245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 2002; 277(2): 1531–7. [DOI] [PubMed] [Google Scholar]
- [38].Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature 2002; 420(6913): 333–6. [DOI] [PubMed] [Google Scholar]
- [39].del Barco Barrantes I, Coya JM, Maina F, Arthur JS, Nebreda AR. Genetic analysis of specific and redundant roles for p38alpha and p38beta MAPKs during mouse development. Proc Natl Acad Sci USA 2011; 108(31): 12764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007; 1773(8): 1358–75. [DOI] [PubMed] [Google Scholar]
- [41].Xie P TRAF molecules in cell signaling and in human diseases. J Mol Signal 2013; 8(1): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dolado I, Swat A, Ajenjo N, De Vita G, Cuadrado A, Nebreda AR. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 2007; 11(2): 191–205. [DOI] [PubMed] [Google Scholar]
- [43].Teramoto H, Coso OA, Miyata H, Igishi T, Miki T, Gutkind JS. Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J Biol Chem 1996; 271(44): 27225–8. [DOI] [PubMed] [Google Scholar]
- [44].Zhou F, Xu Y, Hou XY. MLK3-MKK3/6-P38MAPK cascades following N-methyl-D-aspartate receptor activation contributes to amyloid-beta peptide-induced apoptosis in SH-SY5Y cells. J Neurosci Res 2014; 92(6): 808–17. [DOI] [PubMed] [Google Scholar]
- [45].Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A. Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic beta-cells under glucotoxic conditions: Evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem Pharmacol 2015; 95(4): 301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009; 9(8): 537–49. [DOI] [PubMed] [Google Scholar]
- [47].Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK substrates. Cell 2013; 152(4): 924–e1. [DOI] [PubMed] [Google Scholar]
- [48].Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res 2005; 15(1): 11–8. [DOI] [PubMed] [Google Scholar]
- [49].Koul HK, Pal M, Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer 2013; 4(9–10): 342–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Teodoro T, Odisho T, Sidorova E, Volchuk A. Pancreatic beta-cells depend on basal expression of active ATF6alpha-p50 for cell survival even under nonstress conditions. Am J Physiol Cell Physiol 2012; 302(7): C992–1003. [DOI] [PubMed] [Google Scholar]
- [51].Luo S, Lee AS. Requirement of the p38 mitogen-activated protein kinase signalling pathway for the induction of the 78 kDa glucose-regulated protein/immunoglobulin heavy-chain binding protein by azetidine stress: Activating transcription factor 6 as a target for stress-induced phosphorylation. Biochem J 2002; 366(Pt 3): 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, et al. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 1999; 18(23): 6845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem 1998; 273(49): 33048–53. [DOI] [PubMed] [Google Scholar]
- [54].Loughery J, Cox M, Smith LM, Meek DW. Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res 2014; 42(12): 7666–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schieven GL. The biology of p38 kinase: A central role in inflammation. Curr Top Med Chem 2005; 5(10): 921–8. [DOI] [PubMed] [Google Scholar]
- [56].Shalom-Barak T, Quach J, Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J Biol Chem 1998; 273(42): 27467–73. [DOI] [PubMed] [Google Scholar]
- [57].Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 2004; 53(Suppl 3): S16–21. [DOI] [PubMed] [Google Scholar]
- [58].Wahren J, Kallas A. Loss of pulsatile insulin secretion: A factor in the pathogenesis of type 2 diabetes? Diabetes 2012; 61(9): 2228–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta 2010; 1801(3): 289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kowluru A, Kowluru RA. Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem Pharmacol 2014; 88(3): 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Veluthakal R, Sidarala V, Kowluru A. NSC23766, a known inhibitor of Tiam1-Rac1 signaling module, prevents the onset of type 1 diabetes in the NOD mouse model. Cell Physiol Biochem 2016; 39(2): 760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 2010; 80(6): 874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sidarala V, Veluthakal R, Syeda K, Kowluru A. EHT 1864, a small molecule inhibitor of Ras-related C3 botulinum toxin substrate 1 (Rac1), attenuates glucose-stimulated insulin secretion in pancreatic beta-cells. Cell Signal 2015; 27(6): 1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Veluthakal R, Tunduguru R, Arora DK, Sidarala V, Syeda K, Vlaar CP, et al. VAV2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia 2015; 58(11): 2573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Asahara S, Shibutani Y, Teruyama K, Inoue HY, Kawada Y, Etoh H, et al. Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia 2013; 56(5): 1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Taylor-Fishwick DA. NOX, NOX who is there? The contribution of NADPH oxidase one to beta cell dysfunction. Front Endocrinol (Lausanne) 2013; 4: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, et al. Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 2009; 52(12): 2489–98. [DOI] [PubMed] [Google Scholar]
- [68].Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH. The protein kinases ERK1/2 and their roles in pancreatic beta cells. Acta Physiol (Oxf) 2008; 192(1): 11–7. [DOI] [PubMed] [Google Scholar]
- [69].Frodin M, Sekine N, Roche E, Filloux C, Prentki M, Wollheim CB, et al. Glucose, other secretagogues, and nerve growth factor stimulate mitogen-activated protein kinase in the insulin-secreting beta-cell line, INS-1. J Biol Chem 1995; 270(14): 7882–9. [DOI] [PubMed] [Google Scholar]
- [70].Benes C, Roisin MP, Van Tan H, Creuzet C, Miyazaki J, Fagard R. Rapid activation and nuclear translocation of mitogen-activated protein kinases in response to physiological concentration of glucose in the MIN6 pancreatic beta cell line. J Biol Chem 1998; 273(25): 15507–13. [DOI] [PubMed] [Google Scholar]
- [71].Benes C, Poitout V, Marie JC, Martin-Perez J, Roisin MP, Fagard R. Mode of regulation of the extracellular signal-regulated kinases in the pancreatic beta-cell line MIN6 and their implication in the regulation of insulin gene transcription. Biochem J 1999; 340 (Pt 1): 219–25. [PMC free article] [PubMed] [Google Scholar]
- [72].Longuet C, Broca C, Costes S, Hani EH, Bataille D, Dalle S. Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 beta-cell line and islets of Langerhans. Endocrinology 2005; 146(2): 643–54. [DOI] [PubMed] [Google Scholar]
- [73].Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, et al. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem 2003; 278(35): 32517–25. [DOI] [PubMed] [Google Scholar]
- [74].Trumper J, Ross D, Jahr H, Brendel MD, Goke R, Horsch D. The Rap-B-Raf signalling pathway is activated by glucose and glucagon-like peptide-1 in human islet cells. Diabetologia 2005; 48(8): 1534–40. [DOI] [PubMed] [Google Scholar]
- [75].Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem 2010; 285(44): 33623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yeo RW, Yang K, Li G, Lim SK. High glucose predisposes gene expression and ERK phosphorylation to apoptosis and impaired glucose-stimulated insulin secretion via the cytoskeleton. PLoS One 2012; 7(9): e44988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: Novel blockers of beta-cell death. Diabetes 2001; 50(1): 77–82. [DOI] [PubMed] [Google Scholar]
- [78].Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia 2002; 45(10): 1353–61. [DOI] [PubMed] [Google Scholar]
- [79].Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes. Diabetes 2008; 57(11): 2863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Peng H, Shi M, Zhang L, Li Y, Sun J, Zhang L, et al. Activation of JNK1/2 and p38 MAPK signaling pathways promotes enterovirus 71 infection in immature dendritic cells. BMC Microbiol 2014; 14: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Si X, Luo H, Morgan A, Zhang J, Wong J, Yuan J, et al. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. J Virol 2005; 79(22): 13875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, et al. Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: Role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 2011; 60(11): 2843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, et al. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 2003; 52(12): 2896–904. [DOI] [PubMed] [Google Scholar]
- [84].Kyaw M, Yoshizumi M, Tsuchiya K, Izawa Y, Kanematsu Y, Tamaki T. Atheroprotective effects of antioxidants through inhibition of mitogen-activated protein kinases. Acta Pharmacol Sin 2004; 25(8): 977–85. [PubMed] [Google Scholar]
- [85].Kyaw M, Yoshizumi M, Tsuchiya K, Kirima K, Tamaki T. Anti-oxidants inhibit JNK and p38 MAPK activation but not ERK 1/2 activation by angiotensin II in rat aortic smooth muscle cells. Hypertens Res 2001; 24(3): 251–61. [DOI] [PubMed] [Google Scholar]
- [86].Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 2005; 54(Suppl 2): S97–107. [DOI] [PubMed] [Google Scholar]
- [87].Combette JM, Deloche C New use of cell-permeable peptide inhibitors of the JNK signal transduction pathway for the treatment of various diseases. WO2016055160 (2016).
- [88].Bonny C Cell-permeable peptide inhibitors of the JNK signal transduction pathway. EP1911458 (2014).
- [89].Wang BH, Krum H, Scammells P, Vinh N, Simpson J, Chalmers D. Inhibitors of MAPK. WO2016029263 (2016).
- [90].Goodfellow VS, Nguyen TX, Ravula SB, Gelbard HA Mixed lineage kinase inhibitors and method of treatments. US20140256733 (2014).
- [91].Oommen AM, Verma MK, Bahadur U, Ramu S Use of TAK-1 inhibitor to achieve glycemic control in mammals. US20140154263 (2014).
- [92].Ranayhossaini DJ, Pagano PJ Novel inhibitors of Nox1. US20160089414 (2016).
- [93].Taylor-Fishwick D Methods of preserving and protecting pancreatic beta cells and treating or preventing diabetes by inhibiting Nox-1. WO2014153227 (2014).
- [94].Kim DJ, Jung NY Pharmaceutical composition having antidiabetic and antiobesity activities. W02014017787 (2014).
- [95].Antinozzi PA, Dover EN Compositions and methods for maintaining and improving pancreatic islet cell function and stability. WO2014117080 (2014).