Abstract
Aims/hypothesis
Metabolic abnormalities frequently develop prior to the diagnosis of type 2 diabetes and chronic kidney disease. However, it is not known whether GFR predicts the onset of type 2 diabetes.
Methods
Incident diabetes was ascertained in the Insulin Resistance Atherosclerosis Study (IRAS) (n = 864; age 40–69 years; median follow-up 5.2 years [4.5–6.6 years]; 141 incident cases of diabetes). GFR was estimated by the Modification of Diet in Renal Disease equation. We assessed the relationship between GFR and incident diabetes by logistic regression analysis. Results were adjusted for age, sex, ethnicity, clinic location, BMI, systolic blood pressure, antihypertensive treatment, family history of diabetes, insulin sensitivity and secretion, albumin to creatinine ratio, and levels of triacylglycerols, HDL-cholesterol, plasminogen activator inhibitor-1, and fasting and 2 h glucose.
Results
The relationship between GFR and incident diabetes was not linear. This relationship was statistically significant (p = 0.039) using a restricted cubic polynomial spline for GFR as a regression modelling strategy. Participants were stratified by GFR quintiles. Mean values for GFR from the first to the fifth quintile were 60.8, 71.6, 79.8, 88.2 and 109.0 ml min−1 1.73 m−2. Relative to the fourth quintile, the odds ratios of incident diabetes for the first, second, third and fifth quintiles were 2.32 (95% CI 1.06–5.05), 1.76 (95% CI 0.80–3.88), 1.26 (95% CI 0.56– 2.84) and 2.59 (95% CI 1.18–5.65), respectively.
Conclusions/interpretation
Individuals in the upper and lower ranges of GFR are at increased risk of future diabetes. GFR and type 2 diabetes may share common pathogenic mechanisms.
Keywords: Chronic kidney disease, Glomerular filtration rate, Hyperfiltration, Incidence, Insulin resistance, Type 2 diabetes
Introduction
In the USA, 20–30% of patients with type 2 diabetes develop early stages of nephropathy [1]. Tests for the presence of microalbuminuria and serum creatinine for the estimation of glomerular filtration rate are annually recommended in order to screen for or stage the level of chronic kidney disease [1]. Microalbuminuria and chronic kidney disease are determinants of nephropathy progression [2] and cardiovascular mortality [3, 4] and are associated with insulin resistance and other metabolic abnormalities [5–8]. Microalbuminuria is considered an early marker of atherosclerosis [9, 10] and a feature of the prediabetic state [11] and is associated with an increased risk of type 2 diabetes [12]. However, it is not known whether low GFR predicts the onset of type 2 diabetes.
GFR tends to increase in newly diagnosed patients with type 2 diabetes as an adaptive response to early glomerular haemodynamic changes [13]. Hyperfiltration is a risk factor for future worsening of diabetic nephropathy, even though its presence may not be sufficient for the deterioration of GFR [14]. Hyperfiltration precedes the development of microalbuminuria [15], reflects early target organ damage [16], and correlates with hypertension, insulin resistance and other metabolic disorders [17]. Nevertheless, an association between hyperfiltration and increased risk of diabetes remains to be elucidated.
The aim of this study was to examine the ability of high and low GFR to predict incident diabetes in the Insulin Resistance Atherosclerosis Study (IRAS). The IRAS is a longitudinal epidemiological study designed to analyse the relationship between cardiovascular disease, type 2 diabetes and insulin resistance [18].
Methods
Participants
The IRAS was designed to have an equal representation of participants across glucose tolerance status (normal, impaired glucose tolerance [IGT] and type 2 diabetes), ethnic origin, sex and age (40–49, 50–59 and 60–69 years). Recruitment strategies and protocols have been already published [18]. In brief, the IRAS study was conducted at four clinical centres (Oakland and Los Angeles, CA, USA; San Antonio, TX, USA; San Luis Valley, CO, USA). Sampling strategies were used to yield approximately equal numbers of participants by ethnicity, sex and glucose tolerance categories (type 2 diabetes mellitus, IGT and normal glucose tolerance). Individuals with a history of kidney dialysis or transplant, acute or chronic renal failure or serious illnesses were excluded. Study protocols were approved by local Institutional Review Boards. All participants gave written informed consent.
Baseline data were collected in two visits approximately 1 week apart (range 7–28 days). Baseline examination was completed by 1,624 individuals (48% overall recruitment rate; 56% women), which occurred between October 1992 and April 1994. The present report includes information on 864 participants (81.1% of non-diabetic participants at baseline) for whom diabetes status was ascertained after an average of 5.2 years (range 4.5–6.6 years).
Participant examination
Age, ethnicity, education, diet, family history of diabetes and pharmacological treatment were self-reported. Height, weight and waist circumference were measured following a standardised protocol. Resting blood pressure was measured three times, and the second and third measurements were averaged. Participants were asked before each visit to fast for 12 h, to abstain from intense exercise and alcohol for 24 h and to refrain from smoking during the morning of examination. During the first baseline visit, blood specimens were collected to determine the concentration of serum creatinine, blood urea nitrogen and plasma glucose, albumin, lipoproteins and plasminogen activator inhibitor-1 (PAI-1). Glucose tolerance status was ascertained by oral glucose tolerance test during the first baseline and follow-up visits.
During the second baseline visit, insulin resistance and secretion were measured by the frequently sampled intravenous glucose tolerance test. Insulin sensitivity, expressed as the insulin sensitivity index, was calculated by mathematical modelling methods (MINMOD, version 3.0 [1994], Los Angeles, CA, USA; courtesy of R. Bergman, University of Southern California). Comparisons with the hyperinsulinaemic–euglycaemic clamp indicate that insulin sensitivity index was an adequate estimate of insulin resistance [19]. Insulin secretion, expressed as the acute insulin response, was calculated as the mean of 2 and 4 min insulin concentrations after glucose administration. Acute insulin response correlated well with first-phase insulin response during the hyperglycaemic clamp [20].
Levels of albumin, glucose, triacylglycerols and HDL-cholesterol were measured using standard methods [18]. Serum and urine creatinine levels were determined by a modified kinetic Jaffe reaction [21] and urea nitrogen by standard clinical methods at the central IRAS laboratory with a Paramax PLA instrument (Baxter Diagnostic, Chicago, IL, USA) [18]. Fasting insulin concentration was measured using the dextran—charcoal radioimmunoassay, which had considerable cross-reactivity with proinsulin [22]. PAI-1 was measured with a two-site immunoassay that is sensitive to free active and latent PAI-1 but not to PAI-1 complexed with tissue plasminogen activator (inhouse assay, Laboratory for Clinical Biochemistry Research PAI-1 antigen enzyme immunoassay) [23].
Definition of variables
The urinary albumin to creatinine ratio (ACR) (albumin in mg/l and creatinine in mmol/l) was used as a measure of albumin excretion [2]. Diabetes mellitus was defined as fasting plasma glucose level ≥7.0 mmol/l, 2 h plasma glucose level ≥11.1 mmol/l and/or treatment with hypoglycaemic medication. IGT was defined as 2 h plasma glucose level ≥7.8 mmol/l in the absence of diabetes.
GFR categories
GFR was not measured by definitive methods (e.g. inulin or radioisotope studies), which are difficult to apply to epidemiological studies. Neither was cystatin C available for analysis. We chose the Modification of Diet in Renal Disease (MDRD) equation based on six variables to estimate GFR [2, 24]:
In this equation, GFR was expressed in ml min−1 1.73 m−2 body surface area, serum creatinine and blood urea nitrogen in mg/dl, albumin in g/dl and age in years. The MDRD equation tends to be more accurate than the Cockcroft—Gault equation in obese adults [2]. In addition, weight is not included in the MDRD formula; therefore, a confounding effect of adiposity on the relation of GFR to incident diabetes is less likely.
We created five strata from low renal function through to high renal function (GFR quintiles). We also generated categories with known clinical relevance such as low GFR (<60 ml min−1 1.73 m−2), high GFR (>95% CI upper limit of GFR in healthy participants [1.96 standard deviations above the mean]), and normal/near-to-normal GFR (any value not classified as high or low). Altman's method was used to calculate the 95% reference range of estimated GFR in healthy participants [25]. Healthy individuals had the following characteristics: no vegetarian diet, no history of cardiovascular disease, BMI ≥18.5 and <25 kg/m2, normal glucose tolerance and blood pressure, and ACR <17 mg/g in men and <25 mg/g in women (n = 132). Because of the decline of GFR with age, linear regression analysis was used to determine the relationship between GFR and age (GFR = 95.03 ml min−1 1.73 m−2 − [0.29 × age], p = 0.045). Absolute residuals were calculated and were found to be unrelated to age. Because of the distribution of absolute residuals (half normal), 95% of the GFR data in healthy controls were expected to fall within 0 and . Therefore, the upper limit of the 95% CI was calculated (122.89 ml min−1 1.73 m−2 − [0.29 × age]) and used as the cut-off point for high GFR.
Statistical methods
The analysis was carried out using the SAS statistical software (version 9.1, SAS Institute, Cary, NC, USA) and R project statistical software (version 2.8.1; The R Foundation for Statistical Computing, Vienna, Austria). Stratified by GFR categories, baseline characteristics were analysed by one-way analysis of covariance (continuous variables) and logistic regression analysis (rates). The relation of GFR to incident diabetes was examined by multiple logistic regression analysis in order to take into consideration the effect of potential determinants of diabetes and GFR. In separate logistic regression models, appropriate interaction terms were introduced to model interactions between GFR category and sex (GFR category and age or GFR category and ethnicity and so forth) in relation to incident diabetes. We performed another logistic regression to model incident diabetes with a restricted cubic polynomial spline for GFR to estimate the varying effects of GFR over its full range [26]. Loge− transformed values of ACR, acute insulin response and levels of triacylglycerols and PAI-1 were used in all analyses to improve discrimination and calibration of the models and to minimise the influence of extreme observations. We also used the loge transformation of (insulin sensitivity index +1) given that some participants had insulin sensitivity index =0. These variables were then back-transformed to their units for presentation in tables.
Results
A total of 1,065 non-diabetic individuals were potentially eligible for analysis; however, baseline GFR was not estimated in 43 participants and incident diabetes was not ascertained in 158 additional participants. Therefore, we used information on 864 participants (81.1% of non-diabetic participants at baseline) for whom diabetes status was ascertained at follow-up. These participants had similar baseline characteristics to counterparts who did not return to follow-up including age, sex, ethnicity, glucose tolerance status, BMI, insulin sensitivity, albuminuria and estimated GFR (all comparisons, p > 0.2).
At the baseline examination, 283 participants had IGT and 581 participants had normal glucose tolerance. Only six participants had an albumin excretion rate ≥200 mg/g (range 1.1–558.4 mg/g) and none of the participants had an estimated GFR <39 ml min−1 1.73 m−2 (range, 39.9–239.1 ml min−1 1.73 m−2). Measurement variability of GFR was not equal across GFR categories. A change in serum creatinine concentration of 8.84 µmol/l modified the estimated GFR by 2.1 to 6.0 (median 5.0), 4.5 to 18.3 (median 8.4) and 11.1 to 79.7 (median 17.6)ml min−1 1.73 m−2 in the low, normal/near-to-normal and high GFR categories, respectively.
Baseline rates of low and high GFR were 7.6% (95% CI 6.0–9.6) and 8.6% (95% CI 6.9–10.6), respectively. Low GFR was less common in African-Americans and more common in older individuals and women (Table 1). High GFR was more frequent in African-Americans and less frequent in non-Hispanic whites. In addition, low GFR was associated with antihypertensive treatment and high GFR with diastolic blood pressure (DBP), albuminuria and PAI-1 concentration.
Table 1.
Baseline characteristics by GFR categories after adjusting for age, sex, ethnicity and clinic location
| Characteristic | Low GFR | Normal/near-to- normal GFR |
High GFR | Low vs normal/ near-to-normal p value |
High vs normal/ near-to-normal p value |
|---|---|---|---|---|---|
| n | 66 | 724 | 74 | – | – |
| Age (years)a | 60.0±1.0 | 54.5±0.3 | 52.6±1.0 | <0.001 | 0.071 |
| Female sex (%)a | 75.8 (64.0−84.6) | 55.2 (51.6−58.8) | 54.1 (42.7−65.0) | 0.002 | 0.844 |
| Ethnicity | |||||
| African-American (%)a | 10.6 (5.1−20.6) | 24.9 (21.8−28.1) | 41.9 (31.2−53.4) | 0.012 | 0.002 |
| Hispanic (%)a | 33.3 (23.1−45.5) | 34.5 (31.2−38.1) | 35.1 (25.2−6.6) | 0.846 | 0.917 |
| Non-Hispanic white (%)a | 56.1 (44.0−67.5) | 40.6 (37.1−44.2) | 23.0 (15.8−33.9) | 0.016 | 0.004 |
| BMI (kg/m2) | 28.4±0.7 | 28.4±0.2 | 27.8±0.6 | 0.982 | 0.321 |
| Waist circumference (cm) | 89.5±1.5 | 90.3±0.4 | 90.3±1.4 | 0.633 | 0.947 |
| Triacylglycerol concentration (mmol/l) | 1.39±0.10 | 1.28±0.03 | 1.25±0.08 | 0.296 | 0.697 |
| HDL-cholesterol (mmol/l) | 1.25±0.04 | 1.20±0.01 | 1.26±0.04 | 0.223 | 0.135 |
| SBP (mmHg) | 120.8±1.9 | 121.4±0.6 | 124.7±1.8 | 0.691 | 0.067 |
| DBP (mmHg) | 78.2±1.1 | 77.4±0.3 | 79.6±1.0 | 0.498 | 0.041 |
| Antihypertensive medications (%) | 33.5 (22.6−46.5) | 19.2 (16.4−22.4) | 20.2 (12.5−31.0) | 0.011 | 0.799 |
| Fasting glucose concentration (mmol/l) | 5.35±0.07 | 5.44±0.02 | 5.43±0.06 | 0.232 | 0.896 |
| 2 h glucose concentration (mmol/l) | 6.58±0.23 | 6.91±0.07 | 7.08±0.21 | 0.197 | 0.457 |
| Insulin sensitivity index (MINMOD) (×10−4 min−1 µU−1 ml−1)b,c |
1.80±0.20 | 1.77±0.06 | 1.64±0.16 | 0.903 | 0.432 |
| Acute insulin response (pmol/l)b | 337.0±35.4 | 298.9±9.1 | 292.9±27.6 | 0.221 | 0.895 |
| PAI-1 concentration (ng/ml)b | 14.0±1.5 | 15.8±0.5 | 19.7±1.8 | 0.190 | 0.030 |
| ACR (mg/mmol)b | 0.98±0.11 | 0.84±0.02 | 1.11±0.12 | 0.138 | 0.006 |
| Dietary protein (g/day) | 80.4±3.9 | 77.4±1.1 | 76.8±3.7 | 0.155 | 0.891 |
| GFR (ml min−1 1.73 m−2)a | 55.4±1.4 | 80.3±0.4 | 121.3±1.4 | <0.001 | <0.001 |
Non-adjusted values
Loge-transformed variable and then back-transformed to its natural units for presentation
To convert values to SI units (×10−4 min−1 pmol−1 1−1 ) multiply by 0.167
SBP, systolic blood pressure
There were 141 incident cases of diabetes during an average of 5.2 years. Follow-up time ranged from 4.5 to 6.6 years, but 90% of the participants had the follow-up examination 4.8 to 5.8 years after the initial examination. Follow-up time was not associated with incident diabetes (OR × 1 year increase 0.89 [0.49–1.61]).
Crude rates of incident diabetes were 24.2% (95% CI 15.4–36.0), 14.9% (95% CI 12.5–17.7) and 23.0% (95% CI 14.8–33.9) for the low, normal/near-to-normal and high GFR categories, respectively. Compared with the normal/near-to-normal GFR category, the conversion rate in the low GFR category was marginally significant (p = 0.049), but that in the high GFR category was not (p = 0.072). High GFR was associated with increased odds of incident diabetes independently of the effect of confounding variables (Table 2). Diabetic risk associated with low GFR was near significance. There was no significant interaction of age, sex, race/ethnicity or glucose tolerance status on the association of high and low GFR with incident diabetes (p > 0.2).
Table 2.
Adjusted OR for predicting a 5.2 year incidence of diabetes associated with low and high GFR
| Model | Adjusted for | Low vs normal/near normal | High vs normal/near normal |
|---|---|---|---|
| 1 | Unadjusted OR | 1.82 (1.00−3.32) | 1.70 (0.95−3.03) |
| 2 | Adjusted for age, sex, race/ethnicity, clinic location and duration of follow-up |
1.40 (0.74−2.63) | 1.96 (1.08−3.56) |
| 3 | Adjusted for age, sex, race/ethnicity, clinic location, duration of follow-up, BMI, SBP, antihypertensive treatment, history of diabetes in first degree relatives, insulin sensitivity index, acute insulin response, and levels of triacylglycerols, HDL-cholesterol, PAI-1 and fasting and 2 h glucose |
2.24 (1.00−5.16) | 2.44 (1.15−5.17) |
| 4 | Adjusted for all variables in model 3 plus ACR | 2.14 (0.94−4.86) | 2.29 (1.06−4.97) |
Values are OR (95% CI)
SBP, systolic blood pressure
From the first to the fifth quintile, mean GFR values were 60.8, 71.6, 79.8, 88.2 and 109.0 ml min−1 1.73 m−2, respectively. Corresponding crude rates of incident diabetes were 20.3% (95% CI 15.0–27.0), 16.2% (95% CI 11.4–22.4), 12.7% (95% CI 8.5–18.6), 11.6% (95% CI 7.6–17.2) and 20.8% (95% CI 15.4–27.5). The first and fifth quintiles had higher conversion rates than the fourth quintile (Table 3). We observed no significant interaction of age, sex, race/ethnicity or glucose tolerance status on the relationship between GFR by quintiles and incident diabetes (p > 0.2).
Table 3.
Adjusted OR for predicting a 5.2 year incidence of diabetes associated with quintiles of GFR
| Model | 1st quintile (n = 172)a | 2nd quintile (n = 173)b | 3rd quintile (n = 173)c | 4th quintile (n = 173)d | 5th quintile (n = 173)e |
|---|---|---|---|---|---|
| Model 1 | 1.95 (1.08–3.55) | 1.48 (0.80–2.74) | 1.11 (0.58–2.13) | Reference | 2.01 (1.11–3.64) |
| Model 2 | 1.69 (0.90–3.15) | 1.46 (0.78–2.74) | 1.12 (0.58–2.15) | Reference | 2.34 (1.27–4.32) |
| Model 3 | 2.35 (1.08–5.12) | 1.74 (0.79–3.84) | 1.19 (0.53–2.69) | Reference | 2.59 (1.19–5.64) |
| Model 4 | 2.32 (1.06–5.05) | 1.76 (0.80–3.88) | 1.26 (0.56–2.84) | Reference | 2.59 (1.18–5.65) |
Values are OR (95% CI)
Model 1: unadjusted OR
Model 2: OR adjusted for age, sex, race/ethnicity, clinic location and duration of follow-up
Model 3: OR adjusted for age, sex, race/ethnicity, clinic location, duration of follow-up, BMI, systolic blood pressure (SBP), antihypertensive treatment, history of diabetes in first-degree relatives, insulin sensitivity index, acute insulin response and levels of triacylglycerols, HDLcholesterol, PAI-1 and fasting and 2 h glucose
Model 4: OR adjusted for all variables in model 3 plus ACR
GFR (ml min−1 1.73 m−2):
39.9–67.5;
67.6–75.2;
75.3–83.9;
84.0–92.9;
93.0–239.1
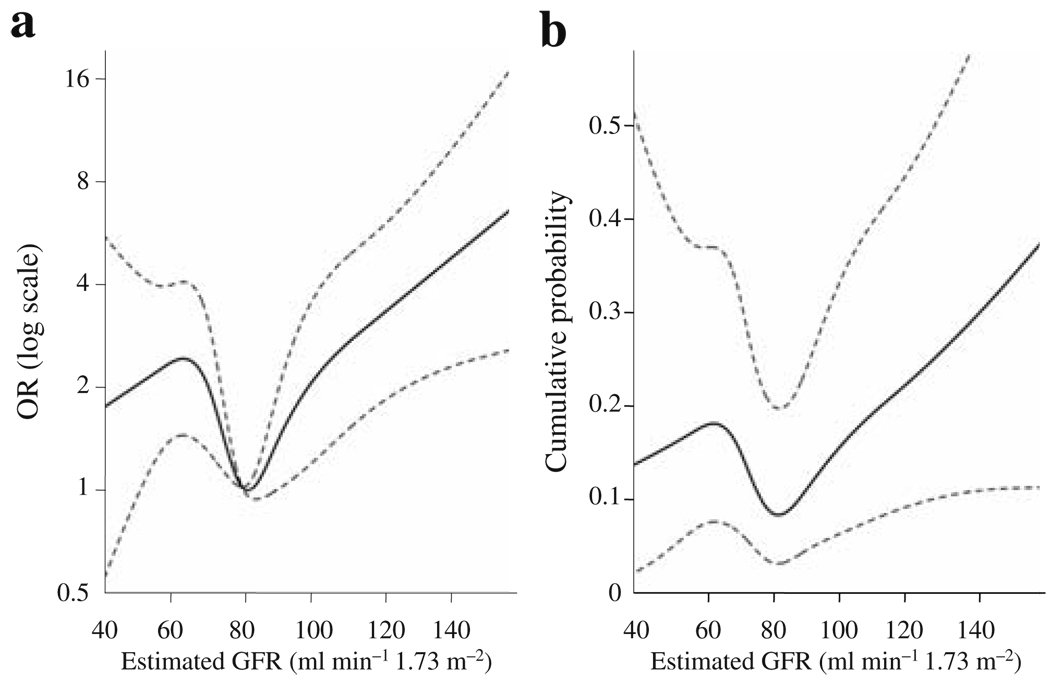
As the relationship between GFR and incident diabetes was not linear, we fitted a logistic regression model with the relevant risk factors in it and a smoothed effect of GFR (Fig. 1). The relationship between GFR and incident diabetes was statistically significant (p = 0.039). Figure 1a presents the OR relative to an estimated GFR of 80 ml min−1 1.73 m−2. The OR is higher in participants with GFR below 65 and above 100 ml min−1 1.73 m−2. Figure 1b shows the cumulative probability for a hypothetical individual with a relevant set of values for the confounders.
Fig. 1.
Relationship between the 5 year risk of type 2 diabetes and GFR modelled by a smooth function. a Probability of incident diabetes adjusted for age, sex, race/ethnicity, clinic location, BMI, systolic blood pressure (SBP), antihypertensive treatment, history of diabetes in first-degree relatives, insulin sensitivity index, acute insulin response and ACR, and levels of triacylglycerols, HDL-cholesterol, PAI-1 and fasting and 2 h glucose. The OR has a reference level GFR of 80 ml min−1 1.73 m−2. b The 5 year cumulative probability for a hypothetical individual with a relevant set of values for the confounders. The participant modelled is a 65 year old Hispanic man at the San Antonio clinic with the following risk factors: SBP 140 mmHg, BMI 30 kg/m2, fasting glucose 6.66 mmol/l and 2 h glucose 6.66 mmol/l. All other risk factors are at the study averages
Discussion
To our knowledge, this is the first study that relates GFR to the development of diabetes. Hyperfiltration-related characteristics are present in non-diabetic participants whose estimated GFR is high. High GFR is associated with arterial pressure, chronic inflammation and albuminuria. These associations are not driven by obesity or insulin resistance and do not explain the increased risk of diabetes associated with high GFR. Low GFR is also a predictor of future diabetes independently of the effect of determinants of GFR and diabetes.
Tomaszewski et al. were the first to describe a relationship between hyperfiltration and metabolic risk [17]. Our study validates, in part, their observations. High GFR is directly related to several components of the insulin resistance syndrome, including arterial pressure, chronic inflammation and albuminuria. Therefore, insulin resistance is an attractive causative factor for the relationship between high GFR and incident diabetes. Insulin has been shown to exert a direct effect on glomerular podocytes [27]. Acute insulin infusion stimulates the sympathetic nervous system [28] and produces a vasodilator hypotensive effect [29]. In our study, however, high GFR is not associated with insulin resistance. This unexpected finding has to be evaluated in view of the limitations of the MDRD equation. For example, the high GFR category may group a heterogeneous pool of individuals. Some of these individuals may have low generation of creatinine and, consequently, falsely elevated GFR. Our analysis also demonstrates that the relation of GFR to metabolic abnormalities is independent of the effect of obesity. Obesity could largely explain the relationship between hyperfiltration and metabolic risk in the study by Tomaszewski et al., because the Cockcroft–Gault equation was used to estimate GFR [17]. Thus, studies with definitive measures of both insulin resistance and GFR are needed to re-examine the relationship between hyperfiltration and insulin resistance.
Epidemiological studies have linked low GFR to insulin resistance and other metabolic abnormalities [7, 8]. In the IRAS, low GFR is associated with hypertensive treatment, which may be a marker for higher blood pressure and/or longer duration of hypertension. However, low GFR was not associated with any other metabolic abnormality. To explain this fact, we advance three possible explanations. First, the number of participants with low GFR is relatively small and none of them has a GFR <39 ml min−1 1.73 m−2. Second, an initial increase in GFR is common in individuals with IGT [13] and obesity-related glomerulopathy [30]. Both obesity and IGT are very prevalent in the IRAS cohort. Finally, worsening GFR in diabetic nephropathy tends to occur after the onset of macroalbuminuria [13].
Mechanisms other than insulin resistance may be important for explaining the relationship between GFR and development of type 2 diabetes. Potential mechanisms are the dysregulation of the autonomic nervous system [31, 32], increased renal gluconeogenesis [33], endothelial dysfunction/chronic inflammation [34–37], oxidative stress [37] and activation of the renin–angiotensin system [38–40]. A marker of chronic inflammation, PAI-1 concentration, is increased in individuals with high GFR. However, PAI-1 concentration does not explain the relationship between GFR and incident diabetes. Future studies need to determine the role of these potential contributory mechanisms.
Using a restricted cubic polynomial spline as a regression modelling strategy is a better method of assessing the non-linear effect of GFR than using GFR categories [26]. Categories may have the advantage of presenting the results in terms of clinically relevant cut-off points. Our results indicate that the increased risk of future diabetes associated with high and low GFR is not the result of arbitrary cut-off points.
Although changes in GFR precede the development of diabetes in men, women and all three ethnic groups, a number of limitations must be considered. First, the assessment of GFR is indirectly estimated. Definitive methods (e.g. with inulin or radioisotope studies) are difficult to apply to epidemiological studies. Second, creatinine concentration is not calibrated for the MDRD equation in the IRAS. However, creatinine concentration was measured by the same laboratory and method for all participants. Third, the MDRD equation may not generate definite GFR estimates in participants with normal or high GFR. Biological and measurement variability associated with normal or high GFR is greater than that associated with low GFR [40]. In the IRAS, a .84 µmol/1 change in serum creatinine concentration represents a 2.1 to 6.0 ml min−1 1.73 m−2 change in participants with low GFR and a 11.1 to 79.7 ml min−1 1.73 m−2 change in participants with high GFR. Finally, the MDRD equation underestimates GFR measurement in individuals with normal or low serum creatinine concentration [41]. For example, the mean GFR (using the six-variable MDRD equation) was 77 ml min−1 1.73 m−2 among adult non-diabetic participants in the National Health and Nutrition Examination Survey III [42]. Cystatin C may be a better filtration marker [43], but is unavailable in the IRAS. Therefore, an incorrect conclusion may derive from our results: that a GFR level of 80 ml min−1 1.73 m−2 is associated with the lowest diabetic risk. To reach this type of conclusion, a definitive method to measure GFR is needed.
In summary, low and high GFR predict incident diabetes independently of confounding risk factors. The exact mechanism is unknown but our results suggest that GFR changes and type 2 diabetes share common pathogenic mechanisms. Studies that use definitive measures of GFR are needed to confirm the hypothesis that changes in GFR may precede the onset of type 2 diabetes independently of ongoing changes in albumin excretion rate.
Acknowledgements
This study was supported by National Heart, Lung, and Blood Institute Grants (HL-47887, HL-47889, HL-47890, HL-47892, HL-47902), the General Clinical Research Centers Program (NCRR GCRC, M01 RR431, M01 RR01346), and CTSA Award (KL2 RR025766) from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Center for Research Resources of the National Institutes of Health
Abbreviations
- ACR
Albumin to creatinine ratio
- DBP
Diastolic blood pressure
- IGT
Impaired glucose tolerance
- IRAS
Insulin Resistance Atherosclerosis Study
- MDRD
Modification of Diet in Renal Disease
- PAI-1
Plasminogen activator inhibitor-1
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contributor Information
C. Lorenzo, Email: lorenzo@uthscsa.edu, Department of Medicine, Division of Clinical Epidemiology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78284-7873, USA.
S. D. Nath, Division of Nephrology, University of Texas, Health Science Center, San Antonio, TX, USA
A. J. G. Hanley, Nutritional Sciences and Medicine and Leadership Sinai Centre, for Diabetes, Mt. Sinai Hospital and the University of Toronto, Toronto, ON, Canada
H. E. Abboud, Division of Nephrology, University of Texas, Health Science Center, San Antonio, TX, USA
J. A. L. Gelfond, Department of Epidemiology and Biostatistics, University of Texas Health Science Center, San Antonio, TX, USA
S. M. Haffner, Department of Medicine, Division of Clinical Epidemiology, University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78284-7873, USA
References
- 1.American Medical Association. Standards of medical care in diabetes—2006. Diabetes Care. 2006;29:S4–S42. [PubMed] [Google Scholar]
- 2.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 3.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110:32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 5.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58:1703–1710. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 9.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 10.Mykkanen L, Zaccaro DJ, O'Leary DH, Howard G, Robbins DC, Haffner SM. Microalbuminuria and carotid artery intimamedia thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28:1710–1716. doi: 10.1161/01.str.28.9.1710. [DOI] [PubMed] [Google Scholar]
- 11.Mykkanen L, Haffner SM, Kuusisto J, Pyorala K, Laakso M. Microalbuminuria precedes the development of NIDDM. Diabetes. 1994;43:552–557. doi: 10.2337/diab.43.4.552. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Hoy WE. Albuminuria as a marker of the risk of developing type 2 diabetes in non-diabetic Aboriginal Australians. Int J Epidemiol. 2006;35:1331–1335. doi: 10.1093/ije/dyl115. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RG, Bennett PH, Beck GJ, et al. Diabetic Renal Disease Study Group. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med. 1996;335:1636–1642. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 14.Rudberg S, Persson B, Dahlquist G. Increased glomerular filtration rate as a predictor of diabetic nephropathy—an 8-year prospective study. Kidney Int. 1992;41:822–828. doi: 10.1038/ki.1992.126. [DOI] [PubMed] [Google Scholar]
- 15.Palatini P, Mormino P, Dorigatti F, et al. Glomerular hyperfiltration predicts the development of microalbuminuria in stage 1 hypertension: the HARVEST. Kidney Int. 2006;70:578–584. doi: 10.1038/sj.ki.5001603. [DOI] [PubMed] [Google Scholar]
- 16.Schmieder RE, Messerli FH, Garavaglia G, Nunez B. Glomerular hyperfiltration indicates early target organ damage in essential hypertension. JAMA. 1990;264:2775–2780. [PubMed] [Google Scholar]
- 17.Tomaszewski M, Charchar FJ, Maric C, et al. Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int. 2007;71:816–821. doi: 10.1038/sj.ki.5002160. [DOI] [PubMed] [Google Scholar]
- 18.Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS): objectives, design and recruitment results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 19.Saad MF, Anderson RL, Laws A, et al. A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance. Insulin Resistance Atherosclerosis Study. Diabetes. 1994;43:1114–1121. doi: 10.2337/diab.43.9.1114. [DOI] [PubMed] [Google Scholar]
- 20.Korytkowski MT, Berga SL, Horwitz MJ. Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism. 1995;44:1121–1125. doi: 10.1016/0026-0495(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 21.Kroll MH, Chesler R, Hagengruber C, Blank DW, Kestner J, Rawe M. Automated determination of urinary creatinine without sample dilution: theory and practice. Clin Chem. 1986;32:446–452. [PubMed] [Google Scholar]
- 22.Herbert V, Lau K, Gottlieb C, Bleicher S. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25:1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- 23.Declerck P, Collen D. Measurement of plasminogen activator inhibitor 1 (PAI-1) in plasma with various monoclonal antibody-based enzyme-linked immunosorbent assays. Thromb Res. 1990;10:S3–S9. doi: 10.1016/0049-3848(90)90373-k. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Altman DG. Construction of age-related reference centiles using absolute residuals. Stat Med. 1993;12:917–924. doi: 10.1002/sim.4780121003. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression modeling strategies. New York: Springer; 2001. [Google Scholar]
- 27.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 28.Rowe JW, Young JB, Minaker KL, Stevens AL, Pallotta J, Landsberg L. Effect of insulin and glucose infusions on sympathetic nervous system activity in normal man. Diabetes. 1981;30:219–225. doi: 10.2337/diab.30.3.219. [DOI] [PubMed] [Google Scholar]
- 29.Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990;85:1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henegar J, Bigler S, Henegar L, Tuagi S, Hall J. Functional and structural changes in the kidney in the early stages of obesity. Am J Soc Nephrol. 2001;12:1211–1217. doi: 10.1681/ASN.V1261211. [DOI] [PubMed] [Google Scholar]
- 31.Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of type 2 diabetes patients and control subjects. Diabet Med. 2003;20:399–405. doi: 10.1046/j.1464-5491.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 32.Neumann J, Ligtenberg G, Klein IH, et al. Sympathetic hyperactivity in hypertensive chronic kidney disease patients is reduced during standard treatment. Hypertension. 2007;49:506–510. doi: 10.1161/01.HYP.0000256530.39695.a3. [DOI] [PubMed] [Google Scholar]
- 33.Eid A, Bodin S, Ferrier B, et al. Intrinsic gluconeogenesis is enhanced in renal proximal tubules of Zucker diabetic fatty rats. J Am Soc Nephrol. 2006;17:398–405. doi: 10.1681/ASN.2005070742. [DOI] [PubMed] [Google Scholar]
- 34.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 36.Dogra G, Irish A, Chan D, Watts G. Insulin resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis. 2006;48:926–934. doi: 10.1053/j.ajkd.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol. 2008;19:593–599. doi: 10.1681/ASN.2007030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the renin–angiotensin system in the ZDF rat. Diabetes. 2004;53:989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 39.Jacob S, Henriksen EJ, Fogt DL, Dietze GJ. Effects of trandolapril and verapamil on glucose transport in insulin-resistant rat skeletal muscle. Metabolism. 1996;45:535–541. doi: 10.1016/s0026-0495(96)90021-9. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Nakagawa T, Suzuki H, et al. Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13:1338–1349. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- 43.Premaratne E, MacIsaac RJ, Finch S, Panagiotopoulos S, Ekinci E, Jerums G. Serial measurements of cystatin C are more accurate than creatinine-based methods in detecting declining renal function in type 1 diabetes. Diabetes Care. 2008;31:971–973. doi: 10.2337/dc07-1588. [DOI] [PubMed] [Google Scholar]