Abstract
Background
Temporary vascular shunting to restore flow after vascular injury has been advocated. The effectiveness of this adjunct in protecting against ischemic injury has not been established. This study will assess the temporal impact of shunts on ischemic injury and arterial flow.
Methods
A porcine model of hind-limb ischemia via iliac artery occlusion was used (N = 36; weight [kg] ± SD: 89 ± 4.4). Animals were randomized into one control (Iscctrl) and four study groups (Isc0, Isc1, Isc3, and Isc6) according to ischemic time. Shunt placement followed ischemia, and flow and circulating injury markers were collected incrementally during 18 hours of reperfusion. Flow proportions and a calculated Ischemia Injury Index were used to characterize group differences.
Results
There were no intergroup differences concerning initial weight, hemodynamic, or laboratory values. Shunt patency was 92% in the absence of anticoagulation. The proportion of common femoral arterial flow to baseline flow in the Isc6 group was lower than the Iscctrl group (p = 0.02). There was a similar trend with the Isc1 and Isc3 groups. The Ischemia Injury Index demonstrated that there was a difference in the Isc3 and Isc6 groups (late shunt placement) compared with the Iscctrl, Isc0, and Isc1 groups (early shunt placement) (p < 0.001).
Conclusion
This study provides physiologic insight into the benefit of shunts in a model of extremity ischemia. Early shunting protects the extremity from further ischemic insult and reduces circulating markers of tissue injury. Additionally, the presence of a shunt does not increase the Ischemic Injury Index and patency is maintained in the absence of heparinization.
Keywords: Temporary vascular shunt, Vascular injury, Vascular trauma, Temporary shunt, Wartime vascular injury, Vascular adjunct
Since 2001, military operations in Iraq and Afghanistan have resulted in as many as 30,000 extremities injuries, which represents more than 75% of all war-related injuries.1,2 Preliminary reports from the war have suggested that the rate of extremity vascular injury is now three to five times that reported from previous wars.3–6 To improve life and limb saving capability, forward surgical teams have been positioned at level II facilities, which enable lifesaving procedures, exploration of wounds, and the application of surgical adjuncts often within 30 minutes to 60 minutes of injury.7
The ability to save an injured extremity is based on adequate perfusion, and hence early repair of associated vascular injuries is optimal. However, forward levels of care often experience casualty surges or have limited resources, either of which may limit the ability to accomplish vascular reconstruction in a timely manner. Furthermore, life-threatening injuries or medical evacuation often take priority over the treatment of extremity vascular injury, which may also extend ischemic time. Finally, delayed revascularization after prolonged warm ischemia may result in more severe reperfusion injury and negatively impact limb salvage and patient physiology.
Recent wartime reports have demonstrated the feasibility and early efficacy of temporary vascular shunts (TVS) in the setting of extremity injury.8–10 This adjunct, which has been used in up to 25% of extremity vascular injuries treated in Iraq, serves to quickly restore perfusion until definitive repair is accomplished.11 Placed expeditiously, shunts maintain limb perfusion while life-threatening injuries are treated or medical evacuation occurs and in this context may reduce warm ischemic time. Contemporary experience suggests that these shunts are currently in place for 1 hour to 4 hours before removal and vascular repair, although anecdotal cases of shunts being used for up to 6 hours or longer exist.12
Although reports from the war reflect favorably on the clinical use of TVS, no animal data exist on shunt patency in the setting of different warm ischemic times. Additionally, the importance of time to shunt placement (immediate, early, or delayed) and its relationship to extremity blood flow and circulating markers of injury has not been characterized in an animal model. The objective of this study is to establish a large animal model of extremity ischemia and reperfusion using TVS currently used in the war. Further, the aim of this report is to characterize the temporal relationship of time from injury to shunt placement, with extremity blood flow and systemic markers of injury.
MATERIALS AND METHODS
Protocol Overview
A porcine (Sus scrofa) model of hind-limb ischemia via iliac artery occlusion and reperfusion using TVS was established using male subjects (N = 36; weight [kg] ± SD: 89 ± 4.2) (Fig. 1, A–C). All animal procedures were performed in accordance with the Institutional Animal Care and Use Committee and adhered to the national and state guidelines concerning animal research. The study was conducted at an accredited animal research facility under the supervision and support of licensed veterinarians.
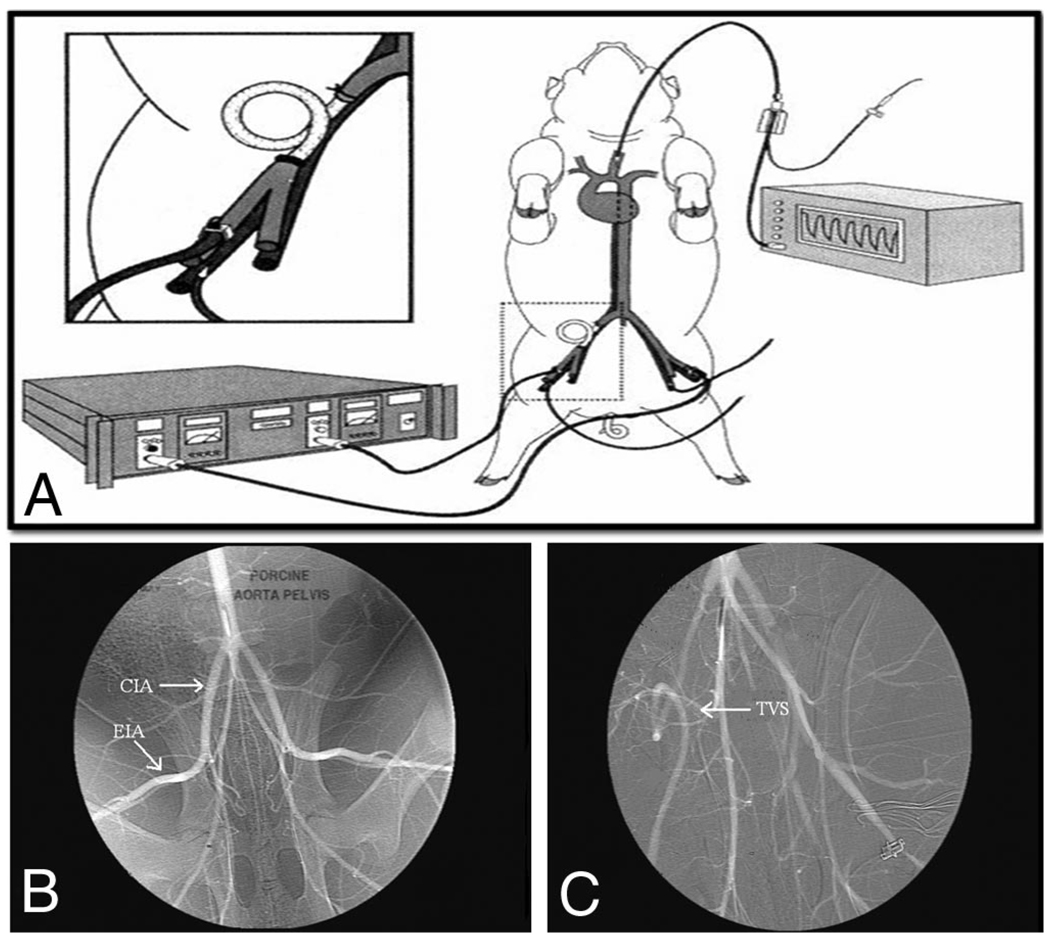
Figure 1.
(A–C)Experimental animal set up (A). The right external iliac artery is exposed and ligated. After a predetermined ischemic time, a temporary vascular shunt (TVS) is placed to reestablish flow (inset). Continuous ultrasonic flow confirms shunt patency and continued reperfusion. Arteriography shown here demonstrates pre- and post-TVS placement. Normal anatomy is shown with common iliac (CIA) and external iliac (EIA) arteries identified (B). After the predetermined ischemic time, a TVS (arrow) was placed in the artery and distal perfusion reestablished (C). (Reprinted with permission from J Trauma. 1999;47:64–71).
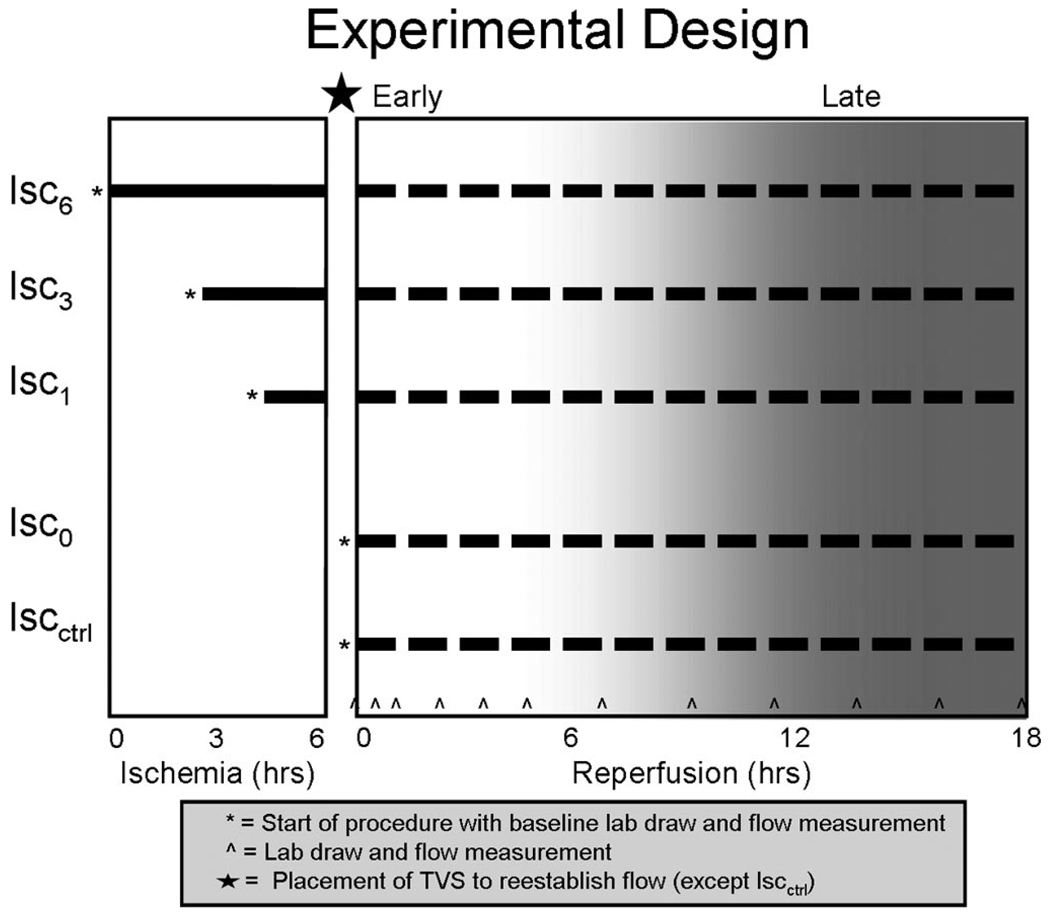
A control group (IscCtrl) underwent iliac artery exposure with no occlusion or shunt placement to account for the effect of the operation, anesthesia, and phlebotomy. The four remaining groups (Isc0, Isc1, Isc3, and Isc6) underwent retroperitoneal right iliac artery exposure and occlusion for increasing increments of time: 0, 1, 3, and 6 hours (Fig. 2). After each interval of warm ischemia, hind-limb perfusion was reestablished using a temporary vascular shunt (Sundt Shunt, Integra, Plainsboro, NJ).
Figure 2.
Experimental design. Five study groups with varying degree of ischemia and shunt utilization underwent 18 hours of reperfusion to determine the temporal impact of shunt placement on flow and circulating markers of tissue injury.
Reperfusion with the TVS was performed for 18 hours during which blood was collected to assess circulating markers of reperfusion, muscle injury, inflammation, and apoptosis. The reperfusion period was divided into early (0–6 hours) during which measures of reperfusion and muscle injury were assessed, and late (8–18 hours) during which markers of inflammation and apoptosis were evaluated (Fig. 2).
Inclusion and Exclusion Criteria
Animals that died before completion of the protocol for reasons unrelated to extremity ischemia (e.g., pneumothorax, ventilator malfunction, or unidentified thrombosis and continued ischemia) were excluded from analysis (N = 6). To maintain projected statistical power, six animals were added to replace these inadvertent deaths. In addition, six animals died before completion of an 18-hour reperfusion protocol from causes, which could not be determined to be independent from reperfusion. In each case, the values from those animals were used until the time of death and additional animals were added to that particular study group to maintain statistical power of the later data points.
Study Groups
The groups used in the final analysis were a control group.
(IscCrtl; N = 8) and four groups with increasing warm ischemia times:
Isc0 = 0 hours ischemia (i.e., opening of artery with immediate placement of TVS) (n = 6);
Isc1 = 1 hour of ischemia followed by placement of TVS (n = 7);
Isc3 = 3 hours of ischemia followed by placement of TVS (n = 6); and
Isc6 = 6 hours of ischemia followed by placement of TVS (n = 9).
Operative Procedure
Animals were sedated with acepromazine 0.11 mg/kg to 0.22 mg/kg, ketamine 15 mg/kg to 20 mg/kg, and atropine 0.04 mg/kg to 0.4 mg/kg administered intramuscularly, induced via mask ventilation with isoflurane, intubated, and maintained under general anesthesia using 2.0% to 2.5% isoflurane in a 1:2 mixture of oxygen and air. Initial ventilator settings were weight based and adjusted as needed to maintain PCO2 at 40 mm Hg. A continuous weight-based infusion of 0.9% normal saline was given through a peripheral ear vein and adjusted accordingly to maintain a urine output of 0.5 mL · kg−1 · hr−1 to 1.0 mL · kg−1 · hr−1. External warming blankets were used to keep the core temperature at a range of 36°C to 38.5°C.
Animals were placed supine on the operating room table and external monitoring devices applied. A carotid line was inserted for continuous hemodynamic monitoring. Bilateral femoral cut downs were performed and 5.0 F microcatheters placed into the femoral veins for blood collection. Ultrasonic flow probes (Transonic Systems. Ithaca, NY) were placed around both common femoral arteries for continuous flow measurement. A low midline incision was performed and the right common iliac artery exposed using a retroperitoneal approach. Proximal and distal vessel loops were placed around the common and external iliac arteries, pulled up to make occlusive, and the limb rendered ischemic. Of note, the internal iliac artery and a lateral circumflex branch arising from the common iliac were also ligated to maximize hind-limb ischemia (Fig. 1, B and C).
After the predetermined ischemic time (0, 1, 3, or 6 hours), an arteriotomy was created between the vessel loops on the common iliac artery. A Fogarty balloon catheter was used to remove any apparent thrombus from the artery and a 4 × 5 Sundt shunt (Integra, Plainsboro, NJ) was placed and secured with vessel loops. Shunt patency and restoration of flow in the extremity were confirmed and monitored with continuous wave Doppler as well as flow probes positioned on the femoral artery distal to the TVS. Primary patency of TVS during the protocol was defined by maintenance of a Doppler signal in the shunt and continuous distal flow without need for reintervention (i.e., thrombectomy).
Reperfusion and Data Collection
Limb reperfusion was performed for 18 hours during which intermittent blood samples were collected and ultrasonic flow rates recorded. Blood draws were accomplished at baseline (i.e., before limb ischemia) and then at the following reperfusion intervals: 0 and 30 minutes and 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, and 18 hours. Basic blood measures included complete blood count, electrolyte panel, and blood gas analysis. Measures of ischemia and reperfusion injury were the focus of the early reperfusion period (0–6 hours) and included arterial blood gas, serum lactate, myoglobin, potassium (K), creatine phosphokinase (CPK), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH). Local and systemic measures of inflammation and apoptosis were the focus of the late reperfusion period (8–18 hours) and included serum levels of tumor necrosis factor-α, interleukin-6, caspase 3, and annexin V.
Vital signs including blood pressure, heart rate, oxygen saturation, and end-tidal CO2, urine output, and core temperature were monitored throughout the protocol. Limb compartment pressures in the ischemic and nonischemic limbs were recorded in the anterior compartment using a Stryker Intra-Compartmental Pressure Monitor (Stryker, Berkshire, UK) at baseline and 6, 12, and 18 hours postreperfusion. After completion of the 18 hours of reperfusion, animals were killed using potassium chloride administered centrally per Institutional Animal Care and Use Committee-directed protocol.
Analysis and Reporting
Data consisted of baseline and repeated measures across reperfusion time points, spanning 18 hours, and continuous ultrasonic flow. Analysis of results was stratified into baseline, early reperfusion period (0–6 hours), and late reperfusion period (8–18 hours). Using the circulating markers lactate (LAC), AST, LDH, potassium (K), and creatine kinase (CPK), an overall Ischemia Index Score (IIS) was produced for each of the groups using individual variable Z scores. Z scores are scale free, which allows the combination of information from multiple markers on different measurement scales.
The index was created by standardizing each of the observations to the average control value at time 0 using the equation Zbiomarker = [(observed − healthy average)/SD healthy]. The IIS was (1) computed for each reperfusion time and group from all variables in the model, (2) averaged according to respective group [(ZAST + ZCPK + ZLDH + ZK + ZLAC)/5], and (3) plotted against reperfusion time. These IIS plots demonstrate the gross deviations from control values of all markers over time.
The IscCtrl and Isc0 groups were tested for differences in the IIS at each postreperfusion time using the Wilcoxon’s rank sum test. Spearman’s rank correlation method was used to test for an association between ischemia time (Isc0–Isc6) and the ischemia index at each reperfusion time point. Proportion of flow was calculated for each reperfusion time as the flow relative to baseline. Comparisons among groups were tested using a repeated-measures linear mixed model.
RESULTS
Group Attributes
The baseline attributes, hemodynamic, and ventilator characteristics of each of the groups are provided in Table 1. There was no difference among the groups’ weight, urine output, systolic blood pressure, oxygenation, or end-tidal CO2 during the baseline phase of the study. Additionally, there was no difference in the baseline laboratory values among groups including myoglobin, AST, LDH, CPK, potassium, LAC, and hemoglobin (Table 1). Each of the groups had similar resuscitation requirements throughout the protocol to maintain urine output at 0.5 mL · kg−1 · hr−1 to 1 mL · kg−1 · hr−1. There was no significant difference in compartment pressures in any of the groups measured throughout the reperfusion period (data not shown).
TABLE 1.
Descriptive Statistics of Baseline Measurements by Treatment Group
| Ischemia Time Group | ||||||
|---|---|---|---|---|---|---|
| Characteristic | IscControl (n = 8) | Isc0 (n = 6) | Isc1 (n = 7) | Isc3 (n = 6) | Isc6 (n = 9) | p* |
| Weight (kg) | 89.1 ± 2.3 | 86.8 ± 3.3 | 93.1 ± 3.7 | 89.4 ± 4.2 | 89 ± 6 | 0.13 |
| Heart rate (bpm) | 103.5 ± 24.9 | 88.8 ± 9.1 | 90.2 ± 13.6 | 89 ± 9.5 | 83.9 ± 8.1 | 0.48† |
| Systolic BP (mm Hg) | 77 ± 9.8 | 85 ± 7.6 | 86 ± 4.4 | 85 ± 10.7 | 85.1 ± 11.2 | 0.76 |
| O2 saturation (%) | 94.3 ± 4.3 | 98.6 ± 2.2 | 93.4 ± 2.3 | 95.8 ± 1.6 | 97 ± 2.9 | 0.06† |
| ETCO2 | 48 ± 9 | 43 ± 6.8 | 43.2 ± 6.7 | 47 ± 8.7 | 42.6 ± 4.4 | 0.63 |
| Temperature (°C) | 36.4 ± 1.3 | 37.1 ± 1.1 | 37.3 ± 0.6 | 37.5 ± 0.8 | 37.3 ± 1.7 | 0.67 |
| Urine output (mL · kg−1 · hr−1)‡ | 0.7 ± 0.2 | 0.8 ± 0.2 | 1.1 ± 0.8 | 0.9 ± 0.2 | 0.7 ± 0.2 | 0.28 |
| Hgb (g/dL) | 10.3 ± 0.7 | 9.4 ± 1.4 | 9.7 ± 0.8 | 9 ± 1.3 | 9.9 ± 0.6 | 0.18 |
| pH | 7.4 ± 0.1 | 7.4 ± 0 | 7.5 ± 0.1 | 7.4 ± 0.1 | 7.5 ± 0 | 0.2† |
| AST (U/L) | 43.5 ± 3.9 | 38.8 ± 7.9 | 38.9 ± 6 | 43.8 ± 9.3 | 57.6 ± 24.7 | 0.14† |
| Creatine kinase (U/L) | 1251.8 ± 569.4 | 973 ± 504.9 | 1820.3 ± 1760.6 | 696.3 ± 144 | 1297.9 ± 620.8 | 0.15† |
| Potassium (mEq/L) | 3.4 ± 0.2 | 3.9 ± 0.4 | 3.5 ± 0.5 | 4.1 ± 0.9 | 3.5 ± 0.4 | 0.26† |
| Lactate (mmol/L) | 1 ± 0.4 | 1.4 ± 0.7 | 1.3 ± 0.5 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.33 |
| Lactate dehydrogenase (U/L) | 474.1 ± 113 | 429.8 ± 152 | 485.4 ± 210.1 | 350.2 ± 53.5 | 457.8 ± 104.6 | 0.21† |
| Myoglobin (ng/mL) | 193.5 ± 124.3 | 111.8 ± 55.7 | 198.4 ± 85 | 121.7 ± 52.4 | 180.2 ± 81.3 | 0.2† |
Comparison of group attributes at baseline. None of the variables were statistically significant between groups as demonstrated by all p values > 0.05.
ANOVA.
Kruskal-Wallis.
Measured at experiment termination.
ETCO2, end-tidal carbon dioxide; Hgb, hemoglobin; AST, aspartate aminotransferase; BP, blood pressure.
Patency of TVS and Flow Characteristics
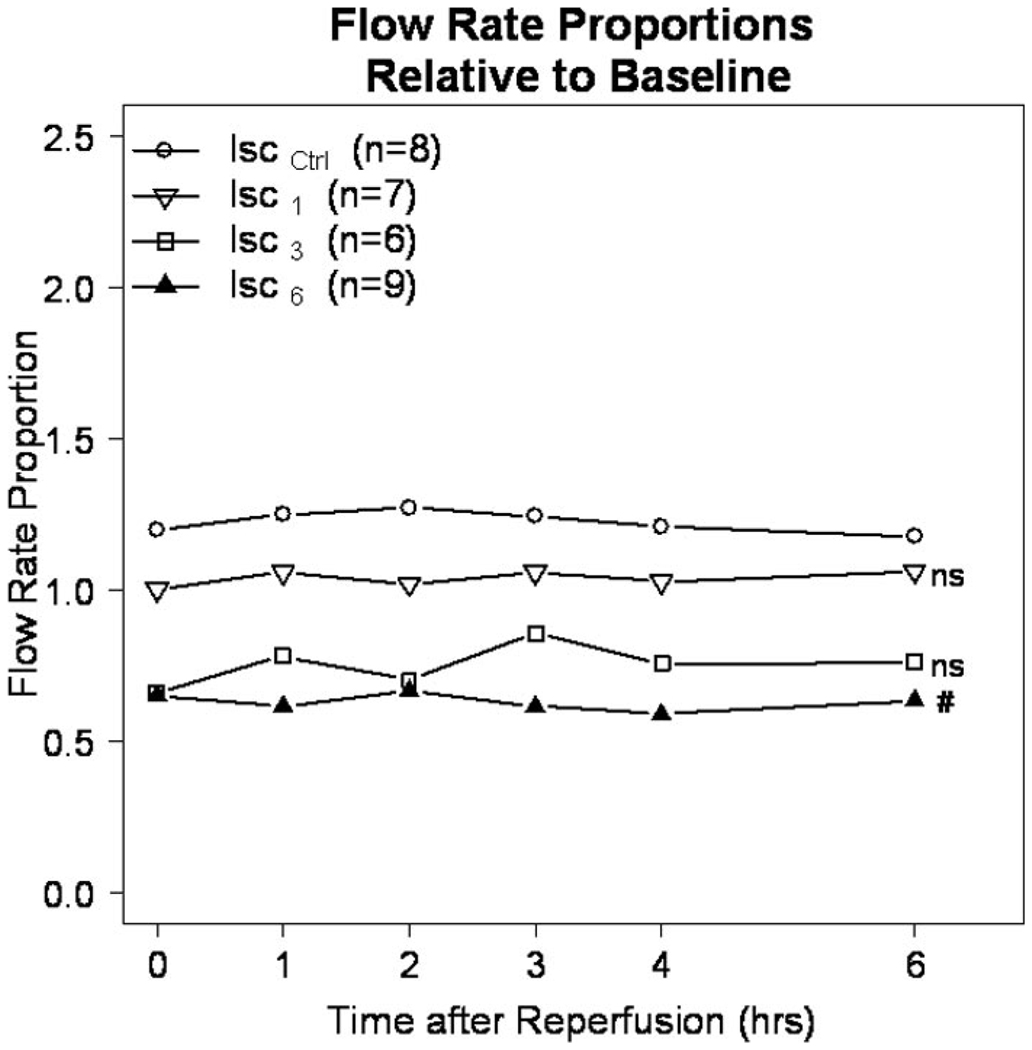
The primary patency of TVS in all groups was 91.7% (33 of 36) at 18 hours of reperfusion. There was no difference in the primary patency among groups because shunt thrombosis occurred one time in each of the Isc1, Isc3, and Isc6 groups. Shunt placement in the absence of ischemia had no detrimental effect on extremity flow during the reperfusion period. Specifically flow in the extremity was not different between the Isc0 group (immediate shunt placement) and the IscCtrl group, which had no shunt (p = 0.79). Similarly, and as illustrated in Figure 3, extremity flow as a proportion of baseline flow (y axis) was the same with early TVS placement after 1 hour of ischemia (Isc1) as that in the IscCtrl group. In contrast, delayed shunt placement at 3 hours (Isc3) and 6 hours (Isc6) resulted in stepwise decrements in extremity flow reaching statistical significance in the Isc6 group (p = 0.04) (Fig. 3).
Figure 3.
Flow rate proportion. The proportion of flow in the common femoral artery compared with the baseline flow for each group. (NS) = p > 0.05 for the Isc1 and Isc3 groups compared with the Iscctrl group; (#) = p = 0.02 for the Isc6 versus Iscctrl groups.
Markers of Muscle Injury
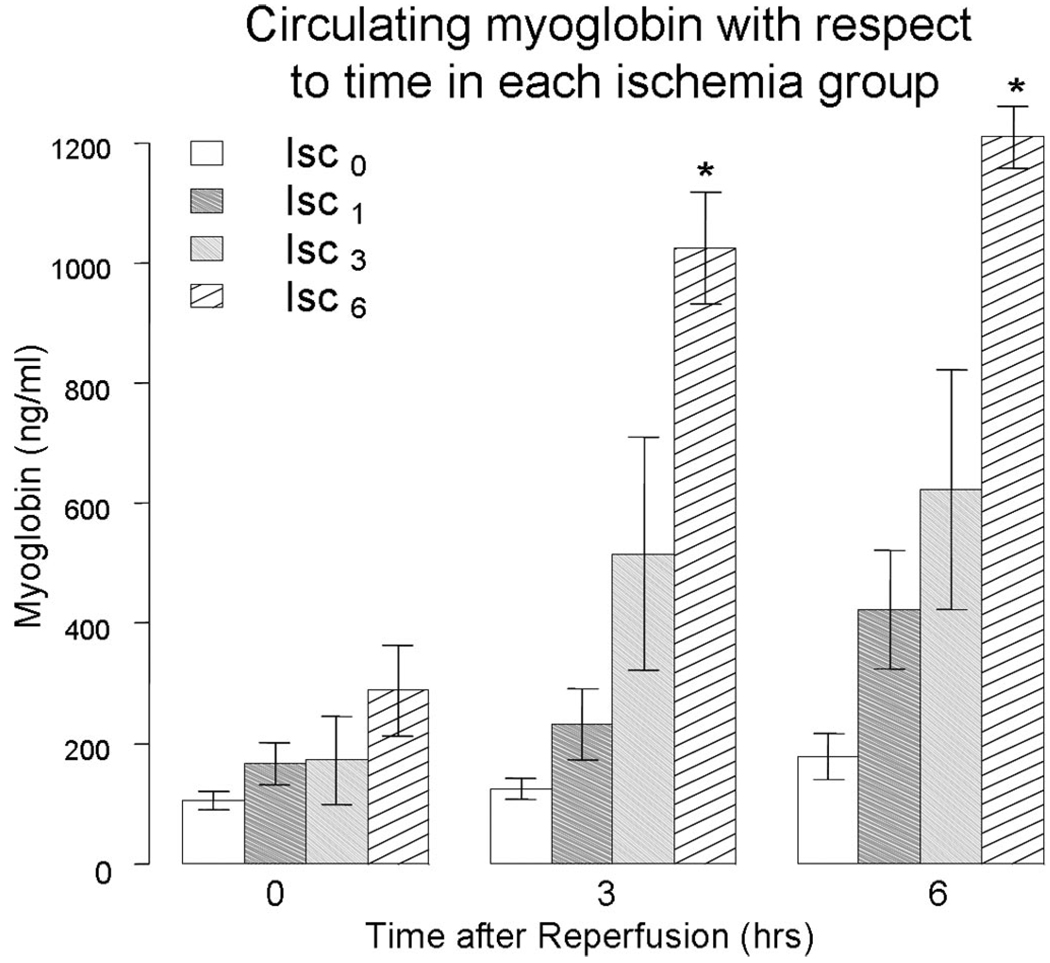
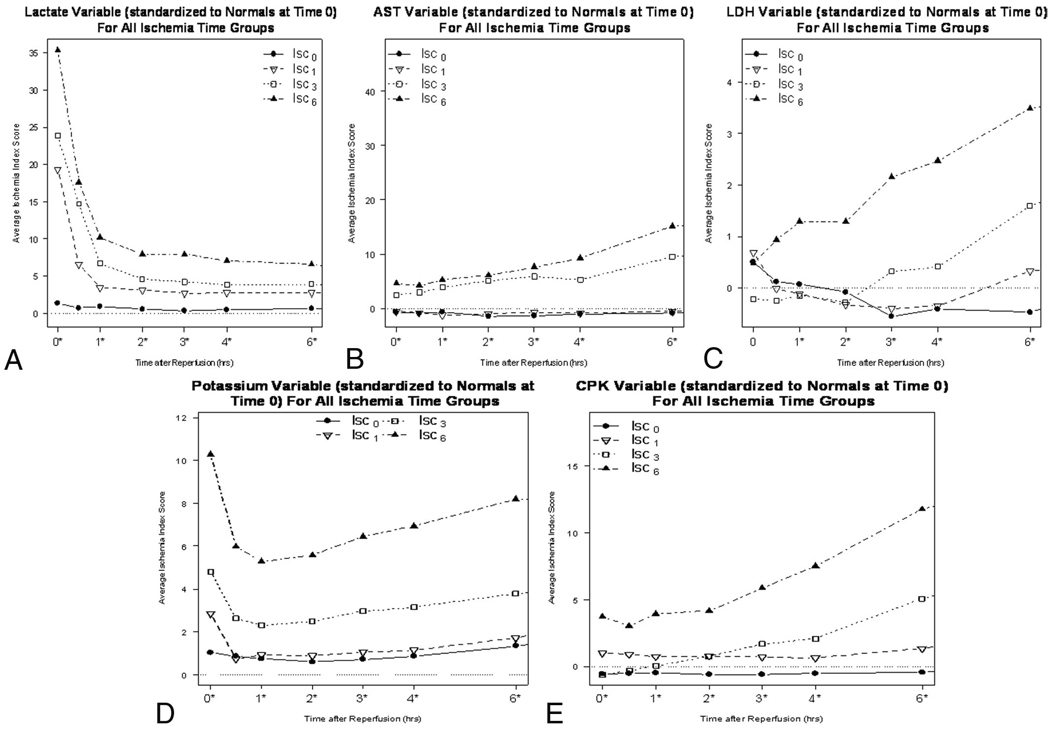
Immediate shunt placement in the absence of ischemia (Isc0) resulted in no increase in circulating myoglobin compared with the control group without TVS (IscCtrl) at 6 hours of reperfusion (177 ng/mL vs. 243 ng/mL, respectively; p = 0.35). Similarly, and as illustrated in Figure 4, circulating levels of myoglobin at 0, 3, and 6 hours were the same with early TVS placement after 1 hour of warm ischemia (Isc1) as the Isc0 or Iscctrl group. In contrast, delayed shunt placement at 3 hours (Isc3) and 6 hours (Isc6) resulted in the stepwise increases in circulating myoglobin during the early reperfusion period (Fig. 4). Additionally, the circulating markers LAC, AST, LDH, potassium (K), and CPK all had a similar trend to myoglobin and were used to construct the IIS as described in the Methods section (Fig. 5, A–E).
Figure 4.
Serum myoglobin, a specific skeletal muscle protein released during injury, is found in greater quantities in the serum as the ischemia time is prolonged. #Significant increase in circulating myoglobin for delayed shunt placement group (Isc6) compared with early shunt placement (Isc0 and Isc1), p < 0.05.
Figure 5.
(A–E) Individual variable Ischemia Index Scores (ISS) are shown. All variables had a similar response to increased ischemic time and were used to calculate the overall ISS for each experimental group. Lactate (A), aspartate aminotransferase (B), lactate dehydrogenase (C), potassium (D), and creatine kinase (E).
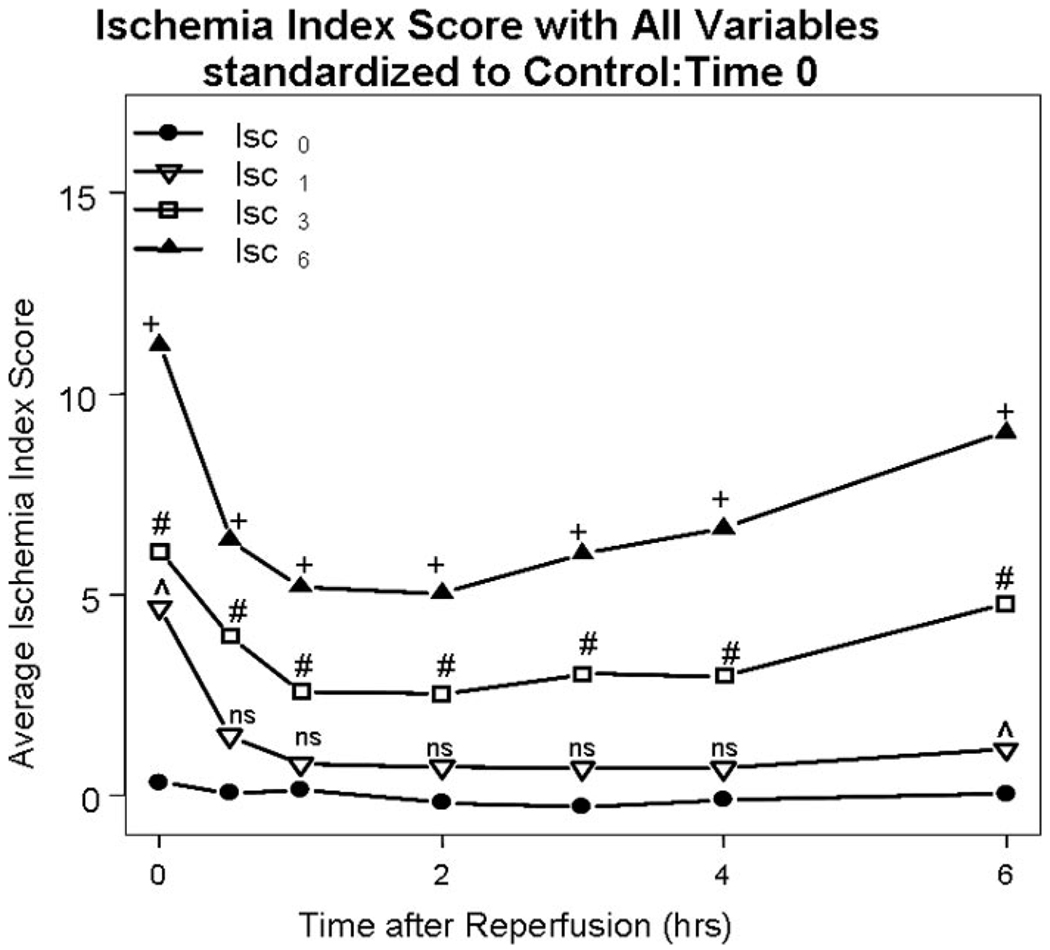
Placement of TVS in the absence of ischemia (Isc0) resulted in a significantly reduced average ischemic index score compared with control (IscCtrl) at reperfusion times 2, 4, 8, and 10 hours (p values: 0.04, 0.02, 0.02, 0.01). Early placement of TVS at 1 hour of warm ischemia (Isc1 group) resulted in the IIS scores, which were the same as those in the Isc0 group (Fig. 6). In contrast, delayed TVS placement at 3 hours (Isc3 group) and then 6 hours (Isc6 group) resulted in incrementally worse IIS compared with the Isc0 group throughout the early reperfusion period (p < 0.001) (Fig. 6). The significant findings observed in the IIS during the early reperfusion period were maintained throughout the late reperfusion period (data not shown). There was no significant difference in levels of tumor necrosis factor-α, interleukin-6, caspase 3, or annexin V among the study groups during the late reperfusion period (data not shown).
Figure 6.
Ischemia Index Score. As ischemic times are increased in all groups utilizing a temporary vascular shunt (TVS), the calculated Ischemia Index Score (IIS) score is significantly increased over time. (+) = p < 0.001 for Isc0 versus Isc6; (#) = p < 0.001 for Isc0 versus Isc3; (ˆ) = p < 0.05 for Isc0 versus Isc1; and (NS) = nonsignificant for Isc0 versus Isc1.
A similar transition point was observed in all three measures of extremity reperfusion injury (serum myoglobin, flow, and IIS) between early or 1 hour of warm ischemia and delayed or 3 hours and 6 hours of warm ischemia. Specifically, there was no significant difference in any of these measures between the early 1-hour ischemic time group (Isc1) and controls. However, in each measure there was an incremental worsening in the groups with delayed shunt placement at 3 hours (Isc3) and then 6 hours (Isc6) of warm ischemia.
DISCUSSION
This study confirms the feasibility, safety, and patency of temporary vascular shunts used to restore extremity perfusion in a large animal model. Technical success of shunt placement in this experiment was 100% and primary shunt patency without anticoagulation was 91.7%. Additionally, shunt placement resulted in no measurable adverse effects during the protocol. This investigation is the first to demonstrate that early application of this adjunct within an hour of extremity ischemia results in decreased measures of injury compared with delayed placement at 3 hours or 6 hours. Importantly, a similar benefit of shunt placement within an hour of ischemic time was observed in the context of limb perfusion where flow was preserved with early placement. In contrast, delayed shunt placement at 3 hours and then 6 hours of warm extremity ischemia resulted in incremental decreases in extremity flow.
This experiment is in contrast to previous animal research that has focused on small animals such as the mouse. There is an abundance of literature from small animal models that has pioneered our understanding of the molecular and mechanistic descriptions of ischemia reperfusion injury. 13–15 Bonheur et al. 13 using a tourniquet model of hind-limb ischemia in mice showed that incremental increases in ischemia time led to decreased flow, edema, and muscle viability after reperfusion, findings that are largely corroborated in this study. Conrad et al.14 further characterized biochemical markers of tissue injury and inflammation to varying ischemic time again showing that even limited amounts of warm ischemia led to detrimental effects.
Although small animal models like these have significant value, they accomplished limb reperfusion by release of a tourniquet from a rodent extremity. Therefore, they lack translational wartime applicability and are unsuited to validate the technique of using TVS to restore extremity perfusion. The iliac artery of the animals in the current experiment averaged 6 mm in diameter, the same size as a human femoral artery. This porcine model is, therefore, a suitable operative environment for realistic assessment of the effectiveness of TVS to restore perfusion. Results from this investigation establish a large animal model that has more direct translation to human vascular injury and allow a platform from which more specific investigations may occur.
The results of this study confirm and extend previous reports examining TVS in a porcine model by defining a temporal profile showing benefit from shunt placement within 1 hour of the onset of warm ischemia.16 The current findings provide novel large animal data to support the importance of early restoration of extremity flow even within a 1-hour to 3-hour time window. These results refute the occasional lax approach to extremity ischemia, which assumes that restoration of flow at any time during the traditionally accepted 6 hours to 8 hours is adequate.17–19 Indeed, this study provides evidence that early restoration of axial flow is critical and that this can be accomplished using the readily available shunt adjunct. In this context, the findings of this study validate efforts to place forward surgical teams equipped with shunt capability in close proximity to areas where extremity vascular injuries are likely to occur.9,10
The results of this investigation are well timed given the rapidly growing interest in and experience with temporary vascular shunts in wartime and the civilian setting.8–10,17 Separate publications this year in the August and September issues of the Journal of Trauma reported on the use of TVS at Grady Memorial Hospital in Atlanta and at a level II Forward Resuscitative Surgical Suite in Iraq’s Western Al Anbar province. Both articles advocate the use of TVS in select vascular injury patterns but acknowledge little understanding about the importance of timing to shunt placement. Whether on the battlefield or in US trauma centers, surgeons are typically confronted with decisions regarding extremity ischemia within an hour of injury. Options at that point include tolerance of warm ischemia for an additional 1 hour to 2 hours while orthopedic injuries are stabilized, more urgent torso or head injuries addressed, or the patient is evacuated to a location more able or better equipped to perform vascular repair. Results from this study demonstrate that quantifiable differences in measures of extremity injury are seen if reperfusion is delayed beyond an hour and provide support for early action including injury exploration, removal of thrombus (i.e., thrombectomy), and restoration of flow.
Reports from Iraq demonstrate that most shunts are placed at level II forward surgical units before transport to level III hospitals, where shunts are removed and repair completed.7–10 In this setting, total warm ischemia time of the extremity begins at the time of injury, which is typically 30 minutes to 60 minutes from initial surgical evaluation.20 In separate reports from different US Navy Forward Resuscitative Surgical Suites, Chambers, and more recently Taller et al.9,10 documented time from initial injury to presentation to be about an hour (61 and 46 minutes, respectively). The opportunity to place a shunt confronts the surgeon at this time understanding that additional warm ischemia will be encountered with MEDEVAC to a level III facility if no intervention is performed. Although MEDEVAC is most often 1 hour to 2 hours (unpublished data from the Joint Theater Trauma Registry), more remote level II facilities showed the average time from injury to arrival at a level III facility to be 5 hours to 6 hours.10 Despite current practice, to date, no large animal data exist to support or refute the importance of early restoration of extremity flow (within 1–2 hours) using TVS. Results from this study support early placement of TVS to limit total ischemia time to <3 hours which, in this study, was the point where evidence of significant injury and decrements in flow occurred.
This study has several limitations worth noting. Foremost is the limited ability to translate information from this nonshock animal model to human extremity injury. Additionally, the lack of tissue study (skeletal muscle and peripheral nerve) limits the ability to translate measures of circulating markers to actual tissue damage and poor functional outcome. Finally, the lack of positive findings in the late reperfusion period with regard to the markers of oxidative stress and apoptosis are unfortunate. However, this limitation is likely secondary to the relatively brief period of reperfusion during which data were collected.
Despite these drawbacks, this experiment establishes a reproducible large animal model of ischemia reperfusion that is translatable to human vascular injury. Additional studies to include tissue analysis and more effective endpoints of ischemia and reperfusion injury are needed to more accurately assess the effect of early reperfusion on muscle and nerve. Finally, this experiment may allow development of a survival model in which functional significance of these varied ischemic times can be assessed.
CONCLUSION
Restoration of extremity blood flow with a temporary vascular shunt currently used in humans on the battlefield can be accomplished safely and effectively in this large animal model. Furthermore, early application of this adjunct provides measurable protective effects against injury and diminished flow compared with delayed placement. These findings provide original large animal data that support recent publications from civilian and military institutions advocating the use of temporary vascular shunts. Additional studies using this and other models will be necessary to define in more detail the impact that varied warm ischemia times or other factors associated with extremity vascular injury such as systemic shock may have on functional or quality limb salvage.
Acknowledgments
Supported by US Air Force Surgeon General, Department of Clinical Investigations intramural funding.
Footnotes
Presented in poster format at the 2008 American Association for the Surgery of Trauma (AAST) meeting in Maui, HI.
The views expressed in this report are those of the authors and do not reflect the official policy of the Department of Defense or other departments of the US Government.
REFERENCES
- 1.Starnes BW, Beekley AC, Sebasta JA, Andersen CA, Rush RM., Jr Extremity vascular injuries on the battlefield: tips for surgeons deploying to war. J Trauma. 2006;60:432–442. doi: 10.1097/01.ta.0000197628.55757.de. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, McMullin NR, Pearse L, et al. Causes of death in US special operations forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Debakey ME, Simeone FA. Battle injuries of the arteries in World War II: an analysis of 2,471 cases. Ann Surg. 1946;123:534–579. [PubMed] [Google Scholar]
- 4.Hughes CW. The primary repair of wounds of major arteries; an analysis of experience in Korea in 1953. Ann Surg. 1955;141:297–303. doi: 10.1097/00000658-195503000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich NM, Baugh JH, Hughes CW. Acute arterial injury in Vietnam: 1,000 cases. J Trauma. 1970;10:359–369. doi: 10.1097/00005373-197005000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Rich NM, Hughes CW. Vietnam vascular registry: a preliminary report. Surgery. 1969;65:218–226. [PubMed] [Google Scholar]
- 7.Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. Echelons of care and the management of wartime vascular injury: a report from the 332nd EMDG/Air Force Theater Hospital, Balad Air Base, Iraq. Perspect Vasc Surg Endovasc Ther. 2006;18:91–99. doi: 10.1177/1531003506293374. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen TE, Clouse WD, Jenkins DH, Peck MA, Eliason JL, Smith DL. The use of temporary vascular shunts as a damage control adjunct in the management of wartime vascular injury. J Trauma. 2006;61:8–12. doi: 10.1097/01.ta.0000220668.84405.17. [DOI] [PubMed] [Google Scholar]
- 9.Chambers LW, Green DJ, Sample K, et al. Tactical surgical intervention with temporary shunting of peripheral vascular trauma sustained during Operation Iraqi Freedom: one unit’s experience. J Trauma. 2006;61:824–830. doi: 10.1097/01.ta.0000197066.74451.f3. [DOI] [PubMed] [Google Scholar]
- 10.Taller J, Kamdar JP, Greene JA, et al. Temporary vascular shunts as initial treatment of proximal extremity vascular injuries during combat operations: the new standard of care at Echelon II facilities? J Trauma. 2008;65:595–603. doi: 10.1097/TA.0b013e31818234aa. [DOI] [PubMed] [Google Scholar]
- 11.Woodward E, Clouse WD, Eliason JE, et al. Penetrating femoropopliteal injury during modern warfare: experience of the Balad Vascular Registry. J Vasc Surg. 2008;47:1259–1264. doi: 10.1016/j.jvs.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Granchi T, Schmittling Z, Vasquez J, et al. Prolonged use of intraluminal arterial shunts without systemic anticoagulation. Am J Surg. 2000;180:493–496. doi: 10.1016/s0002-9610(00)00508-0. discussion 496–497. [DOI] [PubMed] [Google Scholar]
- 13.Bonheur JA, Albadawi H, Patton GM, Watkins MT. A noninvasive murine model of hind limb ischemia-reperfusion injury. J Surg Res. 2004;116:55–63. doi: 10.1016/s0022-4804(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 14.Conrad MF, Stone DH, Albadawi H, et al. Local inflammatory and thrombotic responses differ in a murine model of partial and complete hind limb ischemia/reperfusion. Surgery. 2005;138:375–381. doi: 10.1016/j.surg.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Eberlin KR, McCormack MC, Nguyen JT, Tatlidede HS, Randolph MA, Austen WG., Jr Ischemic preconditioning of skeletal muscle mitigates remote injury and mortality. J Surg Res. 2008;148:24–30. doi: 10.1016/j.jss.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Dawson DL, Putnam AT, Light JT, et al. Temporary arterial shunts to maintain limb perfusion after arterial injury: an animal study. J Trauma. 1999;47:64–71. doi: 10.1097/00005373-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian A, Vercruysse G, Dente C, Wyrzykowski A, King E, Feliciano DV. A decade’s experience with temporary intervascular shunts at a civilian level 1 trauma center. J Trauma. 2008;65:316–324. doi: 10.1097/TA.0b013e31817e5132. discussion 324–326. [DOI] [PubMed] [Google Scholar]
- 18.Malan E, Tattoni G. Physio- and anatomo-pathology of acute ischemia of the extremities. J Cardiovasc Surg (Torino) 1963;4:212–225. [PubMed] [Google Scholar]
- 19.Eger M, Golcman L, Goldstein A, Hirsch M. The use of a temporary shunt in the management of arterial vascular injuries. Surg Gynecol Obstet. 1971;132:67–70. [PubMed] [Google Scholar]
- 20.Chambers LW, Rhee P, Baker BC, et al. Initial experience of US Marine Corps forward resuscitative surgical system during Operation Iraqi Freedom. Arch Surg. 2005;140:26–32. doi: 10.1001/archsurg.140.1.26. [DOI] [PubMed] [Google Scholar]