Version Changes
Revised. Amendments from Version 1
In this revised version of the article we have included an analysis of gene expression patterns for several AD risk factor genes corresponding to the APOE4-positive and APOE4-negative female and male groups.
Abstract
Background: The APOE gene encodes apolipoprotein ε (ApoE), a protein that associates with lipids to form lipoproteins that package and traffic cholesterol and lipids through the bloodstream. There are at least three different alleles of the APOE gene: APOE2, APOE3, and APOE4. The APOE4 allele increases an individual's risk for developing late-onset Alzheimer disease (AD) in a dose-dependent manner. Sex differences have been reported for AD susceptibility, age of onset, and symptom progression, with females being more affected than males.
Methods: In this study, we use a systems biology approach to examine gene expression patterns in the brains of aged female and male individuals who are positive for the APOE4 allele in order to identify possible sex-related differences that may be relevant to AD.
Results: Based on correlation analysis, we identified a large number of genes with an expression pattern similar to that of APOE in APOE4-positive individuals. The number of these genes was much higher in APOE4-positive females than in APOE4-positive males, who in turn had more of such genes than APOE4-negative control groups. Our findings also indicate a significant sex* genotype interaction for the CNTNAP2 gene, a member of the neurexin family and a significant interaction for brain area*sex* genotype for PSEN2, a risk factor gene for AD.
Conclusions: Profiling of these genes using Gene Ontology (GO) term classification, pathway enrichment, and differential expression analysis supports the idea of a transcriptional role of APOE with respect to sex differences and AD.
Keywords: APOE4, Alzheimer's Disease, Systems Genetics
Introduction
The APOE gene encodes the apolipoprotein E protein (ApoE), which associates with lipids to form lipoproteins that package and traffic cholesterol and lipids through the bloodstream ( de Chaves & Narayanaswami, 2008; Eichner et al., 2002; Puglielli et al., 2003). In the central nervous system, ApoE is primarily produced by astrocytes and carries cholesterol to neurons via ApoE receptors of the low-density lipoprotein receptor (LDLR) family ( Getz & Reardon, 2009; Holtzman et al., 2012). There are at least three different alleles of the APOE gene: APOE2, APOE3, and APOE4. In each of these variants, there are two distinct amino acid substitutions at positions 112 and 158 that alter the structure and function of the proteins ( Ghebranious et al., 2005; Mahley et al., 2009).
Of the three alleles, APOE3 is the most common and is believed to have a neutral role in Alzheimer disease (AD), whereas APOE2, the least common, is believed to have a protective effect in AD and general cognitive decline. Brains of patients with AD and APOE2 had fewer fibrillary tangles and lesser amyloid beta deposition. The APOE2 allele has also been positively associated with better memory performance in a cohort of dementia-free, elderly patients. ( Wu & Zhao, 2016). The APOE4 polymorphism, which is found in around 20% of the population but 50% of all patients with AD, increases an individual’s risk for developing late-onset AD in a dose-dependent manner ( Ashford, 2004; Raber et al., 2004; van der Flier et al., 2006; Zuo et al., 2006).
Evidence suggests that the APOE4 allele may contribute to the risk of developing AD due to an increased amount of amyloid plaques in the brain tissue of affected individuals. In turn, a buildup of amyloid plaques may lead to neuronal degeneration and death, resulting in some of the symptoms associated with AD: memory loss, motor function impairment, dementia, and changes in personality ( Barral et al., 2012; Deary et al., 2002; Kim et al., 2009).
Having the APOE4 mutation does not guarantee that a person will develop AD, indicating that there are other genetic risk factors as well as environment and lifestyle elements that probably contribute to the etiology and progression of the disease.
Differences have been reported between females and males carrying the APOE4 allele. Women who are positive for APOE4 have a greater risk of developing AD, show accelerated progression of the disease, and have more severe memory and cognitive decline than men with this allele ( Altmann et al., 2014; Farrer et al., 1997). In a large scale meta-analysis that considered 58,000 research participants, it was also found that females with the APOE4 allele had a greater risk of developing AD earlier in life than males but that the sex difference was smaller than previously thought ( Neu et al., 2017).
In this study, we examined gene expression patterns associated with aged female and male individuals who are positive for the APOE4 polymorphism in order to identify possible sex-related differences that may be relevant to the onset and progression of AD. Our hypothesis was that gene expression patterns based on correlation analysis with APOE expression in these individuals are different.
Methods
Data collection
The RNA sequence data used in this study is derived from the brains of an aged population obtained from the Aging, Dementia and Traumatic Brain Injury Study, accessed through the Allen Brain Atlas ( http://aging.brain-map.org/). To obtain the data, a gene search specifying APOE was first performed. APOE was then selected and used to extract correlates for each brain region through drop down menus located on the same page. The drop-down menus allowed for the selection of brain region, APOE4-positive or -negative data, and sex. Once the selections were made, the “find correlates” button was used to retrieve the data. The datasets consist of gene expression data together with a clinical diagnosis comparison between males and females. The cohorts used in this study are as follows: APOE4-positive: females 7, males 13; and APOE4-negative: females 31, males 49. In this study only the APOE4/4 genotype was taken into account. The other possible APOE genotypes were not considered in the analysis because the genotype information was not available.
APOE gene correlates
Genes whose expression correlated with that of the APOE gene were collected for the four available brain regions: frontal white matter (FWM) (associated with cognitive function, learning, dementia, and personality changes in AD), hippocampus (HIP), parietal cortex (PCx) (associated with sensory processing, attention, motor function, executive function, and spatial reasoning), and temporal cortex (TCx) (involved in sensory processing as well as declarative and long term memory). Data for each brain region was collected separately for all groups. For information about the RNA-sequence data generation and analysis, see the Quantitative Data Generation white paper in Documentation NA-Seq.
Correlations evaluation
Correlates to the APOE gene were obtained by querying the database and specifying these parameters: brain region, APOE4 positive and APOE4 negative, and sex. Correlations equal to or greater than |0.7| were considered in the analysis. The rationale was to investigate genes with a similar expression pattern as APOE in these different groups to identify genes specific and common to each group, as well as possibly identify genes related to sex differences. Keyword search of the Database for Annotation, Visualization and Integrated Discovery (DAVID) version 6.8 ( Huang da et al., 2009) table output was used to identify genes associated with biological processes related to APOE, sex, and AD. Venny 2.0, an online program that compares lists of items, was used to determine the common and unique genes between groups, sex and brain regions.
Statistical analysis
Chi square analyses were used to compare the numbers of gene correlates between the APOE4-positive and APOE4-negative groups and between females and males (Microsoft Office Professional Plus, Excel 2013, Version 15). Gene expression patterns of AD risk factor genes [ CNTNAP2, PSEN2, APOE, PSEN1, APP, ADAM10, and TREM2] for APOE4-postive and APOE4-negative females and males were analyzed by repeated measures ANOVA with genotype and sex as between-subject factors and brain area (FWM, HIP, PCx and TCx) as a within-subject factor. As post hoc test for interactions, Least Squares Means were used (SAS Studio, Release 3.8, Basic Edition). The relationship between CERAD score, Braak stage, and gene expression patterns of AD risk factor genes in APOE4-females and males were analyzed using Pearson product-moment correlations with the HMISC package in R, version 3.6.1. For all statistical analyses α was set to 0.05.
Gene expression sex differences
The differential search function of the RNA-Seq page of the Allen Brain Database was used to find genes that show enrichment between females and males, and between males and females for each group and each brain region to identify sex related differences in gene expression patterns. Genes with a 1.5-fold difference expression and greater were evaluated.
Gene Ontology characterization
DAVID was used to obtain functional information based on Gene Ontology (GO) annotations for the gene correlates. Genes associated with a keyword search term related to APOE, AD, and sex were identified and noted.
Pathway enrichment
KEGG pathway enrichment (using DAVID) was used to further characterize the positive and negative APOE4 gene correlates for each group. Pathways were assessed manually and partitioned based on common themes.
Results
The APOE4 allele alters gene expression patterns
We observed a difference in transcription patterns associated with genes correlating with APOE expression in male and female individuals with the APOE4 allele as compared to APOE4-negative individuals. A difference in transcription patterns between APOE4-positive females and males was also observed.
As assessed by chi square analyses, the proportions of both positive and negative gene correlates to APOE larger than |0.7| were significantly higher in all examined brain regions in both males and females in the APOE4-positive than in the APOE4-negative groups (Extended data Workbooks 1 and 2; Delprato et al., 2019a; Delprato et al., 2019b).
Positive correlates: APOE4-positive vs APOE4-negative groups (all χ 2 have 2 df and p<0.001), females: FWM: χ 2=4470.1; HIP: χ 2=1285.4; PCx: χ 2=4050.4; TCx: χ 2=3906.60; males: FWM: χ 2=574.8; HIP: χ 2=602.8; PCx: χ 2=187.4; TCx: χ 2=318.5.
Negative correlates: APOE4-positive vs APOE4-negative groups (all χ 2 have 2 df and p<0.001), females: FWM: χ 2=2863.1; HIP: χ 2=2654.5; PCx: χ 2=2555.1; TCx: χ 2=2335.1; males: FWM: χ 2=470.8; HIP: χ 2=351.4; PCx: χ 2=220.3; TCx: χ 2=671.1.
In the APOE4-positive groups we also observed sex differences. Females carrying the APOE4-positive allele had significantly more gene correlates than APOE4-positive males. For all brain regions, differences between female and male groups in the numbers of correlates obtained at or above the cutoff value (r=>|0.7|) were assessed with chi square tests (2x2, df=2).
Positive correlates: males vs females (all χ 2 have 2 df and p<0.001): FWM: χ 2=1926.7; HIP: χ 2=237.9; PCx: χ 2=2693.4; TCx: χ 2=2565.1.
Negative correlates: males vs females (all χ 2 have 2 df and p<0.001): FWM: χ 2=1051.8; HIP: χ 2=1660.9; PCx: χ 2=2125.8; TCx: χ 2=826.0.
In the APOE4-negative groups, sex differences were also observed, but they were less strong than in the APOE4 positive groups. For the positive correlates, females had significantly more gene correlates than males. However, for the negative correlates, this was reversed for the FWM, whereas no differences were found for the HIP and TCx.
Positive correlates: males vs females (all χ 2 have 2 df and p<0.001 unless indicated otherwise): FWM: χ 2=73.6; HIP: χ 2=5.3 (0.10>p>0.05); PCx: χ 2=6.5 (p<0.05); TCx: χ 2=21.8.
Negative correlates: males vs females (all χ 2 have 2 df and p<0.001 unless indicated otherwise): FWM: χ 2=150.0; HIP: χ 2=3.0 (ns); PCx: χ 2=84.1; TCx: χ 2=0.9 (ns).
The results indicate that for all brain regions and for both sexes, the number of APOE correlates, and therefore gene expression patterns, are significantly different between the groups carrying the APOE4 allele as compared to APOE4-negative individuals and that expression patterns also differ between females and males of the APOE4-positive groups.
Common and unique genes
For the common gene correlates to APOE in APOE4-positive and APOE4-negative individuals, the results for the female and male comparison are as follows: TCx: 47/37 (positive/negative) common genes between females and males, 3263/2532 genes unique to females, 420/577 genes unique to males; HIP 22/16 common genes between females and males, 1164/1984 genes unique to females, 544/323 genes unique to males; FWM 48/22 common gene between females and males, 3465/2335 genes unique to females, 952/818 genes unique to males; PCx 54/42 common gene between females and males, 3432/2605 genes unique to females, 213/175 genes unique to males (Extended data Workbook 3; Delprato et al., 2019c).
GO enrichment
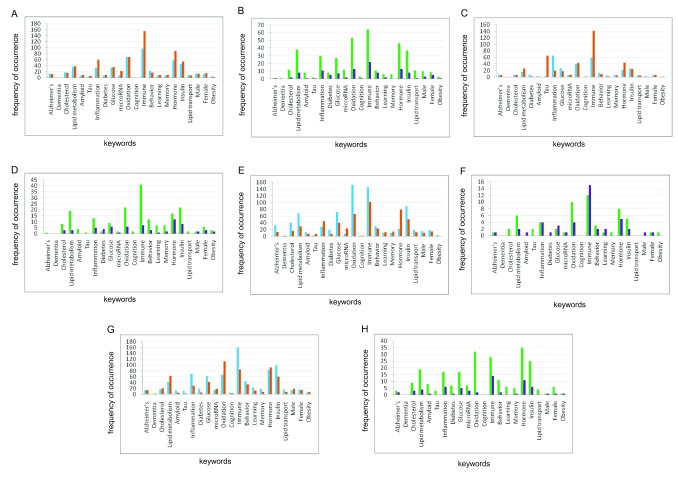
Results from a keyword search of GO terms obtained for positive and negative gene correlates for both the APOE4-positive and APOE4-negative male and female groups across the four brain regions show the same trend of enrichment ( Figure 1 and Extended data Workbook 4; ( Delprato et al., 2019d). Several of the keywords used to analyze the correlates, were associated with a greater number of genes in each brain region and for both positive and negative correlates. The keyword categories are: “immune”, “oxidation”, “inflammation”, “lipid metabolism”, “hormone”, and ”insulin”. Despite the common categories between females and males, there are very few common genes (Extended data Workbook 4; Delprato et al., 2019d).
Figure 1. Keyword enrichment.
Representative keyword enrichment based on GO Term classification. ( A) Females and ( B) males, frontal white matter; ( C) females and ( D) males, hippocampus; ( E) females and ( F) males, parietal cortex; ( G) females and ( H) males, temporal cortex. X-axis, keyword categories; Y-axis, frequency of occurrence.
For the keyword categories, there were no common genes between males and females in the FWM correlates, For the other brain regions common genes between males and females are as follows: HIP positive correlates: “inflammation”, CD4 and CXCL1; negative correlates PPP1CC; PCx negative correlates “Lipid metabolism” PTGES3; “Inflammation” PTGER4; “Oxidation” MTHFD2L; “Immune”, FSD1L, PTGER4; “Hormone” PGRMC2; TCx negative correlates “Inflammation” TBC1D23; “Hormone” GNRH1.
Whether either of the female or male groups had more of any one type of gene is not clear, due to the greater number of gene correlates observed for females. In this respect, the female groups generally had more genes in each of these keyword categories overall. The females also have a substantial number of Alzheimer-related genes associated with each brain region (Extended data Workbook 4; Delprato et al., 2019d).
Pathway enrichment
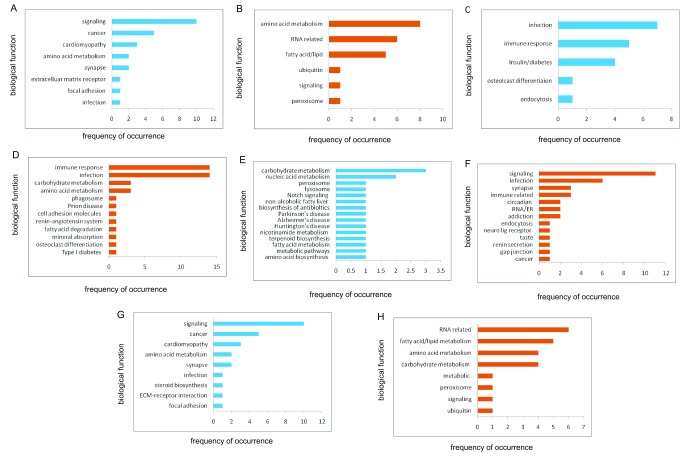
Female pathways. To gain further insight into the function of the gene correlates, a pathway analysis was performed for each group ( Figure 2 and Extended data Workbook 5; Delprato et al., 2019e). For APOE4-positive females, the strongest pathway themes, when combined, are associated with various types of signaling cascades, such as MAPK (cell cycle, transcription, stress response), Chemokine (immune response), cAMP (2nd messenger signaling), Jak-STAT (immune function, cell division, apoptosis, TNF (immune system function, inflammation, infection response, apoptosis), Toll-like receptor (immune function, innate immunity), neurotrophin (growth factors, protection, development and function of neurons), VEGF (growth factors, vascularization, cancer), hedgehog (cell differentiation and cancer), infection, immune system processes, amino acid metabolism, lipid metabolism, energy metabolism, RNA related processes, cancer, trafficking and recycling organelles (endosome, lysosome, peroxisome).
Figure 2. Pathway enrichment.
KEGG pathway categories for female gene correlates. ( A) Positive correlates and ( B) negative correlates, frontal white matter; ( C) positive correlates and ( D) negative correlates, hippocampus; ( E) positive correlates and ( F) negative correlates; parietal cortex; ( G) positive correlates and ( G) negative correlates, temporal cortex. X-axis, frequency of occurrence; Y-axis, biological function.
The PCx-positive correlates are associated with the AD, Parkinson, and Huntington pathways. There are 16 genes ( ATP5D, ATP6, ATP8, COX1, COX2, COX3, COX6A2, CYTB, NDUFA1, NDUFA13, NDUFA4L2, NDUFB7, NDUFS6, NDUFS7, NDUFS8, UQCR11) in common among these pathways and all are related to energy production, mitochondria related-electron transport and oxidative phosphorylation.
Five genes from the HIP negative correlates associated with the Prion disease pathway: C1QA, C7, C1QB, C6 and C1QC; these are all related to the complement pathway of the innate immune system.
Male pathways. For males, the overall number of pathways associated with the APOE4-positive gene correlates were much fewer than for the females, and in some cases, such as for the PCx-positive correlates and TCx-negative correlates, there were no pathways identified. The greatest number of pathways for males were obtained for the TCx-positive correlates and these were related to fatty acid metabolism, amino acid biosynthesis, cell attachment/cytoskeleton, neuro-related processes such as GABA axon guidance, viral infection, and immune function. The HIP positive correlates were related to fatty acid metabolism, amino acid metabolism, Lupus, and viral carcinogenesis. For the HIP-negative correlates two pathways were obtained: alcoholism and RNA transport. For the PCx-negative correlates there were four pathways: spliceosome, RNA degradation, NF-κβ signaling and pertussis. For the other brain regions in males there was one pathway for both positive and negative correlates associated with the FWM: Fatty acid/lipid, carbohydrate metabolism, degradation and glycosphingolipid biosynthesis—lacto and neolacto series (Extended data Workbook 5; Delprato et al., 2019e).
Comparison of female and male pathways
Pathways that were exactly the same between female and males were found for the TCx region only, and these are: hsa04510: Focal adhesion, hsa04512: ECM-receptor interaction, hsa05412: Arrhythmogenic right ventricular cardiomyopathy hsa05410: Hypertrophic cardiomyopathy, hsa05414: Dilated cardiomyopathy, hsa04724: Glutamatergic synapse, and hsa00330: Arginine and Proline metabolism.
For the other brain regions, some of the associated pathways were similar in biological function. Common themes between females and males were related to fatty acid, processes, cardiomyopathy, energy metabolism, amino acid metabolism, cell attachment, ECM (extracellular matrix) receptor interaction, glutamatergic synapse, and pathways related to immune function.
Several themes associated with the APOE4-positive females stand out as being distinct from male pathways and these are related to cancer, signaling, RNA processes, and a myriad of bacterial, viral, and parasitic infectious disease pathways, which include components of endocytosis, intracellular traffic, and immune system function.
Differential gene expression between females and males in APOE4-positive and APOE4-negative groups
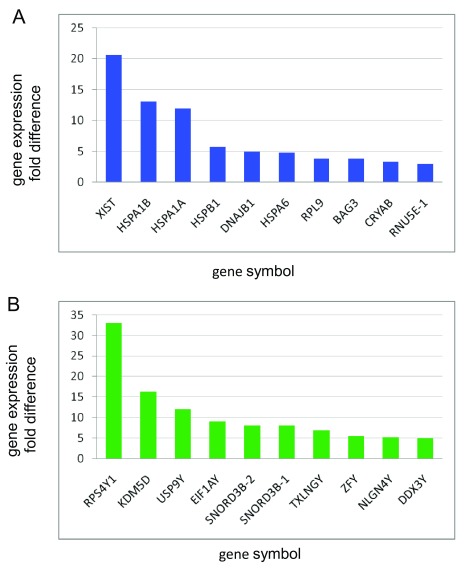
Genes expressed higher in females. For all groups and for each brain region in the female and male comparison there is one gene, XIST, associated with X chromosome inactivation, that is highly expressed and female specific ( Ji et al., 2015). For the APOE4-positive group and for each brain region there are several genes that have higher expression values for females over males. Seven of these genes are heat shock proteins, which are involved in the cellular stress response, and chaperones, which assist in protein folding and unfolding: BAG3, DNAJB1, CRYAB1, HSPA1A, HSPA1B, HSPA6, HSPB1. The two highest expressed genes aside from XIST are HSPA1A and HSPA1B. The latter two are present in female datasets but not in males. The other top expressing genes in the female and male comparison are RPL9, which is down regulated in severe AD ( Kong et al. 2009) and RNU5E-1, small nuclear RNA ( Figure 3 and Extended data Workbook 6; Delprato et al., 2019f).
Figure 3. Sex differences in gene expression.
( A) Top differentially expressed genes in the frontal white matter of APOE4-positive females and ( B) representative top genes differentially expressed in APOE4-positive males. X-axis, gene symbol; Y-axis, fold difference values.
Genes expressed higher in males. For all groups and brain regions, the same top gene, RPS4Y1, which is on the Y chromosome and encodes for the 40S ribosomal subunit, is highly expressed. RPS4Y1 is an RNA binding protein and it is involved in rRNA processing, translation, and protein targeting. Several other genes are expressed at high levels in males. These include KDM5D (immune system function, oxidation reduction, T-cell antigen processing and presentation, regulation of androgen receptor signaling pathway), USP9Y (ubiquitin/deubiquitination), SNORD3B-2/1 small nuclear RNAs (RNA biogenesis/transport), TXLNGY (syntaxin binding, taxin family), ZFY (transcription regulation), NLGN4Y (lipid metabolism, synapse assembly, neuron cell-cell adhesion), DDX3Y (regulation of gene expression, RNA helicase). With the exception of SNORD3B-2/1, all of the other genes are linked to the Y chromosome and are male specific. Other male genes expressed at lower levels but above the cutoff value have diverse functions, including splicing, immune response, and ubiquitination. Based on GO term annotation, none of the top expressing genes in the male and female comparison are heat shock or chaperone proteins but there is one chaperone protein in males just expressed at a 2.0-fold difference, HBB, which is specific for hemoglobin ( Figure 3, Extended data Workbook 6; Delprato et al., 2019f).
We also examined the differential gene expression pattern of transcription factors associated with APOE4-positive and APOE4-negative individuals and we observed distinct expression of transcription factors between these groups and also between females and males (Extended data Workbook 7; Delprato et al., 2019g).
There are an increased number of transcription factors expressed in APOE4-positive females as compared with APOE4-positive males. This is also the case for APOE4-positive females as compared to the APOE4-negative female group. For males, the APOE4-negative and APOE4-positive groups have more or less the same set of transcription factors expressed in each brain region with the exception of the FWM in the APOE4-negative group.
Transcription factors overexpressed in females as compared to males for APOE4-positive groups by brain region are as follows. FWM: FOS, JUN, JUNB, S100A8, A100A9, ATF3, HSPA1A, HSPA1B, HMOX1, IL1B, NPAS4, SIK1, ZSCAN31, HIP: FOS, JUNB, S100A12, NME1-NME2, S100A8, S100A9, ATF3, HSPA1A, HSPA1B, HMOX1, NR4A1, VEGFA; PCx: FOS, FOSB, JUNB, NKX6-2, S100A8, S100A9, ATF3, HSPA1A, HSPA1B, ID3 NR4A1, NR4A2, SIK1; TCx: S100A12, S100A9, HSPA1A, HSPA1B.
For APOE4-negative females, there were no differentially expressed transcription factors observed for the FWM, PCx, and TCx. The exception was for the HIP where HSPA1A and HSPA1B were expressed above the threshold value of 1.5-fold difference.
In males there are generally the same set of transcription factors for APOE4-positive and APOE4-negative groups associated with the HIP, PCx, and TCx, and three of these are Y chromosome specific: KDM5D, ZFY, and HSFY1.
An exception was observed for the male APOE4-negative group. Associated with the FWM, there were several additional transcription factors expressed above the threshold value ( FOS, CAPN3, ENPP2, GREM1, KDM5D, VEGFA, ZFY and two of these, FOS and VEGFA, also occur in the female APOE4-positive groups ( FOS: FWM, HIP, PCx; VEGFA: HIP). Otherwise, the expression of transcription factors observed between females and males is distinct.
Gene expression patterns of AD risk factor genes. For CNTNAP2 we observed significant effects of sex [F(1,72)=6.52, P=0.0128] and brain area [F(3,216)=68.23, P<0.0001] with highest expression in APOE4-positive males in the TCx (Extended data Figure 1). Sex interacted significantly with genotype [F(1,72)=5.66, P=0.0316] and brain area [F(3,216)=3.00, P=0.0329].
For PSEN2 we observed a significant effect for brain area [F(3,213)=20.72, P<0.0001], with a significant brain area*sex*genotype triple interaction [F(3,213)=2.67, P=0.0484]. The highest expression occurred in the TCx for APOE4-positive females (Extended data Figure 2).
APOE expression was significantly different between brain areas [F(3,207)=49.83, P<0.0001]. There was also a marginally significant sex*genotype interaction [F(1,69)=33.19, P=0.0784] with the highest expression levels in APOE4-positive females for the HIP (Extended data Figure 3).
A marginally significant sex*genotype interaction was also observed for PSEN1 expression levels [F(1,73)=3.26, P=0.0751] and expression differed significantly between brain areas [F(3, 219)=72.11, P<0.0001] with the highest levels observed in APOE4-positive females in the FWM (Extended data Figure 4).
For the other risk factor genes, only significant differences between brain areas were obtained: APP [F(3,231)=3.92, Pr=0.0105] with highest expression in APOE4-positive males for the TCx (Extended data Figure 5), ADAM10 [F(3,219)=42.01, P <0.0001] with highest expression for APOE4-positive males in the FWM (Extended data Figure 6), and TREM2 [F(3,219)=33.53, P<0.0001] with highest expression for APOE4-positive males in the HIP (Extended data Figure 7).
Relationship between clinico-pathological stages and expression patterns of AD risk factor genes. The possible connection between clinico-pathological stages and gene expression patterns for the AD risk factor genes among the APOE4-positive and APOE4-negative females and males was investigated (Extended data Workbooks 8 to 14, Workbook 1: CNTNAP2, Workbook 2: PSEN2, Workbook 3: APOE, Workbook 4: PSEN1, Workbook 5: APP, Workbook 6, ADAM10, Workbook 7: TREM2). The strongest correlations (r |0.7|) occurred between Braak stage and expression of APOE: FWM, APOE4-positive females: r=0.87, df=5, P=0.054 and CNTNAP2, HIP, APOE4-positive females, r=0.80, df=5, P=0.057. There were no correlations > |0.7| between CERAD score and the expression of AD risk factor genes. The correlation tables for each gene are included in the extended data workbooks.
Discussion
The APOE4 allele is the most significant genetic risk factor for late onset AD ( Corder et al., 1993; Neu et al., 2017; Raber et al., 2004). It is also associated with aging, atherosclerosis, Lewy Body Dementia, and cardiovascular disease ( de Chaves & Narayanaswami 2008; Dickson et al., 2018; Eichner et al., 2002; Riedel et al., 2016). In this study we used a correlation analysis approach to examine gene expression patterns in an aged population consisting of APOE4-positive and APOE4-negative women and men. The goal of this study was to possibly identify sex-related differences in individuals carrying the APOE4 allele. This is because many studies have reported differences in the effects of the APOE4 allele on females particularly, in the context of AD with respect to age of onset and accelerated disease progression associated with dementia and cognitive decline ( Farrer et al., 1997; Podcasy & Epperson, 2016; Riedel et al., 2016; Vest & Pike, 2013). It is important to consider, however, that the male and female differences observed in AD may also be related to the average life span of females who generally live longer than males. Whether this would actually contribute is not directly evident but could be addressed in these types of studies by accounting for the longevity difference in the experimental design.
Our hypothesis was that gene expression patterns in APOE4-positive and APOE4-negative individuals would differ. We found a large number of genes with the same expression pattern as APOE in APOE4-positive, but not APOE4-negative individuals. This trend was larger in APOE4-positive females as compared with APOE4-positive males and with APOE4-negative control groups.
We did not anticipate that such a large number of gene correlates observed within the significance range would differ between the APOE4-positive and APOE4-negative groups and also between APOE4-positive females and males, even though the expectation had been that there would be differences related to gene function. Despite the differences in the overall numbers of correlates between the groups, the same major categories of genes were observed for both positive and negative correlates as well as for all of the four brain regions considered in this study. The categories of genes relate to immune processes, oxidation, inflammation, lipid metabolism, and hormones.
The results are largely consistent with what is known about APOE function. It is interesting, but in retrospect not surprising, that the same gene categories would emerge for all groups despite the substantial differences in the numbers of gene correlates which may be associated with the different biological processes affected by the APOE4 allele.
The results of the pathway analysis were somewhat similar, but some differences were also observed. A large number of pathways were obtained for the female gene correlates, but many fewer for the males, which is not surprising given the larger number of gene correlates for females. Those that could be compared between the two sexes show that there are common themes such as immune function, lipid metabolism, and inflammation. Interestingly, many of the female pathways were related to microbial infection, major signalling circuits, and amino acid metabolism. Several other pathways were concerned with RNA, cancer, and cardiovascular processes. These are very diverse but all relatable to APOE function and biological programs.
In the evaluation of genes expressed at higher levels in APOE4-positive females than in males, several genes were observed exclusively in females; these were either chaperones that function in protein folding and unfolding or heat shock proteins that play a protective role in cellular stress. The role of these proteins in AD has been investigated ( Campanella et al., 2018; Hamos et al., 1991; Marino Gammazza et al., 2016). In contrast, the genes expressed higher in males than in females were Y-chromosome specific. Distinct patterns of transcription factor expression were also observed between the groups. The transcription factors that are expressed in APOE4-positive females are involved in the regulation of a multitude of diverse processes. Several of these have established roles in the regulation of immune system response, inflammation, oxidative stress, aging, and estrogen signaling, which may be of particular relevance to this study (Extended data Workbook 7; Delprato et al., 2019g).
We also observed a sex*genotype interaction for the CNTNAP2 gene. This gene is a member of the neurexin family; is strongly associated with autism spectrum disorders, and has also been linked to AD ( Lazaro et al., 2019). In one study, CNTNAP2 was down regulated in the hippocampus of AD patients ( van Abel et al., 2012). Interestingly, CNTNAP2 been has identified as a dyslexia susceptibility gene and several variants are associated with gender specific differences ( Gu et al., 2018). We observed a significant triple interaction of brain area*sex*genotype for PSEN2, which is strongly linked to AD and is believed to have a role in APP processing. Disease linked variants of PSEN2 result in increased production of the longer form of amyloid beta, the major component of amyloid plaques in AD patients (reviewed in Cai et al., 2015).
Others have reported differences in gene expression patterns associated with the APOE4 allele ( Huang et al., 2017; Lin et al., 2018; Theendakara et al., 2018). The APOE4 allele has also been shown to alter the transcriptional profile of 857 genes with similar expression patterns to APOE in induced neurons and glia ( Lin et al., 2018). The major pathways identified for these genes are consistent with what we observe: lipid processes, immune system, inflammation, energy metabolism, and transcription. There is a growing body of evidence indicating that APOE has transcription factor activity. Most of the studies in support of this were done in vitro and in mouse models. Further testing in humans is imperative and may provide a clearer understanding of the relationship between APOE, aging, and AD.
The results from our study are consistent with known APOE-related processes and provide further support for the regulatory role of APOE as a transcription factor. That our findings are consistent with other reports that have used different models to study the relationship of APOE with AD and aging support the approach that we have taken ( Huang et al., 2017; Lin et al., 2018; Theendakara et al., 2018). We provide further relevance and insight for the differential transcription-related effects of the APOE4 allele in APOE4-positive males and females, which may be related to the overall heightened immune response and increased risk for AD observed in females ( Klein, 2012; Taneja, 2018). The identification of specific genes, gene families, and pathways that are affected by the APOE4 allele will help to dissect its complex role in diverse but interrelated physiological processes. It would be interesting to compare the results from our study with APOE4 expression patterns in a younger population to see if these differences between APOE4-positive and -negative groups and between females and males are already present and detectable early on or are age dependent.
Data availability
Underlying data
Underlying RNA sequence data used in this study was obtained from the Aging, Dementia and Traumatic Brain Injury Study, accessed through the Allen Brain Atlas ( http://aging.brain-map.org/).
Extended data
Figshare: Workbook 1. Positive and negative gene correlates for APOE4+ and APOE4- females. Frontal white matter, Hippocampus, Parietal cortex, and Temporal cortex. https://doi.org/10.6084/m9.figshare.7849175 ( Delprato et al., 2019a)
Workbook 2. Positive and negative gene correlates for APOE4+ and APOE4- males. Frontal white matter, Hippocampus, Parietal cortex, and Temporal cortex. https://doi.org/10.6084/m9.figshare.7849190 ( Delprato et al., 2019b)
Workbook 3. Common and unique genes for APOE4+ and APOE4- males and females. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.7849196 ( Delprato et al., 2019c)
Workbook 4. Keywords for gene correlates APOE4+ and APOE4- females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.7849214 ( Delprato et al., 2019d)
Workbook 5. KEGG Pathways for positive and negative correlates associated with APOE4+ and females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.7849229 ( Delprato et al., 2019e)
Workbook 6. Differential gene expression in APOE4+ and APOE4- females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.7849238 ( Delprato et al., 2019f)
Workbook 7. Differentially expressed transcription factors in females and males. GO Biological Process. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.7849253 ( Delprato et al., 2019g)
Workbook 8. CNTNAP2 gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867789 ( Delprato et al., 2019h)
Workbook 9. PSEN2 gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867774 ( Deplrato et al., 2019i)
Workbook 10. APOE gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867771 ( Delprato et al., 2019j)
Workbook 11. PSEN1 gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867786 ( Delprato et al.,2019k)
Workbook 12. APP gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867780 ( Delprato et al., 2019l)
Workbook 13. ADAM10 gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867783 ( Delprato et al., 2019m)
Workbook 14. TREM2 gene expression and correlation patterns in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex https://doi.org/10.6084/m9.figshare.8867777 ( Delprato et al., 2019n)
Extended data Figure 1. Gene expression graphs for CNTNAP2, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867849 ( Delprato et al., 2019o)
Extended data Figure 2. Gene expression graphs for PSEN2, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867852 ( Delprato et al., 2019p)
Extended data Figure 3. Gene expression graphs for APOE, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867858 ( Delprato et al., 2019q)
Extended data Figure 4. Gene expression graphs for PSEN1, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867840 ( Delprato et al., 2019r)
Extended data Figure 5. Gene expression graphs for APP, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867846 ( Delprato et al., 2019s)
Extended data Figure 6. Gene expression graphs for ADAM10, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867843 ( Delprato et al., 2019t)
Extended data Figure 7. Gene expression graphs for TREM2, in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. A. APOE4-positive females, B. APOE4-negative females, C. APOE4-positive males, D. APOE4-negative males. Bar graphs represent mean expression +/- SE https://doi.org/10.6084/m9.figshare.8867855 ( Delprato et al., 2019u)
Extended data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- Altmann A, Tian L, Henderson VW, et al. : Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford JW: APOE genotype effects on Alzheimer's disease onset and epidemiology. J Mol Neurosci. 2004;23(3):157–165. 10.1385/JMN:23:3:157 [DOI] [PubMed] [Google Scholar]
- Barral S, Bird T, Goate A, et al. : Genotype patterns at PICALM, CR1, BIN1, CLU, and APOE genes are associated with episodic memory. Neurology. 2012;78(19):1464–1471. 10.1212/WNL.0b013e3182553c48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, An SS, Kim S: Mutations in presenilin 2 and its implications in Alzheimer's disease and other dementia-associated disorders. Clin Interv Aging. 2015;10:1163–72. 10.2147/CIA.S85808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella C, Pace A, Caruso Bavisotto C, et al. : Heat Shock Proteins in Alzheimer's Disease: Role and Targeting. Int J Mol Sci. 2018;19(9): pii: E2603. 10.3390/ijms19092603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, et al. : Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. 10.1126/science.8346443 [DOI] [PubMed] [Google Scholar]
- de Chaves EP, Narayanaswami V: Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 2008;3(5):505–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Pattie A, et al. : Cognitive change and the APOE epsilon 4 allele. Nature. 2002;418(6901):932. 10.1038/418932a [DOI] [PubMed] [Google Scholar]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 1. Positive and negative gene correlates for APOE4+ and APOE4- females. Frontal white matter, Hippocampus, Parietal cortex, and Temporal cortex. figshare.Fileset.2019a. 10.6084/m9.figshare.7849175.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 2. Positive and negative gene correlates for APOE4+ and APOE4- males. Frontal white matter, Hippocampus, Parietal cortex, and Temporal cortex. figshare.Fileset.2019b. 10.6084/m9.figshare.7849190.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 3. Common and unique genes for APOE4+ and APOE4- males and females. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Fileset.2019c. 10.6084/m9.figshare.7849196.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 4. Keywords for gene correlates APOE4+ and APOE4- females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Fileset.2019d. 10.6084/m9.figshare.7849214.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 5. KEGG Pathways for positive and negative correlates associated with APOE4+ females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Fileset.2019e. 10.6084/m9.figshare.7849229.v2 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 6. Differential gene expression in APOE4+ and APOE4- females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Fileset.2019f. 10.6084/m9.figshare.7849238.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 7. Differentially expressed transcription factors in females and males.GO Biological Process. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Fileset.2019g. 10.6084/m9.figshare.7849253.v2 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 8. CNTNAP2 gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019h. 10.6084/m9.figshare.8867789 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 9. PSEN2 gene expression and correlation analysis in APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019i. 10.6084/m9.figshare.8867774 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 10. APOE gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019j. 10.6084/m9.figshare.8867771 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 11. PSEN1 gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019k. 10.6084/m9.figshare.8867786 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 12. APP gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019l. 10.6084/m9.figshare.8867780 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 13. ADAM 10 gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019m. 10.6084/m9.figshare.8867783 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Workbook 14. TREM2 gene expression and correlation analysis for APOE4-positive and APOE4-negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Dataset.2019n. 10.6084/m9.figshare.8867777 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 1. Gene expression graphs for CNTNAP2 in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019o. 10.6084/m9.figshare.8867849 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 2. Gene expression graphs for PSEN2 in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019p. 10.6084/m9.figshare.8867852 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 3. Gene expression graphs for APOE in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019q. 10.6084/m9.figshare.8867858.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 4. Gene expression graphs for PSEN1 in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019r. 10.6084/m9.figshare.8867840.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 5. Gene expression graphs for APP in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019s. 10.6084/m9.figshare.8867846.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data, Figure 6. Gene expression graphs for ADAM10 in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019t. 10.6084/m9.figshare.8867843.v1 [DOI]
- Delprato A, Crusio W, Dedhia M, et al. : Extended data Figure 7. Gene expression graphs for TREM2 in APOE4-positive and APOE4- negative females and males. Frontal white matter, Hippocampus, Parietal cortex, Temporal cortex. figshare.Figure.2019u. 10.6084/m9.figshare.8867855.v1 [DOI]
- Dickson DW, Heckman MG, Murray ME, et al. : APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182–e1195. 10.1212/WNL.0000000000006212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichner JE, Dunn ST, Perveen G, et al. : Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155(6):487–495. 10.1093/aje/155.6.487 [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, et al. : Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. 10.1001/jama.1997.03550160069041 [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA: Apoprotein E as a lipid transport and signaling protein in the blood, liver, and artery wall. J Lipid Res. 2009;50 Suppl:S156–61. 10.1194/jlr.R800058-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebranious N, Ivacic L, Mallum J, et al. : Detection of ApoE E2, E3 and E4 alleles using MALDI-TOF mass spectrometry and the homogeneous mass-extend technology. Nucleic Acids Res. 2005;33(17):e149. 10.1093/nar/gni155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Hou F, Liu L, et al. : Genetic variants in the CNTNAP2 gene are associated with gender differences among dyslexic children in China. EBioMedicine. 2018;34:165–170. 10.1016/j.ebiom.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamos JE, Oblas B, Pulaski-Salo D, et al. : Expression of heat shock proteins in Alzheimer's disease. Neurology. 1991;41(3):345–350. 10.1212/WNL.41.3.345 [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Herz J, Bu G: Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006312. 10.1101/cshperspect.a006312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA: Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, et al. : ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell. 2017;168(3):427–441.e21. 10.1016/j.cell.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Higa KK, Kelsoe JR, et al. : Over-expression of XIST, the Master Gene for X Chromosome Inactivation, in Females With Major Affective Disorders. EBioMedicine. 2015;2(8):909–918. 10.1016/j.ebiom.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM: The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63(3):287–303. 10.1016/j.neuron.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL: Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays. 2012;34(12):1050–1059. 10.1002/bies.201200099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Mou X, Liu Q, et al. : Independent component analysis of Alzheimer's DNA microarray gene expression data. Mol Neurodegener. 2009;4:5. 10.1186/1750-1326-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro MT, Taxidis J, Shuman T, et al. : Reduced Prefrontal Synaptic Connectivity and Disturbed Oscillatory Population Dynamics in the CNTNAP2 Model of Autism. Cell Rep. 2019;27(9):2567–2578.e6. 10.1016/j.celrep.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Seo J, Gao F, et al. : APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018;98(6):1141–1154.e7. 10.1016/j.neuron.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y: Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer's disease to AIDS. J Lipid Res. 2009;50 Suppl:S183–8. 10.1194/jlr.R800069-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino Gammazza A, Bavisotto CC, Barone R, et al. : Alzheimer's Disease and Molecular Chaperones: Current Knowledge and the Future of Chaperonotherapy. Curr Pharm Des. 2016;22(26):4040–4049. 10.2174/1381612822666160518141437 [DOI] [PubMed] [Google Scholar]
- Neu SC, Pa J, Kukull W, et al. : Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. 10.1001/jamaneurol.2017.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podcasy JL, Epperson CN: Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglielli L, Tanzi RE, Kovacs DM: Alzheimer's disease: the cholesterol connection. Nat Neurosci. 2003;6(4):345–351. 10.1038/nn0403-345 [DOI] [PubMed] [Google Scholar]
- Raber J, Huang Y, Ashford JW: ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25(5):641–650. 10.1016/j.neurobiolaging.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, Brinton RD: Age, APOE and sex: Triad of risk of Alzheimer's disease. J Steroid Biochem Mol Biol. 2016;160:134–147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja V: Sex Hormones Determine Immune Response. Front Immunol. 2018;9:1931. 10.3389/fimmu.2018.01931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theendakara V, Peters-Libeu CA, Bredesen DE, et al. : Transcriptional Effects of ApoE4: Relevance to Alzheimer's Disease. Mol Neurobiol. 2018;55(6):5243–5254. 10.1007/s12035-017-0757-2 [DOI] [PubMed] [Google Scholar]
- van Abel D, Michel O, Veerhuis R, et al. : Direct downregulation of CNTNAP2 by STOX1A is associated with Alzheimer's disease. J Alzheimers Dis. 2012;31(4):793–800. 10.3233/JAD-2012-120472 [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Schoonenboom SN, Pijnenburg YA, et al. : The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology. 2006;67(3):526–527. 10.1212/01.wnl.0000228222.17111.2a [DOI] [PubMed] [Google Scholar]
- Vest RS, Pike CJ: Gender, sex steroid hormones, and Alzheimer’s disease. Horm Behav. 2013;63(2):301–7. 10.1016/j.yhbeh.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhao L: ApoE2 and Alzheimer's disease: time to take a closer look. Neural Regen Res. 2016;11(3):412–3. 10.4103/1673-5374.179044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo L, van Dyck CH, Luo X, et al. : Variation at APOE and STH loci and Alzheimer's disease. Behav Brain Funct. 2006;2:13. 10.1186/1744-9081-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]