ABSTRACT
We investigated the clinicopathological role of the PD-1/PD-L1 pathway in primary diffuse large B-cell lymphoma of the central nervous system (PCNS-DLBCL) arising in the immune-privileged site. PD-L1 immunostaining of ≥30% of tumor cells was defined as tPD-L1+, and PD-L1 immunostaining of ≥30% of total cellularity, including tumor and non-tumoral cells, as tmPD-L1+ . PD-1+ and CD8+ tumor-infiltrating lymphocytes (TILs) were enumerated. Thirty-five cases (35.7%) were tPD-L1+ and 47 cases (48%) were tmPD-L1+ . The number of TILs was greater in tmPD-L1+ cases than in tmPD-L1− cases (CD8+, P= .050; PD-1+, P= .019). tPD-L1+ and tmPD-L1+ cases tended to have a poor performance status. In contrast, the numbers of CD8+ and PD-1+ TILs tended to be higher in patients with a good performance status and MYC/BCL2 negativity. Patients with tPD-L1+ had a worse overall survival (P= .026), and those with increased CD8+ or PD-1+ TILs tended to have a better overall survival (P= .081 and 0.044, respectively). Tumoral PD-L1 expression and the number of PD-1+ TILs were independent prognostic factors. tPD-L1+ patients with a small number of CD8+ or PD-1+ TILs had the worst prognosis, and tPD-L1− patients with a large number of CD8+ or PD-1+ TILs had the best prognosis. In validation group, increased CD8+ or PD-1+ TILs were significantly associated with a prolonged survival, but PD-L1 had no prognostic significance. In conclusion, PD-L1 is frequently expressed in tumor cells and the immune microenvironment of PCNS-DLBCL and is correlated with increased TILs. PD-L1 and CD8+ and PD-1+ TILs have potential as prognostic biomarkers and therapeutic targets in PCNS-DLBCL.
KEYWORDS: Primary central nervous system lymphoma, diffuse large Bcell lymphoma, programmed cell death1, programmed cell death ligand1, tumorinfiltrating lymphocytes
Introduction
Primary diffuse large B-cell lymphoma of the central nervous system (PCNS-DLBCL) is an aggressive extranodal non-Hodgkin lymphoma that arises in immune-privileged sites. Although there is no consensus on the optimal treatment regimen for PCNS-DLBCL, high-dose methotrexate-based polychemotherapy with or without radiotherapy is widely used.1 Rituximab as an initial treatment has been reported to be clinically beneficial.2 However, PCNS-DLBCL has a higher rate of recurrence and a worse prognosis than systemic DLBCL despite the application of chemo- and/or radiotherapy; thus, novel therapeutic modalities are needed. Several clinical prognostic models including the International Extranodal Lymphoma Study Group (IELSG) score,3 the Nottingham–Barcelona score,4 and the Memorial Sloan–Kettering Cancer Center (MSKCC) class for PCNS-DLBCL have been developed.5 In addition, efforts have been made to identify pathologic or biological prognostic biomarkers; however, there are no robust prognostic models or biomarkers for PCNS-DLBCL.
Tumor cells use diverse strategies to render their microenvironment friendly to tumor progression and to evade host immune surveillance.6 The programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway is an immune-checkpoint pathway utilized by tumor cells for immune escape. PD-1 is an inhibitory receptor expressed on activated T cells and other immune cells. PD-L1 is expressed on tumor cells and antigen-presenting cells and its expression is regulated by intrinsic (i.e., oncogenic signaling and PD-L1 gene alterations) as well as adaptive (i.e., interferon (IFN)-γ) produced by tumor-infiltrating immune cells) mechanisms. The binding of PD-L1 to PD-1 on activated T cells induces apoptosis, anergy, and functional exhaustion; in this way, tumor cells evade immune surveillance.7,8 PD-1/PD-L1 pathway blockades showed a considerable antitumor effect in patients with many solid tumors and those with refractory or relapsed Hodgkin and non-Hodgkin lymphomas.9
To date, the PD-1/PD-L1 pathway has been primarily investigated in systemic DLBCL. Several clinicopathological features including Epstein-Barr virus (EBV) infection,10 chromosome 9p amplification/translocation,11,12 and activated B-cell-like phenotype were related to increased PD-L1 expression in systemic DLBCL.13,14 In addition, PD-L1 expression on tumor cells and/or macrophages were linked to increased PD-1+ tumor-infiltrating lymphocyte (TIL) infiltration.15,16
PCNS-DLBCL is an entity distinct from systemic DLBCL in terms of its clinical, pathological, and genetic features.17 Moreover, because it resides in an immune-privileged site, PCNS-DLBCL may have a distinct immune microenvironment. However, little is known about the immune microenvironment of this entity. It was reported that primary central nervous system lymphomas (PCNSLs) and primary testicular lymphomas often exhibit a copy number gain at 9p24.1; i.e., the PD-L1 and PD-L2 gene loci, in approximately 50% of PCNS-DLBCL cases as well as PD-L1 gene translocation and subsequent PD-L1 overexpression.18 These findings implicate the PD-1/PD-L1 pathway in the pathogenesis of lymphomas in immune-privileged sites. However, the PD-1/PD-L1 expression status and its association with clinicopathological features in patients with PCNS-DLBCL are unclear. The few previous studies to address this issue used small sample numbers and yielded conflicting results.19–22
Thus, we investigated PD-L1 expression and the numbers of CD8+ or PD-1+ TILs in tumor cells and the immune microenvironment of PCNS-DLBCL and analyzed their clinicopathological and prognostic implications.
Results
PD-L1 expression is positively correlated with the numbers of CD8+ and PD-1+ TILs
Representative IHC images of PD-L1, PD-1, and CD8 expression are shown in Figure 1(a). PD-L1 expression was observed on tumor and/or on non-tumor cells (Figure 1(a)). PD-L1 staining in tumor cells versus non-tumoral cells was validated by double immunostaining for PD-L1 and PAX5 in representative cases (Supplementary Methods; Supplementary Figure S1A; Supplementary Table S1). Most of the non-tumor cells expressing PD-L1 were considered as macrophages in histological basis.
Figure 1.
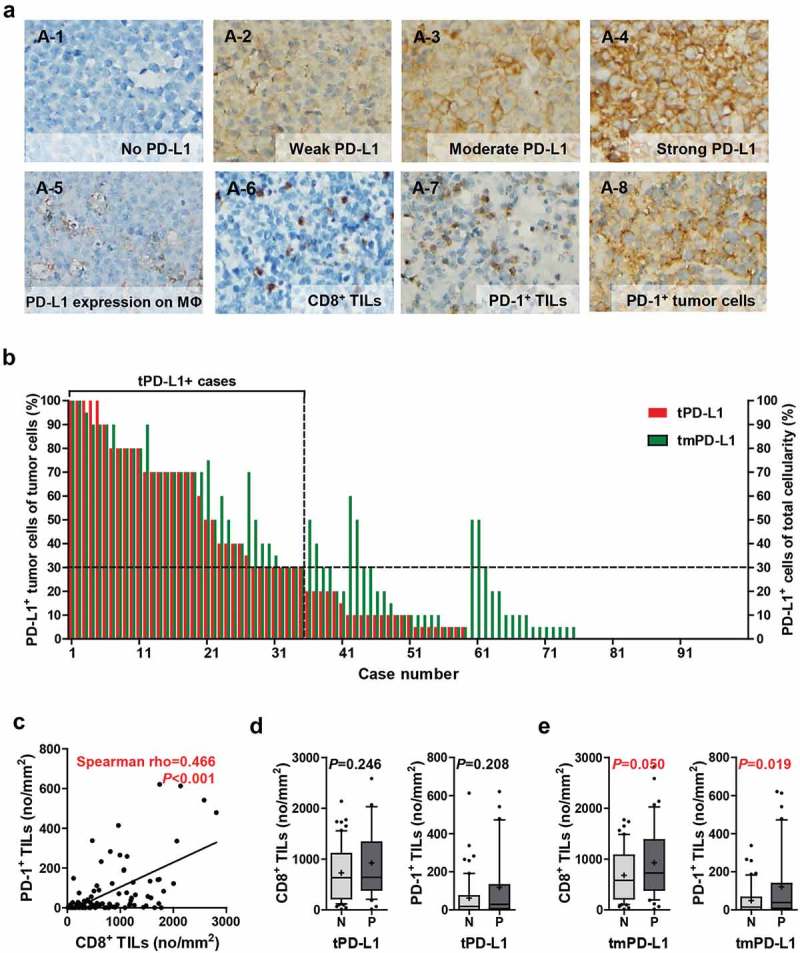
Tumoral and non-tumoral PD-L1 expression is positively correlated with the numbers of CD8+ and PD-1+ TILs. (a) Representative IHC images of PD-L1, PD-1, and CD8. PD-L1 was expressed on tumor cells (A-1–4) and surrounding non-tumoral cells, primarily macrophages (A-5), concurrently or alone. CD8 and PD-1 were expressed on TILs (A-6–7), and PD-1 was expressed on tumor cells (A-8). Percentages of PD-L1+ cells of the tumor cells (tPD-L1, red bar) and those of PD-L1+ cells of the total cellularity including tumor cells and non-tumor cells (tmPD-L1, green bar) in each case are displayed (b). The correlation between the numbers of CD8+ TIL and PD-1+ TIL were assessed by Spearman correlation analysis (c). Numbers of CD8+ TILs and PD-1+ TILs in tPD-L1– and tPD-L1+ cases (d) and tmPD-L1 – and tmPD-L1+ cases (e). Differences were analyzed using the Mann-Whitney U test. Whiskers, 10th to 90th percentiles; midline of the box, median; +, mean. Points below and above the whiskers are individual points. Abbreviations: MΦ, macrophage; N, negative; P, positive.
Thirty-five (35.7%) of the 98 cases were designated as tPD-L1+ (i.e., PD-L1 staining in ≥30% of tumor cells) and 47 (48%) of the 98 cases as tmPD-L1+ (i.e., PD-L1 staining in ≥30% of total cellularity including tumor cells and non-tumoral cells in the microenvironment) (Figure 1(b)). PD-1 and CD8 expression were observed on TILs (Figure 1(a)). However, in some cases, the tumor cells showed PD-1 expression (Figure 1(a) [A-8]). The number of CD8+ and PD-1+ TILs ranged from 14.76 to 2,813.35/mm2 (median 637.16, mean 798.88) and from 0 to 621.15/mm2 (median 19.55, mean 80.50), respectively. The number of CD8+ TILs was positively correlated with that of PD-1+ TILs (Spearman’s rho = 0.466, P < .001) (Figure 1(c)). The numbers of CD8+ and PD-1+ TILs were not significantly different between tPD-L1+ and tPD-L1– cases (Figure 1(d)), but those of CD8+ and PD-1+ TILs tended to be significantly higher in tmPD-L1+ cases compared to tmPD-L1– cases (CD8+ TILs, P = .050; PD-1+ TILs, P = .019) (Figure 1(e)). Moreover, after excluding the tPD-L1+ cases, tmPD-L1 expression was significantly positively correlated with the numbers of CD8+ TILs (Spearman’s rho = 0.340, P = .007) and PD-1+ TILs (Spearman’s rho = 0.366, P= .004) (Supplementary Figure S1B-C).
In the validation group (n = 42), 10 (23.8%) and 13 (31%) cases were tPD-L1+ and tmPD-L1+, respectively. Similar to the experimental group, a strong positive correlation between the numbers of CD8+ and PD-1+ TILs were observed (Spearman’s rho = 0.874, P< .001) (Supplementary Figure S2A). The numbers of CD8+ TILs and PD-1+ TILs were significantly higher in tPD-L1+ cases compared to tPD-L1– cases (P= .010 and 0.006, respectively); and in tmPD-L1+ cases compared to tmPD-L1– cases (P= .001 and 0.001, respectively) (Supplementary Figure S2B and C). In the open datasets of mRNA expression of PCNSL tissues, CD274 (PD-L1) expression tended to be positively correlated with CD8A expression (KAWAGUCHI dataset, Spearman’s rho = 0.348, P= .044; CHAPUY dataset, Spearman’s rho = 0.54, P= .041; CHANGOHK dataset, Spearman’s rho = 0.75, P= .066) (Supplementary Figure S3).
Relationships between PD-L1 expression and clinicopathological features
The overall clinicopathological features of the patients in the experimental and validation group are summarized in Table 1 and Supplementary Table S2, respectively. The relationships between PD-L1 expression and clinicopathological parameters are summarized in Figure 2 (adjusted P) and supplementary Figure S4 (raw P). A poor performance status (ECOG 2–4) tended to be associated with a high tPD-L1 level (raw P= .043; adjusted P= .092). Although the tPD-L1 levels were higher in tumors harboring wild-type CD79B (raw P= .049; adjusted P = .142), other pathological features–including age, cell-of-origin, MYD88 mutation, clinical prognostic models, and MYC/BCL2 expression–were not related to the tPD-L1 or tmPD-L1 level.
Table 1.
Clinicopathological characteristics of patients with PCNS-DLBCL.
| Variablea | n (%) (total = 98) |
|
|---|---|---|
| Age (years) | Median, 61 (range, 10–82) | |
| Sex | Male Female |
56 (57.1) 42 (42.9) |
| Initial symptoms | Headache & vomiting Seizure Neurological deficit |
26 (26.5) 6 (6.1) 66 (67.3) |
| ECOG PSa | 0, 1 2–4 |
61 (62.9) 36 (37.1) |
| KPSa | ≥ 70 < 70 |
83 (86.5) 13 (13.5) |
| B Symptoms | Absent Present |
94 (65.9) 4 (4.1) |
| Serum LDHa | Normal Elevated |
58 (61.7) 36 (38.3) |
| EBV | Negative Positive |
25 (92.6) 2 (7.4) |
| Cell-of-originb | GCB Non-GCB |
23 (23.5) 75 (76.5) |
| Involvement of deep structuresc | Absent Present |
29 (29.6) 69 (70.4) |
| Extent of disease | Unifocal Multifocal |
39 (39.8) 59 (60.2) |
| Ocular involvementa | Absent Present |
87 (89.7) 10 (10.3) |
| CSF proteina | Normal Elevated |
33 (40.7) 48 (59.3) |
| CSF cytologya | Negative Positive |
72 (85.7) 12 (14.3) |
| IELSGa | 0–1 2–3 4–5 |
12 (15.0) 51 (63.8) 17 (21.3) |
| Nottingham-Barcelonaa | 0–1 2–3 |
48 (49.5) 49 (50.5) |
| MSKCC classa | 1 2 3 |
18 (18.6) 68 (70.1) 11 (11.3) |
| Prior steroid therapy | None ≤1 day >1 day, ≤3 days unknown |
77 (78.6) 7 (7.1) 10 (10.2) 4 (4.1) |
| Radiotherapy | Not done Done |
36 (31.6) 78 (68.4) |
| Chemotherapy | MVPd HD-MTX Otherse |
79 (81.4) 14 (14.4) 4 (4.1) |
| Rituximab | Not done Done |
103 (90.4) 11 (9.6) |
| IT-MTX | Not done Done |
89 (78.1) 25 (21.9) |
| Surgery | Biopsy Subtotal resection Gross total resection |
8 (8.2) 74 (75.5) 16 (16.3) |
| MYC expression (% of tumor cells) |
< 40 ≥ 40 |
78 (79.6) 20 (20.4) |
| BCL2 expression (% of tumor cells) |
< 60 ≥ 60 |
41 (41.8) 57 (58.2) |
| MYC/BCL2 expression | Others Double expresser |
82 (83.7) 16 (16.3) |
| MYD88 mutationa | Wild-type Mutation |
49 (64.5) 27 (35.5) |
| CD79B mutationa | Wild-type Mutation |
71 (77.2) 21 (22.8) |
Abbreviations: CSF, cerebrospinal fluid; ECOG PS, Eastern Cooperative Oncology Group Performance GCB, germinal center B cell-like; HD-MTX, high-dose methotrexate; IELSG, International Extranodal Lymphoma Study Group; IT-MTX, intrathecal methotrexate; KPS, Karnofsky Performance Status score; LDH, lactate dehydrogenase; MSKCC, Memorial Sloan Kettering Cancer Center; MVP, chemotherapy regimen comprising high-dose methotrexate, vincristine, and procarbazine.
aSome cases had missing values.
bCell-of-origin was determined by Hans algorithm (Blood 2004;103:275–82).
cDeep structures are the periventricular regions, basal ganglia, brain stem, and/or cerebellum.
dIncluding eight patients who received rituximab-MVP.
eOthers include COPADM and CHOP.
Figure 2.
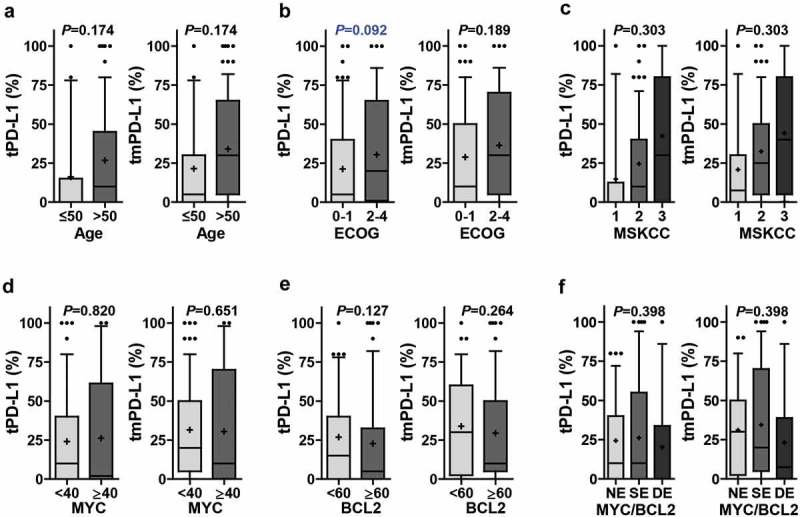
PD-L1 expression according to clinicopathological parameters. The percentages of tumoral PD-L1 (tPD-L1) and tumoral plus non-tumoral PD-L1 (tmPD-L1) were compared according to age (≤50, n = 21; >50, n = 77) (a), ECOG performance status (0–1, n = 61; 2–4, n = 36) (b), MSKCC class (class 1, n = 18; class 2, n = 68; class 3, n = 11) (c), MYC expression (<40, n = 78; ≥40, n = 20) (d), BCL2 expression (<60, n = 41; ≥60, n = 57) (e), and MYC/BCL2 expression status (NE, n = 37; SE, n = 45; DE, n = 16) (f). Differences were analyzed using Mann–Whitney U test and Kruskal–Wallis test and multiple test correction were done by Benjamini and Hochberg method. Adjusted P values were presented on graphs. Whiskers, 10th to 90th percentiles; midline of the box, median; +, mean. Points below and above the whiskers are individual points. Abbreviations: DE, double expresser; ECOG, Eastern Cooperative Oncology Group; MSKCC, Memorial Sloan Kettering Cancer Center; NE, non-expresser; SE, single expresser.
Relationships between the number of CD8+ or PD-1+ TILs and clinicopathological features
The numbers of CD8+ TILs and PD-1+ TILs were not significantly different according to the steroid therapy status prior to the obtaining of tumor tissue (Supplementary Figure S5). The relationships between CD8+ and PD-1+ TILs and clinicopathological parameters are summarized in Figure 3 (adjusted P) and Supplementary Figure S6 (raw P). In contrast to PD-L1, the number of CD8+ TILs tended to be higher in patients with a good performance status (ECOG 0–1) (raw P= .046; adjusted P = .092), MYC negativity (raw P= .012; adjusted P = .048), and BCL2 negativity (raw P= .023; adjusted P = .092). The number of CD8+ TILs was lowest in MYC/BCL2 double expressers and highest in MYC/BCL2 non-expressers (raw P= .006; adjusted P = .024). The number of PD-1+ TILs also tended to be higher in patients with a good performance status and MYC or BCL2 negativity. In the validation group, the numbers of CD8+ and PD-1+ TILs were associated with clinicopathological parameters in a pattern similar to that in the experimental group, but with reduced statistical significance (Supplementary Figure S7).
Figure 3.
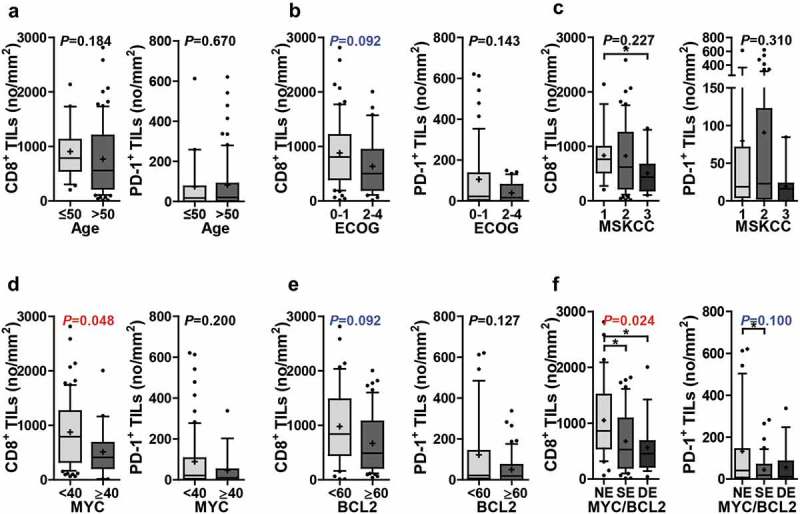
Numbers of CD8+ TILs and PD-1+ TILs according to clinicopathological parameters. The numbers of CD8+ TILs were compared according to age (≤50, n = 21; >50, n = 75) (a), ECOG performance status (0–1, n = 60; 2–4, n = 35) (b), MSKCC class (class 1, n = 18; class 2, n = 66; class 3, n = 11) (c), MYC expression (<40, n = 76; ≥40, n = 20) (d), BCL2 expression (<60, n = 40; ≥60, n = 56) (e), and MYC/BCL2 expression status (NE, n = 36; SE, n = 44; DE, n = 16) (f). The numbers of PD-1+ TILs were compared according to age (≤50, n = 19; >50, n = 71) (a), ECOG performance status (0–1, n = 57; 2–4, n = 32) (b), MSKCC class (class 1, n = 16; class 2, n = 64; class 3, n = 9) (c), MYC expression (<40, n = 72; ≥40, n = 18) (d), BCL2 expression (<60, n = 38; ≥60, n = 52) (e), and MYC/BCL2 expression status (NE, n = 35; SE, n = 40; DE, n = 15) (f). Differences were analyzed using Mann–Whitney U test and Kruskal–Wallis test and multiple test correction were done by Benjamini and Hochberg method. Adjusted P values were presented on graphs. Whiskers, 10th to 90th percentiles; midline of the box, median; +, mean. Points below and above the whiskers are individual points. *P< .05. Abbreviations: DE, double expresser; ECOG, Eastern Cooperative Oncology Group; MSKCC, Memorial Sloan Kettering Cancer Center; NE, non-expresser; SE, single expresser.
PD-1 expression on PCNS-DLBCL tumor B cells
Of the total patients, 16 (16/140, 11.4%) showed PD-1 expression on more than 50% of tumor B cells, mostly with moderate to strong intensity. These patients were characterized by favorable clinical features including the absence of deep-structure involvement (P= .041), unifocal disease (P= .018), and a lower Nottingham–Barcelona score (P= .008) (Supplementary Figure S8).
Prognostic significance of PD-L1 expression and TILs
The results of univariate analyses of survival according to clinicopathological parameters are summarized in Supplementary Table S3. Old age and multifocal disease were associated with a worse prognosis (old age, PFS, P= .006 and OS, P< .001; multifocal disease, PFS, P= .034 and OS, P= .039). Patients with a high Nottingham–Barcelona score or MSKCC class had a poor prognosis (Nottingham–Barcelona score, PFS, P< .001; OS, P< .001; MSKCC class, PFS, P= .017; OS, P= .005). MYC and BCL2 double expressers showed shorter PFS (P= .036) and OS (P= .015). Patients treated with HD-MTX-based chemotherapy plus radiotherapy (59/98, 60.2%) showed a better PFS (P= .033) and OS (P = .008) than those treated with other regimens.
Kaplan-Meier survival analysis demonstrated that tPD-L1+ was significantly associated with a shorter OS (P= .026) and a tendency towards a shorter PFS (P= .088) (Figure 4(a,b)). tmPD-L1+ cases also tended to have a poor prognosis (Supplementary Figure S9A and B). Patients with large numbers of CD8+ or PD-1+ TILs had a longer OS (P= .081 and 0.044, respectively) (Figure 4(c,d); Supplementary Figure S9C and D). tPD-L1+ patients with a small number of CD8+ or PD-1+ TILs showed the worst prognosis, and tPD-L1– patients with a large number of CD8+ or PD-1+ TILs the best prognosis; the difference in OS between these groups was significant (Figure 4(e–h)). In the low CD8+ or PD-1+ TILs group, tPD-L1+ patients tended to have worse PFS and OS than tPD-L1– patients. In the high PD-1+ TIL group, tPD-L1+ patients had a worse OS than tPD-L1– patients (Figure 4(h)). Moreover, in tPD-L1– patients, a large number of PD-1+ TILs were significantly associated with a better prognosis (Figure 4(h)).
Figure 4.
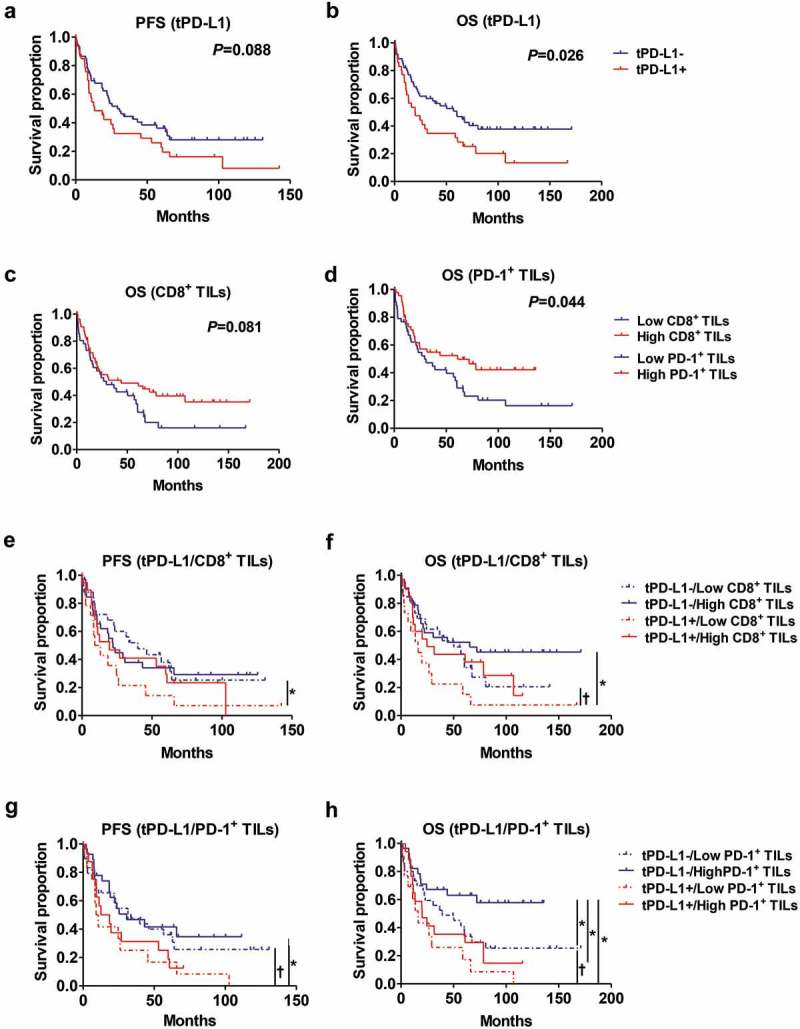
Prognostic significance of tPD-L1, CD8+ TILs, and PD-1+ TILs. Kaplan-Meier analysis of PFS and/or OS according to tPD-L1(tPD-L1-, n = 63; tPD-L1+ = 35) (a-b), CD8+ TILs (Low CD8+ TILs, n = 42; High CD8+ TILs, n = 54) (c), PD-1+ TILs (Low PD-1+ TILs, n = 44; High PD-1+ TILs, n = 46) (d), tPD-L1 plus CD8+ TILs (tPD-L1-/Low CD8+ TILs, n = 27; tPD-L1-/High CD8+ TILs, n = 34; tPD-L1+/Low CD8+ TILs, n = 15; tPD-L1+/High CD8+ TILs, n = 20) (e-f), and tPD-L1 plus PD-1+ TILs (tPD-L1-/Low PD-1+ TILs, n = 31; tPD-L1-/High PD-1+ TILs, n = 29; tPD-L1+/Low PD-1+ TILs, n = 13; tPD-L1+/High PD-1+ TILs, n = 17) (g-h). Differences in survival were analyzed by log-rank test. † P= .05–0.1, *P< .05.
In a multivariate analysis, tPD-L1 expression and the number of PD-1+ TILs were found to be independent prognostic indicators for OS (tPD-L1+, P= .026, HR = 1.896 [95% CI, 1.081–3.325]; high PD-1+ TILs, P= .004, HR = 0.428 [95% CI, 0.241–0.763]) (Table 2 and Supplementary Table S4).
Table 2.
Multivariate analysis of overall survival (OS) in patients with PCNS-DLBCL.
| HR (95% CI) | P | |
|---|---|---|
| Age | 1.045 (1.013–1.077) | 0.005 |
| N-B score 2–3 | 1.977 (1.042–3.750) | 0.037 |
| MSKCC class | - | - |
| HD-MTX+RTx | 0.582 (0.333–1.018) | 0.058 |
| MYC/BCL2 DE | - | - |
| tPD-L1+ | 1.896 (1.081–3.325) | 0.026 |
| High CD8+ TILs | - | - |
| High PD-1+ TILs | 0.428 (0.241–0.763) | 0.004 |
Abbreviations: CI, confidence interval; DE, double expresser; HD-MTX, high-dose methotrexate; MSKCC, Memorial Sloan Kettering Cancer Center; N-B, Nottingham–Barcelona; RTx, radiotherapy.
In the validation group, tPD-L1 and tmPD-L1 had no prognostic significance (data not shown). However, a large number of CD8+ or PD-1+ TILs were significantly associated with a prolonged OS (CD8+ TILs, P = .003; PD-1+ TILs, P = .043) (Supplementary Figure S10A and B). In addition, tPD-L1– patients with a large number of CD8+ or PD-1+ TILs had the longest OS (Supplementary Figure S10C and D).
Additionally, we evaluated “Immunoscore” using CD3+ TILs and CD8+ TILs, because immunoscore has been emerged as a good prognostic marker for cancer, especially for colon cancer.23–26 Consistent with previous studies on colon cancer, PCNS-DLBCL patients with high immunoscore showed significantly better OS than those with low immunoscore, when using the numbers of CD3+ and CD8+ TILs having minimum P value as cutoffs (P = .040) or using the median numbers of CD3+ and CD8+ TILs as cutoffs (P = .044) (Supplementary Figure S11).
Discussion
We investigated PD-L1 expression on tumor cells and in the tumor microenvironment, and CD8+ and PD-1+ TIL status in a large cohort of patients with PCNS-DLBCL. The results demonstrate that PD-L1 expression and CD8+ or PD-1+ TILs are correlated with each other and associated with several clinicopathological parameters. Moreover, high tumoral PD-L1 expression and few CD8+ or PD-1+ TILs on patients with PCNS-DLBCL were predictive of a poor prognosis.
In this study, a substantial proportion of PCNS-DLBCL tumor and/or non-tumor cells expressed PD-L1. Notably, the numbers of CD8+ and PD-1+ TILs were positively correlated with tumoral and/or non-tumoral PD-L1 expression and significantly correlated with the expression of tmPD-L1 rather than tPD-L1. Moreover, after the exclusion of tPD-L1+ patients, significant positive correlations between the tmPD-L1 expression level and the numbers of CD8+ and PD-1+ TILs were observed. A positive correlation between PD-L1 and CD8 in tumor and tumor microenvironment of PCNSL was also validated at the mRNA level using a publicly available database. PD-L1 expression on tumor cells is induced by extrinsic ways, such as cytokines, primarily IFN-γ, secreted by activated immune (especially T) cells; and by intrinsic ways. An increased PD-L1 gene copy number was reported to contribute to tPD-L1 expression in a substantial portion of PCNS-DLBCL patients.18 In contrast, PD-L1 expression on non-tumor cells (e.g., macrophages) is induced by cytokines secreted from surrounding immune cells.7 Therefore, the stronger positive correlation between tmPD-L1+ compared to tPD-L1+ cases with the number of TILs, particularly PD-1+ TILs (putatively T-cell signaling-activated T cells), suggests that adaptive induction of PD-L1 expression in non-tumor cells, as well as adaptive and intrinsic induction of PD-L1 expression in tumor cells, plays a role in immune evasion by PCNS-DLBCL.
In systemic DLBCL, tumoral PD-L1 expression is related to EBV infection and a non-GCB phenotype.10,11 In this study, PD-L1 expression in PCNS-DLBCL tended to be higher in patients who were older, and in those with a poor performance status, and wild-type CD79B, but was not associated with the cell-of-origin. These differences might be attributable to the pathological and genetic features of PCNS-DLBCL, which are distinct from those of systemic DLBCL, e.g., higher rates of non-GCB phenotype, 9p24.1 copy number gain, and MYD88 mutation, and lower rates of EBV infection.17,18,27 In contrast to PD-L1 expression, an increased number of CD8+ or PD-1+ TILs was associated with favorable clinicopathological features, such as good performance status and MYC and/or BCL2 negativity. Because a large number of CD8+ or PD-1+ TILs imply an active immune response in the tumor microenvironment, such an association is reasonable. The small number of TILs in MYC/BCL2 double expressers is intriguing, as it suggests a link between the poor prognostic implications of MYC/BCL2 double expression with the immune microenvironment of systemic and PCNS-DLBCL.
PD-1 expression is induced by T-cell receptor signaling on activated T cells,8 and PD-1 expression was mostly observed on activated T cells in the DLBCL microenvironment. However, PD-1 is expressed on malignant B cells in 2–22% of systemic DLBCL cases, but the clinical relevance of this is unclear.15,28–30 In this study, PD-1 was expressed on malignant B cells in 11% of patients with PCNS-DLBCL. Moreover, tumoral PD-1 expression was significantly associated with several favorable clinical features including the absence of deep-structure involvement, unifocal disease, and a low Nottingham–Barcelona score. It was reported that PD-1 functions as a tumor suppressor in T-cell lymphoma by suppressing oncogenic T-cell signaling.31 Moreover, PD-1 is upregulated in activated human B cells and inhibits B-cell activation upon binding to PD-L1.32,33 Taken together, the findings of this study suggest that PD-1 expressed on malignant B cells could play a tumor-suppressive role in B-cell lymphoma. Larger clinical and functional studies are needed to evaluate the prognostic significance and biological role of PD-1 expression on malignant B cells.
The prognostic implications of PD-L1 expression and PD-1+ TILs in PCNS-DLBCL are disputed. In previous studies of PCNS-DLBCL, high PD-L1 expression was unrelated,21 or related to a favorable prognosis;20,22 an increased PD-1+ TILs were related to an unfavorable prognosis.20,21 This study included the largest population of any similar work to date, analyzed the prognostic implications in experimental and validation groups and according to treatment modality, involved a multivariate analysis, quantified PD-L1 expression and enumerated TILs, and evaluated PD-L1 expression together with CD8+ or PD-1+ TILs. We found that tumoral PD-L1 expression was an independent prognostic indicator of a poor OS. However, tmPD-L1 expression was not significantly related to the prognosis of patients with PCNS-DLBCL. These findings are comparable to those reported in patients with systemic DLBCL in that non-tumoral PD-L1 expression was not significantly correlated with the clinical outcome.14,15 PD-L1 on tumor cells or immune cells contributes to immune escape, but the relative contributions of PD-L1 expression on these cells are context-dependent.34,35 Thus, the prognostic implications of PD-L1 expression on tumor cells versus immune cells may differ among cancer types. Meanwhile, the lack of prognostic significance of PD-L1 in the validation group may be attributable to the small number of cases in this group and/or other confounding factors, such as differences in treatment modalities. In previous studies, high level of soluble PD-L1 in blood was an independent poor prognostic factor for OS in patients with systemic DLBCL.36 Given the poor prognostic implication of tPD-L1 in PCNS-DLBCL observed in this study, it may deserve to investigate the prognostic significance of soluble PD-L1 in blood or cerebrospinal fluid in PCNS-DLBCL.
A large number of CD8+ or PD-1+ TILs were associated with prolonged OS and the latter was an independent prognostic factor for OS. The prognostic implications of CD8+ or PD-1+ TILs were confirmed in the validation group. These findings suggest that the presence of large numbers of TILs, particularly PD-1+ TILs, reflects an active immune response in the tumor microenvironment and is thus related to a favorable clinical outcome of PCNS-DLBCL. The binding of PD-L1 to PD-1 transmits an inhibitory signal to activated T cells; therefore, we analyzed survival according to PD-L1 and TIL status. tPD-L1+ patients with few TILs showed the worst prognosis, whereas tPD-L1– patients with a large number of TILs showed the best prognosis. tPD-L1+ status with low TIL infiltration is regarded as intrinsic induction of PD-L1.37 The worse prognosis of these cases suggests that PD-L1 plays an intrinsic role in tumor progression other than dampening T-cell-mediated immune responses. Alternatively, factors that upregulate the expression of PD-L1, such as chromosome 9p alterations and oncogenic signaling, may contribute to tumor progression. Among the cases with a large number of PD-1+ TILs, tPD-L1+ cases had a shorter OS than tPD-L1– cases. These data suggest that the expression of PD-L1 on tumor cells impairs anti-tumor T-cell responses, leading to a poor clinical outcome.
Different organs have different immune microenvironments. Immune-cell infiltrates are reported to be less marked in PCNS-DLBCL compared to systemic DLBCL,38,39 which may partly explain the poor prognosis of PCNS-DLBCL. We report here that PCNS-DLBCL has substantial immune-cell infiltration, and that PD-L1 expression and TILs are related and have prognostic implications. Moreover, we observed that immunoscore based on CD3+ and CD8+ TILs was associated with a favorable prognosis of patients with PCNS-DLBCL, similar to colon cancer. These findings further suggest that tumor immune microenvironment may be important for the pathobiology of PCNS-DLBCL and the PD-1/PD-L1 pathway contributes to immune evasion by PCNS-DLBCL, even in immune-privileged sites. Consistently, four patients with relapsed/refractory PCNS-DLBCL showed objective responses to PD-1 blockade; three patients exhibited a complete response.40 A clinical trial aiming to confirm the efficacy of PD-1 blockade in PCNS-DLBCL is ongoing (CA209-647, #NCT02857426).
This study has several limitations. First, the discriminative power of the statistical analysis of some of the variables was limited by the sample size. Second, the retrospective nature of this study resulted in disparities in the treatment and follow-up protocols. Third, genetic factors that affect PD-L1 expression, such as 9p24.1/PD-L1 gene alteration, were not evaluated. Fourth, we addressed PD-L1 expression mainly in tumoral and non-tumoral cells, not discriminating various immune cells with a potential to express PD-L1 within the tumor microenvironment.
In summary, our data demonstrate that PD-L1 expression in the PCNS-DLBCL tumor microenvironment is positively correlated with infiltration of CD8+ or PD-1+ TILs, that tumoral PD-L1 expression is associated with a poor prognosis, and that a large number of CD8+ or PD-1+ TILs are associated with a good prognosis. These findings suggest that PD-L1-mediated immune evasion is important for tumor progression in PCNS-DLBCL. Therefore, PD-L1 expression and CD8+ and PD-1+ TIL status have potential as prognostic biomarkers and therapeutic targets.
Materials and methods
Patients
Two cohorts of patients with PCNS-DLBCL were included in this study. The experimental group consisted of 98 patients diagnosed and managed at Seoul National University Hospital (SNUH, Seoul, Republic of Korea) from 2000 to 2012. The validation group consisted of 42 patients treated homogenously with R-MVP (rituximab and chemotherapy with high-dose methotrexate, vincristine, and procarbazine) at SNUH from 2013 to 2015. The patients’ diagnoses were confirmed according to the current World Health Organization classification.17 Patients treated with steroid therapy 4 or more days prior to the obtaining of tumor tissues were excluded. Clinical data were retrieved by hemato-oncologists. This study followed the recommendations of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of SNUH (No. 1609–129-795).
Immunohistochemistry (IHC)
Immunohistochemical staining was performed using whole formalin-fixed paraffin-embedded tissue sections and antibodies against PD-L1 (clone E1L3N, Cell Signaling Technology, Danvers, MA, USA), CD3 (clone 2GV6, Ventana Medical Systems, Tucson, AZ, USA), CD8 (clone SP16, Thermo Fischer Scientific, Rockford, IL, USA), PD-1 (clone MRQ-22), MYC (clone EP121) (Cell Marque, Rocklin, CA, USA), BCL2 (clone 124, DAKO, Carpinteria, CA, USA), CD10 (clone 56C6), BCL6 (clone LN22), and MUM1 (clone Ma695) (Novocastra, Newcastle, UK). Staining was performed using a Ventana Benchmark XT (Ventana Medical Systems) or a Bond-Max autostainer (Leica Microsystems, Melbourne, Australia).
Interpretation of PD-L1 IHC and quantification of CD8+ and PD-1+ TILs
PD-L1 expression was evaluated based on the intensity of membrane staining and the proportion (or level) of immunostained cells. PD-L1 expression on tumor cells was designated tPD-L1, and PD-L1 expression on both tumor and non-tumor cells (primarily macrophages) was designated tmPD-L1. PD-L1 staining of any intensity of ≥30% of total tumor cells was defined as tPD-L1-positive, and PD-L1 staining of any intensity of ≥30% of total cellularity including tumor cells and non-tumoral cells in the microenvironment was considered as tmPD-L1-positive.
CD8+ and PD-1+ TILs were enumerated as detailed in the Supplementary Methods, and the numbers of TILs per unit area (mm2) were calculated. For dichotomization of cases, the cutoff values with the greatest discriminatory power were determined by receiver operating characteristic curve analysis and log-rank test. Briefly, in the experimental group, CD8+ and PD-1+ TILs were manually counted in all tumor areas of biopsied samples and in representative tumor areas of resected samples. Cutoff values of 542.6/mm2 for CD8+ and 18.8/mm2 for PD-1+ TILs were used to classify the cases into the CD8+ and PD-1+ TIL-low and -high groups, respectively. In the validation group, CD8+ and PD-1+ TILs in all tumor areas were enumerated using ImageScope software (Aperio Technologies, Alta Vista, CA, USA). Cutoff values of 760/mm2 for CD8+ and 174.4/mm2 for PD-1+ TILs were adopted.
Assessment of immunoscore
Immunoscore was evaluated in 64 cases of the experimental group. Because there was no solid guideline for scoring the immunoscore of lymphoma, we made some modifications from previous studies of colon cancer.23–25 Mostly, lymphoma tissue is taken by biopsy, so evaluating TILs from the invasive margin is frequently limited. Therefore, we only evaluated the infiltration of CD3+ and CD8+ TILs from tumor center (cells per mm2) and dichotomized the cases into high and low TIL infiltration according to an optimal cutoff value determined using the minimum P value approach and median. Cases are stratified according to a score range from I0 to I2, depending on the sum of high TIL infiltration observed.
Sanger sequencing
The mutation status of MYD88 and CD79B was examined by Sanger sequencing (detailed in Supplementary Methods).
Open database validation
For external validation, three publicly available datasets of RNA expressions of PCNSL were used (detailed in Supplementary Methods).
Statistical analyses
All statistical analyses were conducted using SPSS software (v. 21; IBM Corp., Armonk, NY, USA) and MedCalc software (v. 18.5; MedCalc Software, Ostend, Belgium). Graphs were created using Prism software (v. 5; GraphPad Software, La Jolla, CA, USA). Correlation analysis or comparison between PD-L1 expression, TILs, and clinicopathological parameters were performed using the Mann–Whitney U test, Spearman correlation analysis, and Kruskal–Wallis test. Multiple test correction was done by Benjamini and Hochberg method. The cutoff values of PD-L1, CD8+ TILs, PD-1+ TILs, MYC, and BCL2 were determined by receiver operating characteristic (ROC) curve analysis and log-rank test. Overall survival (OS) was measured from the date of diagnosis to the date of death or the last follow-up. Progression-free survival (PFS) was calculated from the date of chemotherapy initiation to the date of disease progression, death, or the last follow-up. PFS and OS were analyzed using the Kaplan-Meier method and the log-rank test. Univariate and multivariate analyses were performed by Cox regression. Two-sided P-values <0.05 were considered indicative of statistical significance.
Funding Statement
This work was supported by the Ministry of Education, Science and Technology [NRF-2016R1D1A1B01015964].
Acknowledgments
This work was supported by the Basic Science Research Program (grant No.: NRF-2016R1D1A1B01015964) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (MEST), Republic of Korea.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Grommes C, DeAngelis LM.. Primary CNS Lymphoma. J Clin Oncol. 2017;35:2410–2418. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosee PL, Schorb E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri AJ, Blay JY, Reni M, Pasini F, Spina M, Ambrosetti A, Calderoni A, Rossi A, Vavassori V, Conconi A, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 4.Bessell EM, Graus F, Lopez-Guillermo A, Lewis SA, Villa S, Verger E, Petit J.. Primary non-Hodgkin’s lymphoma of the CNS treated with CHOD/BVAM or BVAM chemotherapy before radiotherapy: long-term survival and prognostic factors. Int J Radiat Oncol Biol Phys. 2004;59:501–508. doi: 10.1016/j.ijrobp.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta M, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 6.Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol. 2012;23(Suppl 8):viii6–9. doi: 10.1093/annonc/mds256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 9.Merryman RW, Armand P, Wright KT, Rodig SJ. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017;1:2643–2654. doi: 10.1182/bloodadvances.2017012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgiou K, Chen L, Berglund M, Ren W, de Miranda NF, Lisboa S, Fangazio M, Zhu S, Hou Y, Wu K, et al. Genetic basis of PD-L1 overexpression in diffuse large B-cell lymphomas. Blood. 2016;127:3026–3034. doi: 10.1182/blood-2015-12-686550. [DOI] [PubMed] [Google Scholar]
- 12.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 14.Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, Sugita Y, Yufu Y, Choi I, Abe Y, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon D, Kim S, Kim PJ, Go H, Nam SJ, Paik JH, Kim YA, Kim TM, Heo DS, Kim CW, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2016;68:1079–1089. doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 16.Kwiecinska A, Tsesmetzis N, Ghaderi M, Kis L, Saft L, Rassidakis GZ. CD274 (PD-L1)/PDCD1 (PD-1) expression in de novo and transformed diffuse large B-cell lymphoma. Br J Haematol. 2018;180:744–748. doi: 10.1111/bjh.14432. [DOI] [PubMed] [Google Scholar]
- 17.Kluin PM, Deckert M, Ferry JA. Primary diffuse large B-cell lymphoma of the CNS In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H,Thiele J, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2017. 300–302. [Google Scholar]
- 18.Chapuy B, Roemer MG, Stewart C, Tan Y, Abo RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127:869–881. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berghoff AS, Ricken G, Widhalm G, Rajky O, Hainfellner JA, Birner P, Raderer M, Preusser M. PD1 (CD279) and PD-L1 (CD274, B7H1) expression in primary central nervous system lymphomas (PCNSL). Clin Neuropathol. 2014;33:42–49. [DOI] [PubMed] [Google Scholar]
- 20.Four M, Cacheux V, Tempier A, Platero D, Fabbro M, Marin G, Leventoux N, Rigau V, Costes-Martineau V, Szablewski V. PD1 and PDL1 expression in primary central nervous system diffuse large B-cell lymphoma are frequent and expression of PD1 predicts poor survival. Hematol Oncol. 2017;35:487–496. doi: 10.1002/hon.2375. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Kim SH, Kim SJ, Chang JH, Yang WI, Suh CO, Kim YR, Jang JE, Cheong JW, Min YH, et al. Programmed cell death 1 expression is associated with inferior survival in patients with primary central nervous system lymphoma. Oncotarget. 2017;8:87317–87328. doi: 10.18632/oncotarget.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayano A, Komohara Y, Takashima Y, Takeya H, Homma J, Fukai J, Iwadate Y, Kajiwara K, Ishizawa S, Hondoh H, et al. Programmed cell death ligand 1 expression in primary central nervous system lymphomas: a clinicopathological study. Anticancer Res. 2017;37:5655–5666. doi: 10.21873/anticanres.12001. [DOI] [PubMed] [Google Scholar]
- 23.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 24.Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi: 10.1158/1078-0432.CCR-13-2830. [DOI] [PubMed] [Google Scholar]
- 25.Yomoda T, Sudo T, Kawahara A, Shigaki T, Shimomura S, Tajiri K, Nagasu S, Fujita F, Kinugasa T, Akagi Y. The immunoscore is a superior prognostic tool in stages II and III colorectal cancer and is significantly correlated with programmed death-ligand 1 (PD-L1) expression on tumor-infiltrating mononuclear cells. Ann Surg Oncol. 2019;26:415–424. doi: 10.1245/s10434-018-07110-z. [DOI] [PubMed] [Google Scholar]
- 26.Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–2139. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Nam SJ, Kwon D, Kim H, Lee E, Kim TM, Heo DS, Park SH, Kim CW, Jeon YK. MYC and BCL2 overexpression is associated with a higher class of Memorial Sloan-Kettering Cancer Center prognostic model and poor clinical outcome in primary diffuse large B-cell lymphoma of the central nervous system. BMC Cancer. 2016;16:363. doi: 10.1186/s12885-016-2397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laurent C, Charmpi K, Gravelle P, Tosolini M, Franchet C, Ysebaert L, Brousset P, Bidaut A, Ycart B, Fournie JJ. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4:e1026530. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muenst S, Hoeller S, Willi N, Dirnhofera S, Tzankov A. Diagnostic and prognostic utility of PD-1 in B cell lymphomas. Dis Markers. 2010;29:47–53. doi: 10.3233/DMA-2010-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xerri L, Chetaille B, Serriari N, Attias C, Guillaume Y, Arnoulet C, Olive D. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39:1050–1058. doi: 10.1016/j.humpath.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Wartewig T, Kurgyis Z, Keppler S, Pechloff K, Hameister E, Öllinger R, Maresch R, Buch T, Steiger K, Winter C, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552:121–125. doi: 10.1038/nature24649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, Chetaille B, Olive D. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 33.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau J, Cheung J, Navarro A, Lianoglou S, Haley B, Totpal K, Sanders L, Koeppen H, Caplazi P, McBride J, et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat Commun. 2017;8:14572. doi: 10.1038/ncomms14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, Freeman GJ, Sharpe AH. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossille D, Azzaoui I, Feldman AL, Maurer MJ, Labouré G, Parrens M, Pangault C, Habermann TM, Ansell SM, Link BK, et al. Soluble programmed death-ligand 1 as a prognostic biomarker for overall survival in patients with diffuse large B-cell lymphoma: a replication study and combined analysis of 508 patients. Leukemia. 2017;31:988–991. doi: 10.1038/leu.2016.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang C, Lin CH, Cheng AL, Medeiros LJ, Chang KC. Primary central nervous system diffuse large B-cell lymphoma has poorer immune cell infiltration and prognosis than its peripheral counterpart. Histopathology. 2015;67:625–635. doi: 10.1111/his.12706. [DOI] [PubMed] [Google Scholar]
- 39.Nam SJ, Kim S, Kwon D, Kim H, Kim S, Lee E, Kim TM, Heo DS, Park SH, Lim MS, et al. Prognostic implications of tumor-infiltrating macrophages, M2 macrophages, regulatory T-cells, and indoleamine 2,3-dioxygenase-positive cells in primary diffuse large B-cell lymphoma of the central nervous system. Oncoimmunology. 2018;7:e1442164. doi: 10.1080/2162402X.2018.1490854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MGM, Chapuy B, Armand P, Rodig SJ, Shipp MA. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood. 2017;129:3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


