ABSTRACT
Given the growing interest and promising preliminary results of immunotherapy in malignant pleural mesothelioma (MPM), it has become important to more fully understand the immune landscape in this tumor. This may be especially relevant in deciding who might benefit most from checkpoint blockade or agonist antibody therapy.
Since the phenotype of tumor infiltrating lymphocytes (TILs) in MPM has not been fully described and their function has not been carefully assessed, we collected fresh tumor and blood from 22 patients undergoing surgical resection and analysed single cell suspensions by flow cytometry. The functionality of TILs was assessed by measurement of cytokine expression (IFN-γ) following overnight stimulation ex vivo.
Results showed low numbers of CD8+ TILs whose function was either moderately or severely suppressed. The degree of TIL hypofunction did not correlate with the presence of co-existing macrophages or neutrophils, nor with expression of the inhibitory receptors PD-1, CD39 and CTLA-4. Hypofunction was associated with higher numbers of CD4 regulatory T cells (Tregs) and with expression of the inhibitory receptor TIGIT. On the other hand, presence of tissue-resident memory (Trm) cells and expression of TIM-3 on CD8+ cells were positively associated with cytokine production. However, Trm function was partially suppressed when the transcription factor Eomesodermin (Eomes) was co-expressed.
Understanding the function of TILs in malignant mesothelioma may have clinical implications for immunotherapy, especially in choosing the best immunotherapy targets. Our data suggests that Treg cell blocking agents or TIGIT inhibitor antibodies might be especially valuable in these patients.
KEYWORDS: Mesothelioma, inhibitory receptors, tissue resident memory cells, Eomes, CD8+ TILs, hypofunction
Introduction
Malignant pleural mesothelioma (MPM) is an incurable cancer of the mesothelial membranes, causally associated with asbestos exposure in the majority of patients. MPM incidence has been increasing in Western Europe and in Asia.1,2 Current trimodality options of surgery, chemotherapy and radiotherapy have failed to increase median overall survival beyond 18 months.3,4 Given the poor prognosis and current limited treatment options, novel therapeutic strategies are urgently needed.
There is increasing evidence supporting a role for immunotherapy in MPM. “In situ” vaccination with an adenovirus expressing type 1 interferon-α and dendritic cell vaccination trials have shown promise.5,6 Most recently, antibodies targeting immune checkpoints have been studied in MPM.7 Although the anti-CTLA-4 antibody tremelimumab was not effective in a randomized trial,8 it appears that responses to the anti-PD-1 antibody, pembrolizumab, can be seen in ~20–30% of MPM.9
Given this encouraging data, it is becoming increasingly important to fully understand the mesothelioma immune microenvironment (TME). The purpose of this study was to thus comprehensively characterize the phenotype and function of tumor-infiltrating lymphocytes (TILs) in MPM patients, with the hope that this information will be useful in identifying possible therapeutic approaches and predicting what patients might benefit most from current or newly developed immunotherapeutic agents.
Results
Patient and sample characteristics
We studied the immune landscape and T cell function in blood and tumor specimens of advanced stage MPM patients undergoing pleurectomy (n = 22). Transplant donors’ lung tissue (TFL) and blood were used as controls (n = 12). Selected patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| MPM Patients (n = 22) |
Tumor-Free Lung Donors (n = 21) |
|||
|---|---|---|---|---|
| Number | % | Number | % | |
| Median age (range) | 71 (57–83) | – | 63 (28–72) | |
| Gender | ||||
| Male | 19 | 86 | 15 | 71 |
| Female | 3 | 14 | 6 | 29 |
| Race | ||||
| Caucasian | 22 | 100 | 19 | 90 |
| Black | 2 | 10 | ||
| Smoking | ||||
| Former | 13 | 59 | 11 | 53 |
| Never | 9 | 41 | 10 | 47 |
| Histology | ||||
| Epithelioid | 19 | 86 | ||
| Biphasic | 3 | 14 | ||
| Disease Stage | ||||
| I | 1 | 5 | ||
| II | 0 | 0 | ||
| III | 15 | 68 | ||
| IV | 6 | 27 | ||
| Cause of Death | ||||
| Stroke/CVA | 12 | 57 | ||
| Anoxia | 7 | 33 | ||
| Head Trauma | 2 | 10 | ||
Immune cell content of mesothelioma
Because the phenotype of blood lymphocytes (Suppl. Fig. 1A) is so different than tissue lymphocytes, we primarily compared MPM TILs to T cells from TFLs (TFLLs). An overview of the cell content of TFL and MPM digests revealed the heterogeneous presence of a number of immune cell types (Figure 1(a)), with CD45+ leukocytes comprising approximately 60% of the MPM and around 78% of the TFL digests.
Figure 1.
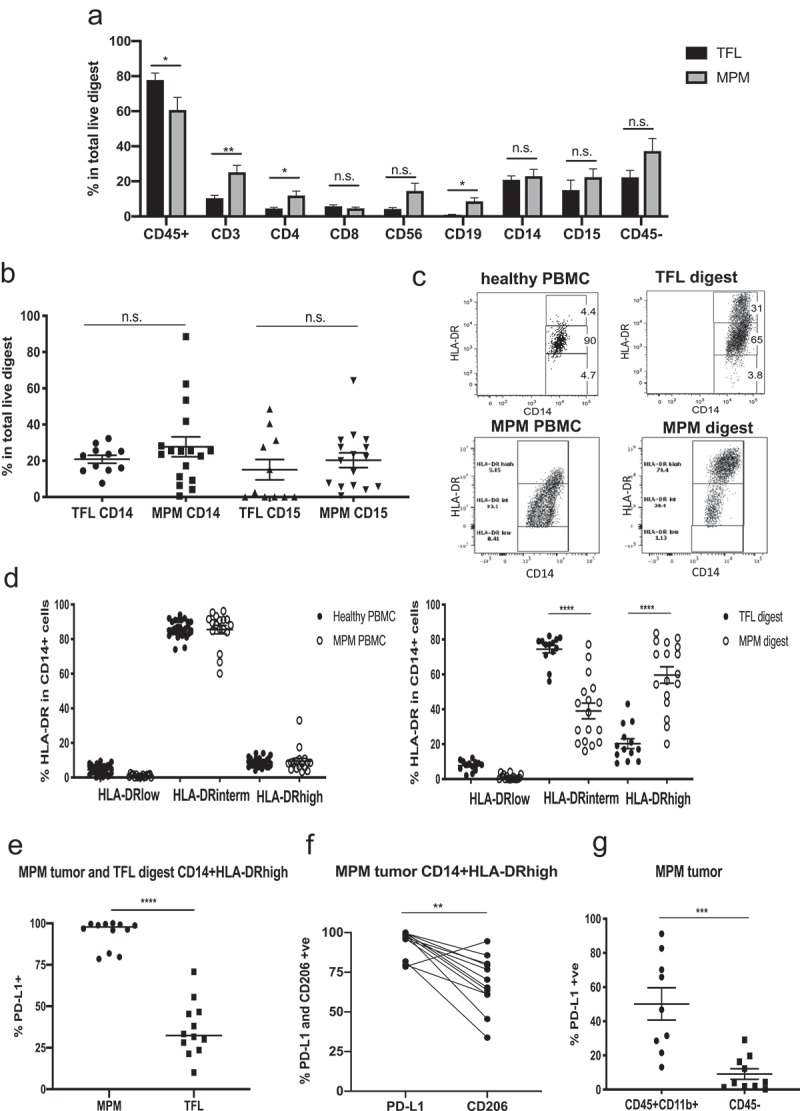
Immune cell frequencies in tumor-free lung (TFL) and malignant pleural mesothelioma (MPM) digests. Flow cytometry was used to characterize cells in the PBMCs of MPM patients or the digests of MPM tumors or tumor-free lungs (TFL). (a) Frequency of main immune cell subsets in total live TFLs versus MPM tumor digests demonstrating significantly less CD45+ immune cell infiltration in the tumors, but more T (CD3+, CD4+, CD56+) and B (CD19+) cell subsets. Statistics by multiple t tests (*p < .05, **p < .01). (b) Frequency of CD14+ and CD15+ cells in TFL and MPM digests. There were no significant differences. (c) Representative flow tracings showing the distribution of HLA-DR levels between healthy PBMC, TLF digest, MPM PBMCs and MPM digest on CD14-gated cells. CD14 cells with intermediate levels of HLA-DR are classified as monocytes, while those with high levels of HLA-DR are classified as macrophages. (d) Frequency of HLA-DR expression in CD14-gated cells in healthy and MPM PBMCs (left graph) showing no differences, and between TFL and MPM digests (right graph) showing HLA-DRhi cells are more frequent in the MPM digests. Statistics by Mann-Whitney t-test (****p < .0001). (e) The level of PDL-1 expression on the CD14+ HLA-DRhigh cells is plotted for MPM and TFL digests. Expression of PDL1 is significantly higher on the MPM CD14+ cells. Statistics by Mann-Whitney t-test (****p < .0001). (f) The expression of PDL1 and CD206 in CD45+ CD11b+CD14+ HLA-DRhigh for each patient is plotted. Whereas PDL1 expression is uniformly high, CD206 expression is variable and significantly lower than PDL1 expression. Statistics by Mann-Whitney t-test (**p < .01). (g) PD-L1 expression in all myeloid cells (CD45+ CD11b+ gated cells) versus the CD45 -ve gated cells in MPM digests. Expression on the myeloid cells is significantly higher. Statistics by Mann-Whitney t-test (***p < .001).
Myeloid cells
In MPM tumors, the most prevalent innate immune cells were myeloid cells, with a mixture of CD14+ and CD15+ (27.7% and 20.3% of live tumor cells respectively) (Figure 1(b)), similar to TFLs (20.8% CD14+ and 15% CD15+ cells respectively). We further phenotyped the CD14+ cells as ‘canonical monocytes’ or as macrophages based on the expression level of the marker HLA-DR (Figure 1(c)).10 In both healthy and MPM PBMCs, the number of CD14+/HLA-DRlow cells (myeloid-derived suppressor cells) was less than 5%. The majority of the CD14+ cells (~85%) were HLA-DRintermediate in both cohorts, defining their status as canonical monocytes (Figure 1(d), left panel). In tissues, HLA-DRintermediate monocytes represented an average of 73.9% of CD14+ cells in TFLs, but only 40.7% in MPM tumors (p < .0001). In both TFL and MPM digests, the remainder of the CD14+ cells were macrophages marked by high expression of HLA-DR (HLA-DRhigh). In TFLs, HLA-DRhigh cells represented 21.1% of the CD14+ cells, however, macrophages were present in much higher fraction in MPM tumors where they comprised 57.6% of all CD14+ cells (p < .0001) (Figure 1(d), right panel). All of the HLA-DRhigh CD14+ cells from MPM digests expressed high (>75% of cells) levels of PDL1, in contrast to the much lower level of expression on TFL macrophages (p < .0001, Figure1(e)). Unlike PDL1 expression, the level of the macrophage marker CD206 on MPM macrophages (thought by some to signal a more suppressive M2 phenotype) was more variable (Figure 1(f)). PD-L1 expression on all tumor myeloid cells (CD45+ CD11b+) was 42.2% versus only 9.1% on the CD45- tumor and stromal cells (p = .004) (Figure 1(g)).
Lymphocytes
We examined the frequency of all T cells (CD3+), CD8+ T cells, helper T cells (CD4+), regulatory T cells (Treg) (CD4+CD25+FOXP3+), natural killer (NK) cells (CD45+CD56+CD3-CD14-) and B cells (CD45+CD19+) (Figure 1(a)). NK cells were increased in MPMs (14.6%) versus TFLs (4.3%), however this was very heterogeneous between samples (Figure 2(a)). B cell frequency was significantly increased in MPM digests (8.5%), compared to TFL digests (1.0%) (p = .032) (Figure 2(b)). T cell infiltration in tumors was quite heterogeneous, averaging 25.2% in total live MPM digests. CD4+ T cells made up 11.9% of the live MPM digest, with most of them being differentiated towards an effector memory (CD45RO+CD62L-) phenotype (Suppl Figs. 1B and 1C). The frequency of regulatory T cells (Tregs) within the CD4+ population was significantly increased in MPMs (12.8%) compared to TFL digests (2.2%) (p = .0001), and MPM tumors had significantly more Tregs than MPM blood (p = .005) (Figure 2(c)). The Tregs expressed high levels of TIGIT (72.5% of cells), CD39 (63.5% of cells), and CTLA-4 (68.5% of cells), moderate levels of PD1 (42.8% of cells), but only low levels of TIM-3 (3.6% of cells) (Figure 2(d)).
Figure 2.
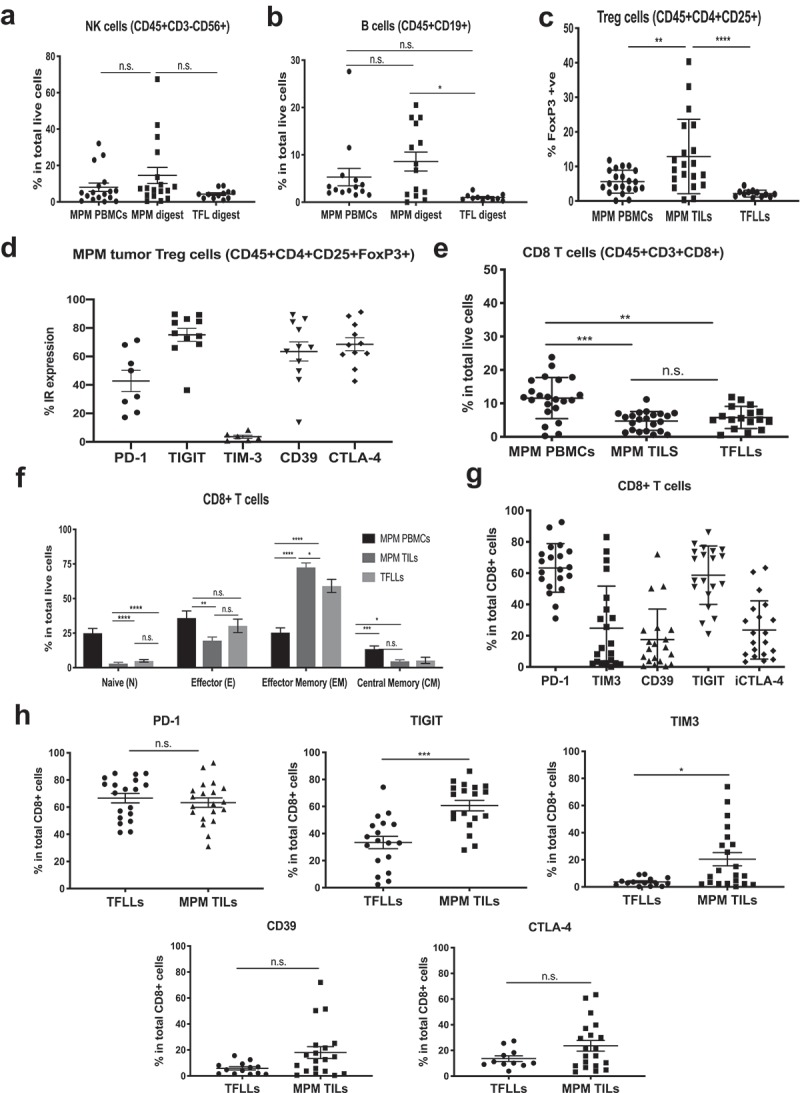
Frequencies and phenotype of lymphocytes in the MPM microenvironment. Flow cytometry was used to characterize cells in the PBMCs of MPM patients or the digests of MPM tumors or tumor-free lungs (TFL). All Statistics by Mann-Whitney test (*p < .05, **p < .01, ***p < .001, ****p < .0001; ns = not significant). (a) Frequency of NK cells was determined in PBMC and total live digests. There were no significant differences. (b) Frequency of B cells was determined in PBMC and total live digests. The percentage of B cells in MPM tumor digest was significantly higher in MPM vs TFL digests. (c) Frequency of FOXP3+ in total CD4+ cells (Tregs) in MPM PBMCs, MPM and TFL digests. MPM digests had significantly increased percentages of Tregs compared to PBMC or TFL digests. (d) Individual inhibitory receptor expression on Tregs from MPM digests was determined. High levels of TIGIT, CD39, and CTLA-4, moderate levels of PD-1 and low levels of TIM3 were observed. (e) Frequency of CD8+ T cells was determined in PBMC and total live digests. Whereas PBMC levels were significantly higher than seen in tissue digests, there were no significant differences noted between MPM and TFL digests. (f) The frequency of CD8+ T cell Naïve, Effector, Central Memory, and Effector Memory frequencies in MPM PBMCs, MPM TILS and TFLLs were determined. Naïve cells were higher in PBMC, while tumor digests had more effector and central memory cells. (g) Expression of IRs (PD-1, TIM-3, CD39, TIGIT and CTLA-4) on CD8+ MPM TILs. High levels of PD-1 and TIGIT, with moderate levels of CD39, TIM3, and CTLA-4, were observed. (h) Inhibitory receptor expression on CD8+ TILs from TFL and MPM digests was determined. There were no significant differences in expression of PD-1, CD39, or CTLA4. The levels of TIGIT and TIM3 were significantly greater on MPM TILs vs TFLLs.
Phenotype of CD8 T cells
CD8+ TILs represented 4.6% of the MPM digests, similar to 5.8% CD8+ cells in TFL digests (Figure 2(e)). The CD8 T cell differentiation status was determined by the expression of the markers CD45RO and CD62L, which delineate the four main different subsets of T cell differentiation (representative tracings, Suppl Fig. 1D). The majority of the T cells in MPM PBMCs were naïve (CD45RO-CD62L+) and effector cells (CD45RO-CD62L-). In contrast, most TFLLs and MPM TILs were CD45RO+, and most were differentiated towards the effector memory phenotype (CD45RO+/CD62L-). The frequency of effector memory CD8+ TILs (72.6%) was higher in MPM tumors than in TFL digests (59.1%) (p = .049) (Figure 2(f)).
Analysis of inhibitory receptors (IRs) showed variable expression levels among different MPM tumor samples, with PD1 and TIGIT being the most prominent (Figure 2(g)). PD-1 expression on CD8+ MPM TILs was high (63.3%), but similar to that seen on TFLLs (66.6%) (Figure 2(h), top left panel). TIGIT was highly expressed on MPM TILs (58.7%), and was significantly lower on TFLLs (33.4%) (p = .0002) (Figure 2(h), top middle panel). TIM-3 expression pattern was highly variable, but significantly higher on MPM TILs (24.4%) compared to TFLLs (3.7%) (p = .023) (Figure 2(h), top right panel). CD39 expression was higher in MPM TILs (17.5%) than in TFLLs (5.9%), however the difference did not quite reach significance (p = .065) (Figure 2(h), bottom left panel). No significant differences in the expression of CTLA-4 were noted between TFLLs (17.0%) and MPM TILs (23.6%) (Figure 2(h), bottom right panel).
Function of CD8 TILs
To test CD8+ T cell baseline function, MPM digests, digests of TFLs, and MPM PBMCs were incubated overnight in the presence of monensin (Golgistop) and brefeldin (Golgiplug). Intracellular cytokine staining at that time showed only minimal spontaneous IFN-γ production (representative tracings from one MPM patient in Figure 3(a), left column panels). We thus utilized an assay to stimulate cytokine secretion by sub-maximal TCR stimulation (induced by plate-bound anti-CD3 antibody) in a non-antigen specific manner.11 Using this assay, we observed that an average of 33.4% of the CD8+ TFLLs produced IFN-γ (Figure 3(b)). Notably, the MPM TILs were significantly less functional (p < .0001), with a range of 0 to 25% and an average of only 11.4% of CD8+ T cells staining positively for IFN-γ (Figure 3(b); representative tracings from one MPM patient in Figure 3(a), middle column panels). The % of cells secreting IFN-γ and TNFα in the TILs were highly concordant (R2 = 0.777; p < .0001), demonstrating polyfunctionality (Figure 3(c)). We therefore used IFN-γ as our primary cytokine readout. Stimulation of all samples using PMA/Ionomycin as a positive control was performed and resulted in uniform and similar IFN-γ production averaging ~80%, in both MPM TILs and TFLLs, and ~75% in MPM PBMCs (Figure 3(d); representative tracings from one MPM patient are shown in Figure 3(a), right column panels).
Figure 3.
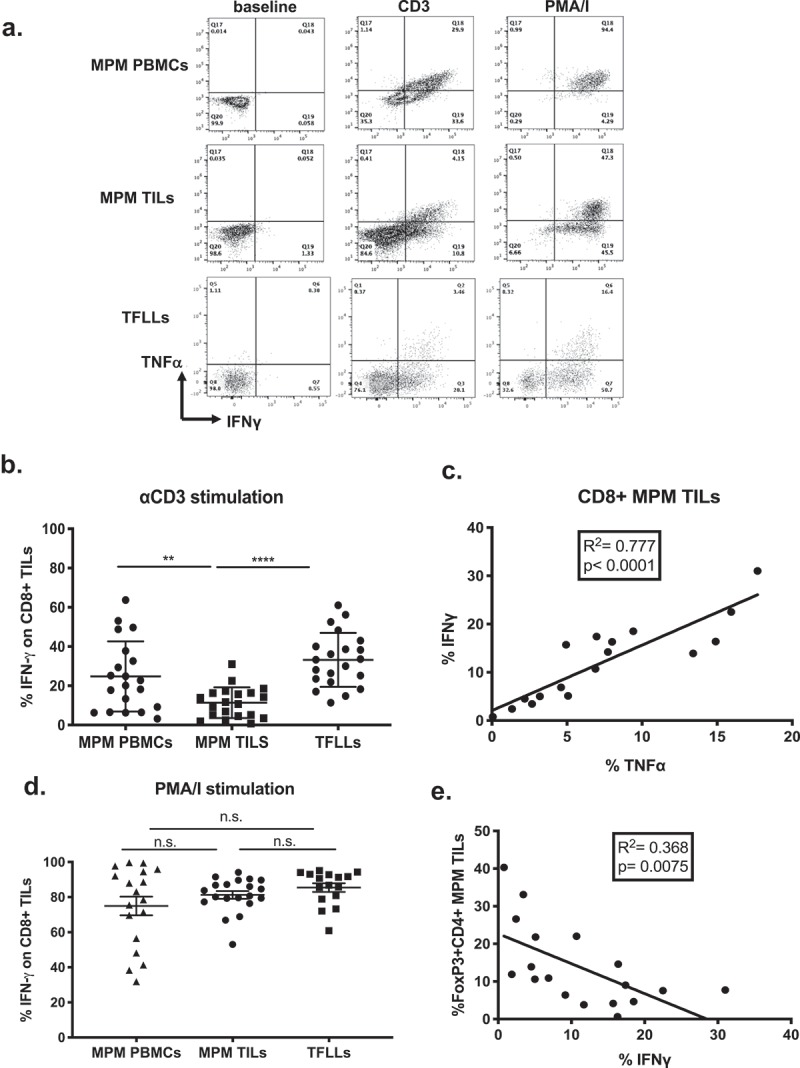
Function of CD8+ T cells is suppressed in MPM TILs. T cells were stimulated with plate-bound anti-CD3 antibody or PMA/Ionomycin overnight and the levels of intracellular IFN-γ or TNF-α expression were measured using flow cytometry. (a) Representative flow tracings of CD8+ T cells of MPM PBMCs, MPM TILs and TFLLs showing cytokine (IFN-γ on x-axis and TNF-α on y-axis) production at baseline, or following overnight stimulation with anti-CD3 or PMA/I. MPM PBMCs and MPM TILs are from the same patient. (b) Percent of CD8 + T cells of MPM PBMCs, MPM TILs and TFLLs producing IFN-γ following overnight stimulation with plate-bound anti-CD3 antibody. MPM TILs made significantly less IFN-γ. Statistics by one-way ANOVA (**p < .01). (c) Intracellular IFN-γ and TNFα production in each patient’s MPM TILs was highly and significantly correlated (R2 = 0.777; p < .0001). (d) The percent of CD8+ T cells producing IFN-γ in MPM TILs and TFLLs following overnight stimulation with PMA/I was high in all groups with no statistically significant differences. Statistics by one-way ANOVA. (e) The percent of Tregs (CD4+ FOXP3+) of total CD4+ cells was significantly negatively correlated with IFN-γ production by CD8+ MPM TILs (R2 = 0.368; p = .0075).
Associations with CD8± TIL hypofunction
We next performed a series of correlation analyses for possible associations with CD8+ production of IFN-γ. No significant correlations were seen between the frequency and proliferation of CD8+ T cells and their function (Suppl Figs. 2A and 2B), nor between IFN-γ production and frequency of CD14+ or CD15+ myeloid cell types (Suppl Fig. 2C and 2D). However, the frequency of CD4+ Tregs was significantly negatively correlated with CD8+ TIL function (R2 = 0.368; p = .0075) (Figure 3(e)).
The current paradigm suggests that T cell exhaustion correlates with expression levels of IRs. We therefore plotted the percentage of CD8+ T cells expressing IFN-γ against the expression levels of specific IR’s. No significant associations with IR frequency and percent of patient’s TILs making IFN-γ were noted, however, there appeared to be trends towards increasing hypofunction and the percentage of TILs expressing PD1, TIGIT, CD39, and CTLA-4 (Suppl. Figs. 3A, 3B, 3C and 3D respectively). The opposite trend (higher IR levels in more functional patients) was seen for TIM-3 (Suppl. Fig. 3E).
To determine how the presence of a specific IR affected the ability of a given T cell to produce cytokine, flow cytometric measurements of intracellular IFN-γ production by CD8+ TILs expressing specific IRs were plotted for each patient (representative tracings are shown in Figure 4(a)). The percentage of IFN-γ produced by CD8+ TILs is plotted on the x-axis versus the expression of a specific IR on the y-axis. The boxed numbers represent the frequency of IR positive cells that produced IFN-γ (top row panels), and the frequency of IR negative cells that produced IFN-γ (bottom row panels). Figure 4(b–e) compares for each patient the percentage of cells making IFN-γ that either express or do not express a specific IR. We found that for each patient, the expression of PD1 (Figure 4(b)) or CD39 (Figure 4(c)) did not significantly affect IFN- γ production at the single cell level. However, for a given patient, cells expressing TIGIT made significantly less IFN-γ (average 8.3%) than those not expressing TIGIT (average 15%) (p = .014) (Figure 4(d)). In contrast, a significantly higher percentage of TIM-3+ CD8 TILs made IFN-γ (19.8%) compared to TIM-3- CD8+ TILs (13.3%) (p = .023) (Figure 4(e)). We saw no differences in the amount of IFN-γ produced by CD8+ effector (CD45RO-CD62L-) cells vs. CD8+ effector memory (CD45RO+CD62L-) cells (data not shown).
Figure 4.
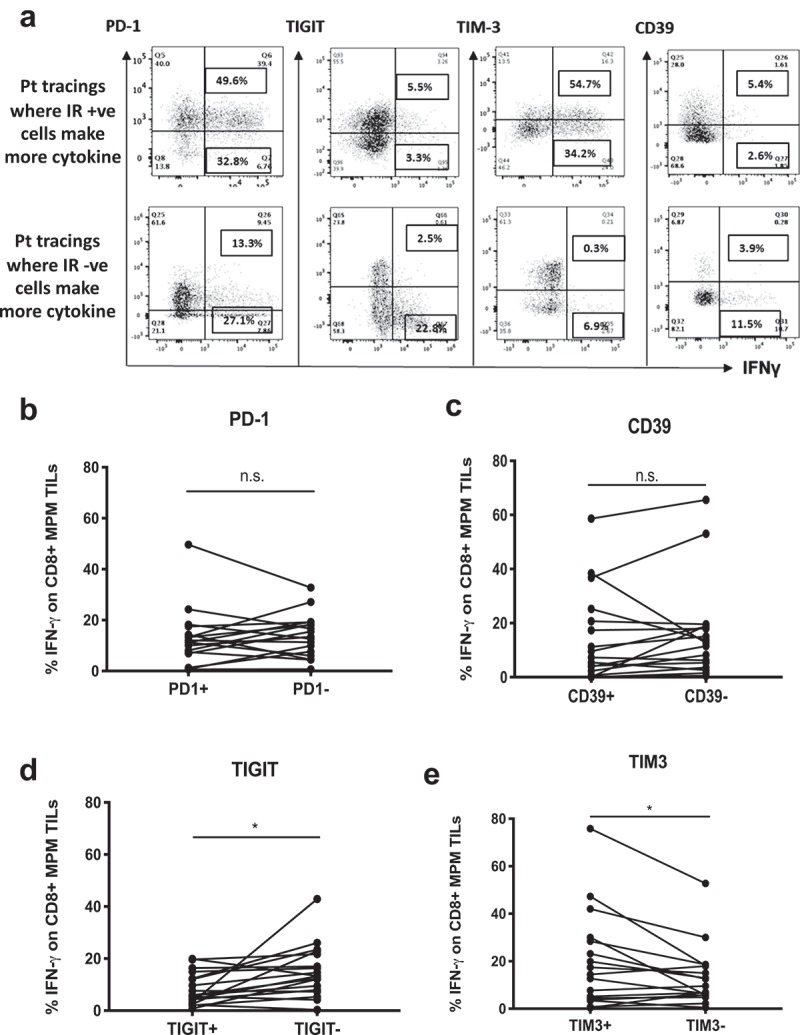
Function of CD8+ T cells in relation to IR expression. TILs from individual patients were stimulated with anti-CD3 antibody overnight and flow cytometry performed with surface and intracellular staining to determine the IR expression levels on the cells making IFN-γ. (a) Representative flow tracings where the IFN-γ expression is plotted on the X axis vs the level of IR expression (PD1, TIGIT, TIM-3 or CD39) on the Y axes. The top row panels show examples where T cells of a given patient with high levels of IRs made more IFN-γ than the T cells of the same patient which lacked IR expression. The bottom row panels show examples where T cells lacking or downregulating IRs produced more cytokine than the T cells of the same patient that expressed IRs. (b) The % of cells making IFN-γ in PD-1+ vs. PD-1- CD8 MPM TILs for each individual patient were compared. No significant differences were observed (Mann-Whitney test, p = ns). (c) The percent of cells making IFN-γ in CD39+ vs. CD39- CD8 MPM TILs for each individual patient were compared. No significant differences were observed (Mann-Whitney test, p = ns). (d) The percent of cells making IFN-γ in TIGIT+ vs. TIGIT- CD8 MPM TILs for each individual patient were compared. A significant difference was observed with TIGIT+ cells making less IFN-γ (Mann-Whitney test, *p = .014). (e) The percent of cells making IFN-γ in TIM-3+ vs. TIM-3- CD8 MPM TILs for each individual patient (n = x) are compared. A significant difference was observed with TIM+ cells making more IFN-γ (Mann-Whitney test, *p = .023).
Tissue-resident memory T cells are the main producers of IFN-γ in MPM TILs
Since lung tissue-resident memory (Trm) CD8+ T cells, identified by co-expression of markers CD45RO, CD103, and CD69, have recently been associated with increased T cell function and improved prognosis in lung cancer,12,13 we evaluated their presence in MPM. As expected, we observed a very low frequency of Trms in MPM PBMCs (3%) and a significantly higher frequency in TFLs (36.8%, p < .0001). The MPM TILs had a highly variable (and somewhat biphasic) expression of CD103 with an average that was similar to that seen in TFLs (35.4%) (representative flow tracings in Figure 5(a); Figure 5(b), left panel). High co-expression of CD69 in CD45RO+CD103+CD8+ MPM TILs and TFLLs confirmed their identity as resident memory T cells (Figure 5(b), right panel).
Figure 5.
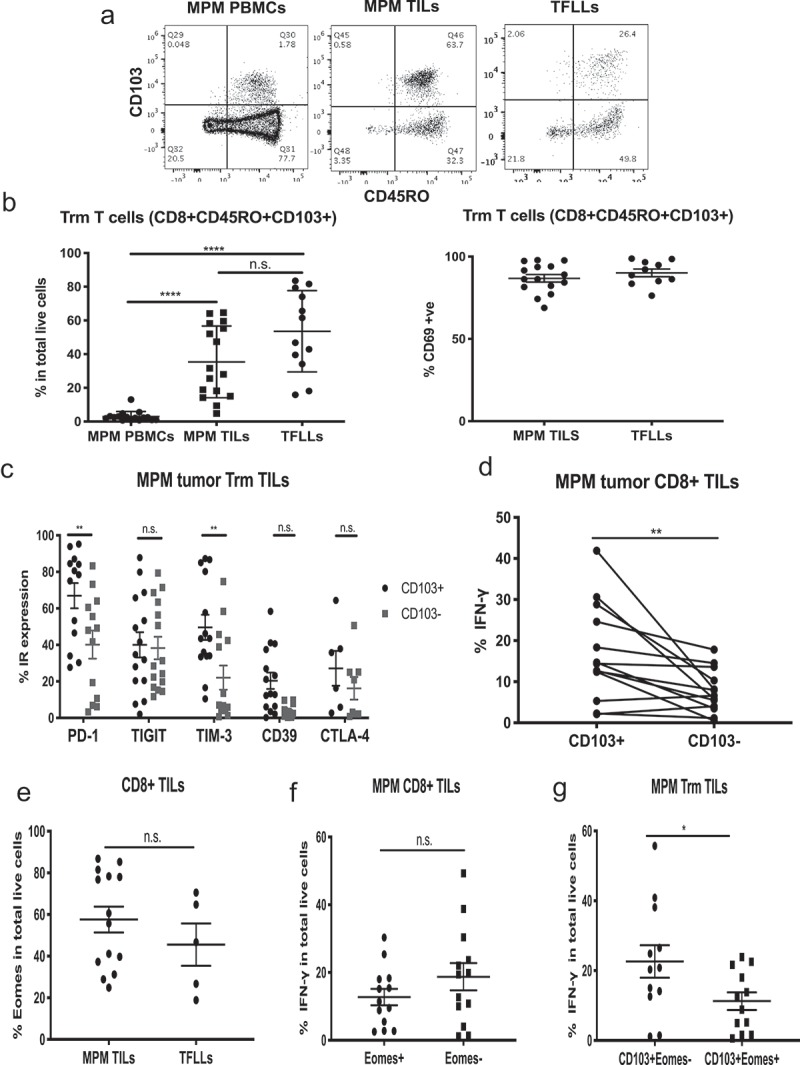
Phenotype and function of tissue-resident memory (Trm) cells in MPM. Trms were defined as CD8+ CD45RO+CD103+ cells. (a) Representative flow tracings of tissue resident memory cells (Trms) of MPM PBMCs, MPM TILs and TFLLs. (b) Left graph: Frequency of Trms in MPM TILs and TFLLs are significantly higher than in PBMCs. Statistics by Mann-Whitney test (****p < .0001). Right graph: TFLL Trms and MPM TIL Trms are characterized by high expression of CD69. (c) IR expression in Trms. Levels of PD1 and TIM3 are statistically higher in the CD103+ vs. CD103- MPM TILs. Statistics by multiple t tests (**p < .01). (d) IFN-γ production was statistically significantly higher in CD103+ vs. CD103- cells in each individual MPM patient (paired parametric t test, **p = .0085). (e) Expression of Eomes in CD8+ MPM TILs and TFLLs shows no significant differences. (Mann-Whitney test, p = n.s.). (f) The IFN-γ production in Eomes+ vs. Eomes- CD8+ MPM TILs was not significantly different. (Mann-Whitney test, p = n.s.). (g) IFN-γ production in CD103+ vs. CD103- cell in relation to Eomes expression was significantly different (Mann-Whitney test, *p = .04).
Consistent with previous reports,14 CD103+ Trm cells showed higher expression of several IRs compared to the CD103- T cells (Figure 5(c)). Specifically, in MPM CD8+ TILs, PD1 and TIM-3 expression levels were significantly higher in the CD103+ cells. In TFLs, PD-1 was shown to be significantly higher in the CD103+ subset, but TIM-3 showed no differences between the CD103+ and CD103- subpopulations and its expression averaged less than 20%. In contrast to MPM Trms, the CD103+ Trm cells of TFLs had significantly lower expression of TIGIT (Suppl. Fig. 4).
Trm cells are reported to retain high levels of effector activity, despite expression of one or more IRs. We therefore assessed the association between expression of CD103 and IFN-γ production at the single cell level in a subgroup of our MPM cohort. We found that, for each patient, the CD103+CD8+ MPM TILs produced significantly more IFN-γ in comparison to the CD103-CD8+ TILs (p = .0085) (Figure 5(d)). Thus, CD103+ TIL expression was a predictor of functional T cells.
Eomes expression in MPM TILs associates with decreased function
In our recent study of early stage human lung cancer TILs, we noted that the expression of Eomes was associated with decreased TIL cytokine function.15 We therefore assessed Eomes expression in MPM TILs and the association between expression of Eomes and IFN-γ production at the single cell level in a subgroup of MPM patients. Eomes was found to be expressed heterogeneously in MPM samples in a somewhat biphasic fashion, with some showing expression levels of around 20% and others showing high expression of 80–90% (Figure 5(e)). We noted that production of IFN-γ by CD8+Eomes+ TILs was lower (12.7%) than by CD8+Eomes- TILs (18.7%), but the difference was not significant (p = .31) (Figure 5(f)). However, when we compared the effect of Eomes expression on IFN-γ production levels in the CD103+ Trm cells (Figure 5(g)), we observed that a significantly higher percent of cells made IFN-γ in the absence of Eomes (22.6%), compared to CD103+/Eomes+ cells (11.2%) (p = .04).
Discussion
Most previous descriptions of the immune environment in MPMs have relied on immunohistochemical (IHC) studies and showed variable T cell and high macrophage and Treg infiltration.16–18 A comprehensive analysis of the MPM immune phenotype using flow cytometry of digested tumor biopsies was recently reported by Awad et al.19 Frequencies of T cells in their 38-patient cohort were highly variable, but showed a similar differentiation status and cellular composition as we observed, including a relatively high percentage of CD4+ T cells that expressed FOXP3 (~20%). In their patient cohort, the percentage of CD45+ cells that were CD4+ and CD8+ cells was ~10–20%, whereas we uniformly observed lower numbers of TILs within MPM tumors (the maximal % being less than 15%). This may be linked to technical factors (i.e. different digestion techniques, whether frozen samples were used, etc.), sampling issues, or having somewhat different patient populations.
Expression of inhibitory receptors (IRs) on TILs is generally thought to indicate T cell exhaustion.20,21 Our IR expression findings are similar to the report by Awad et all,19 who found that the percentage of CD8+ T cells expressing PD-1 was ~50%, and TIM-3 ~ 20%. We extended these findings by noting high levels of TIGIT (~60%), CD39 (~20%), and CTLA4 (~25%). Importantly, we compared the expression levels of these IRs to those expressed on tissue lymphocytes from tumor-free lungs (TFLLs). Expression levels of TIGIT, TIM-3, and CD39 were all higher in MPM TILs; interestingly, TFLLs expressed similarly high levels of PD-1 and moderate levels of CTLA-4.
The most important and novel part of our study was to go beyond phenotype and assess the functional status of the TILs. Following submaximal T cell stimulation, we observed that MPM TILs were consistently hypofunctional. However, the TILs showed uniformly high levels of cytokine production when maximally stimulated with PMA/I, indicating that the cells were capable of responding. Since PMA/I activates the part of the TCR signaling cascade after activation of protein kinase C (PKC), our observed high-level cytokine response indicates that the profound defects we saw after TCR crosslinking were due to a defect in the T cell signaling cascade before activation of PKC.
It is instructive to compare and contrast our TIL findings in MPM with those that we recently described in early stage non-small cell lung cancer (NSCLC) using the same analytic approaches (Suppl Fig. 5).15 We observed that the number of CD8+ TILs in NSCLC was significantly higher (12%) than that observed in the MPM cohort (4.6%) (Suppl Fig. 5A). The distribution of IRs in lung cancer TILs was very similar to MPM TILs (Suppl Fig. 5B). Importantly, however, the profound hypofunction that we observed in virtually all of MPM TILs was quite different than what we noted in early stage NSCLC TILs, where only about a third of the TILs were hypofunctional (Suppl Fig. 5C). The reason for this difference may relate to the stage of tumor evolution when sampling occurred (early stage NSCLC vs late stage MPM tumors) or may be related to intrinsic differences between the tumor types. The presence of Trms may also contribute to this difference in TIL function, as Trm frequency in early stage NSCLC is significantly higher than in MPM (Suppl Fig. 5D).
It was of interest to determine if we could find cell characteristics associated with T cell hypofunction. Using correlation analyses, we noted a highly significant negative association of T cell function with the percentage of CD4+/FOXP3+ T regulatory cells. This finding is consistent with a number of preclinical studies22–24 showing that Tregs support MPM tumor growth and would support the use of Treg-depleting therapy in MPM as it becomes available.
Although the current paradigm in the field is that IRs mark cells that are exhausted and thus hypofunctional,25,26 increasing amounts of evidence suggest that IR expression is contextual and may depend as much on differentiation status as “exhaustion” in human CD8 T cells.27–29 This may be especially true for PD-1 which is known to be a marker of activated T cells30,31 and is also upregulated on highly active Trms.12,30 When we examined PD-1+ TILs compared to PD-1- TILs within the same MPM digests, we found no differences in their ability to make IFNγ after stimulation. Similarly, the ectoenzyme CD39 (which helps produce adenosine), also thought to be a marker of exhausted T cells,32 did not predict the ability of TILs within the same digest to make cytokine. However, we did observe that the presence of TIGIT on MPM TILs marked significantly more hypofunctional cells. TIGIT is an inhibitory receptor that binds to two nectin-like proteins, CD155 and CD112, expressed on some tumors.33 TIGIT has been associated with poor cytokine production and is being investigated in a number of clinical trials.34 Our data suggest that MPM might be a good target tumor for anti-TIGIT antibody therapy.
In contrast, we noted that the presence of TIM-3 identified TILs that made significantly increased amounts of IFNγ. Although TIM-3 is often considered an inhibitory receptor and has been found to be highly expressed on exhausted T cells,35 there are data to show that it can also function as an activating receptor, depending on context and co-expression of other proteins.36–38 It has been reported that in MPM patients that TIM-3 expression in lymphoid aggregates were a prognostic indicator of better outcomes.18 Our data in MPM suggest that blockade of TIM-3 might not be an optimal approach. It is of interest to note that our IR data in MPM TILs is different than what we observed in early stage NSCLC TILs where, like PD-1 and CD39, neither TIGIT nor TIM-3 predicted cytokine production.15
The other factor that was positively associated with cytokine production was the differentiation status of the TILs towards the tissue resident memory (Trm) cell phenotype. Trms are a subset of differentiated effector CD8+ T cells that permanently reside in mucosal tissues and can be characterized in the lung by expression of the integrin αeβ7 (CD103) and the surface T cell activation marker CD69.12,13 Although Trms express high levels of multiple IRs, they also have high levels of “deployment-ready” mRNA encoding effector molecules and appear to be highly cytolytic, presumably to effect rapid immune response to infectious agents. The presence of Trms was found to be a positive prognostic factor in lung cancer13 and other tumors.39–41 We were unable to find any previous reports documenting the presence and function of Trms in MPM. Our data show that approximately 40% of MPM TILs were identified as Trms. In agreement with previous reports,13,14 Trm IR expression was higher in CD103+ than in CD103− cells for all the IRs we analyzed. Despite that, the Trm TILs consistently made more IFNγ than the CD103- cells in the same tumor digest.
Our Trm findings are similar to those in our recently published early stage lung cancer study, where we saw a similar pattern of response and discovered that the expression of transcription factor Eomesodermin (Eomes) appeared to play a role in downregulating Trm TIL function.15 Eomes is a master transcription factor with context-dependent roles in the effector function of CD8 T cells.42 Its expression in TILs seems to be associated with T cell exhaustion.43 Interestingly, a number of papers have noted that Eomes is normally expressed at only very low levels in Trms.44 When we examined the relationship between the presence of Eomes and IFNγ production in MPM Trms, we found that the CD103+ TILs that did not express Eomes made significantly more IFNγ that the CD103+ cells that did express Eomes. These data support our observations in lung cancer showing an association of Eomes with hypofunction in the generally more active Trm cells within tumors.15
It is useful to consider the strengths and limitations of this study. Strengths include sample processing within a 1-hour window following acquisition and, for the first time to our knowledge, assessment of TIL function. Although most previous studies on TILs have used PBMCs for comparisons, T cells from blood are very different than TILs. Accordingly, we used T cells from digests from tumor free lungs as our controls. We also focused on actual mesothelioma tumor tissue rather than effusion samples. Although effusions are easily accessible, they are a unique compartment that does not truly mimic the desmoplastic tumor microenvironment of the actual tumor.
In terms of limitations, our MPM cohort was limited to 22 patients due to the rarity of the disease. This relatively small number of patients limits the power of our negative observations and it is likely that some of the trends we observed would become statistically significant with more subjects. The ideal controls for our study would have been lymphocytes from pleural tissue rather than lung tissue. Unfortunately, the extremely small volume of normal pleural tissue (due to the thinness of this layer) made it impossible for us to obtain enough cells to conduct meaningful flow cytometry. Our functional assays were limited to polyclonal TCR activation rather than specific antigen stimulation. It is possible that the signal strength provided by CD3 ligation is stronger than that of natural antigens, however, T cell hypofunction occurring after direct CD3 crosslinking and in the absence of inhibitory signals strongly suggests that such T cells have an intrinsic deficit in their response capacity and would not respond to antigen-specific stimulation. Most of our patients were treated with surgery and we do not yet have correlations of TIL function with clinical outcomes, although this data is being collected. Finally, we did not address the interesting question of how checkpoint blockade (anti-PD-1 or anti-PD-L1 antibodies) relate to our findings. For that, prospective studies examining phenotypic markers on TILs, as well as TIL functional assays, with PD-L1 signals provided in the presence and absence of anti-PD-1 antibodies are being designed.
In summary, we conducted a comprehensive examination of the immune cell composition of MPM tumors with a focus on the function of CD8 TILs. Mesothelioma tumors possess a microenvironment enriched in myeloid cells, but with relatively low infiltration of CD8+ lymphocytes. The overall function of CD8+ T cells is severely compromised in part due to the presence of Tregs and expression of TIGIT on CD8 T cells. The Trm subset of CD8+ T cells is significantly more functional, although its anti-tumor ability appears to be impaired by the development of an exhaustion program marked by Eomes. Future studies will need to investigate further the role of increased Eomes in association with Trm dysfunction in the MPM tumor microenvironment and whether these markers might predict response to checkpoint blockade. The data from this study suggest that treatment of patients with T-regulatory cell blocking agents and TIGIT inhibitors, when they become available, might be especially valuable in MPM, whereas caution should be taken with TIM3 blocking agents.
Methods
Patient selection, tissue acquisition and processing
Blood and operative biopsy samples were obtained from 22 MPM patients undergoing pleurectomy. We also obtained samples of blood and tumor-free lung (TFL) tissue from 21 organ transplant donors whose lungs were not used for transplant (due to focal areas of pneumonia or donor hypoxemia). These patients died of cerebrovascular accidents (12 pts), anoxia (7 pts), and head trauma (2 pts). In these cases, the areas of most normal appearing lung were selected for processing. The study was approved by the institutional review board at the University of Pennsylvania and informed consent was obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki.
Tissues were digested and processed within an hour of removal using a validated protocol that minimizes cleavage of cell surface markers.45 Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by Ficoll density gradient and red blood cell lysis. All assays were performed immediately after isolation and processing.
Immunophenotyping
Single cell suspensions from healthy PBMCs, TFL digests, MPM PBMCs and MPM digests were stained with Live Dead Blue (Invitrogen, 1:400 in PBS) and cell surface marker antibodies for 45 minutes, 4°C. For intracellular marker and transcription factor staining, cells were treated using the eBioscience FoxP3 staining protocol. Briefly, cells were washed, fixed with eBioscience Fix/Perm for one hour at 4°C, washed twice and stained in presence of Perm/Wash for 45 minutes at 4°C.
All surface marker and intracellular markers used are summarized in Suppl. Table 1. Flow cytometric analysis was performed on a BD LSR Fortessa with FACS Diva Software (BD Biosciences) and analyzed using FlowJo 10.2.
Functional assays
For stimulations to assess cytokine production, single cell tumor digests were stimulated overnight at 37°C, 5% CO2 with PMA/Ionomycin (30ng/ml/1uM) or platebound anti-human CD3 (OKT3, Biolegend, 0.5ug/ml) in presence of GolgiStop (0.66ul/ml) and GolgiPlug (1ul/ml BD Biosciences,). Cells were then stained for surface markers, followed by concurrent intracellular transcription factor and cytokine staining as detailed previously,11 using the eBioscience FoxP3 staining protocol. All surface, transcription factor, and cytokine antibodies used are summarized in Suppl Table 1.
Statistical analysis
Descriptive statistics were computed for all variables. P values were performed either by a non-parametric Mann-Whitney test, non-parametric log-rank test, or two-way ANOVA test, where appropriate. In each figure, variability in the data is shown as standard error of the mean (SEM). Correlations between IFNγ+ CD8+ T cells with other cell types, inhibitory receptors and Eomes were performed using the Pearson correlation coefficient method.
Funding Statement
This project was funded by the University of Pennsylvania’s Abramson Cancer Center and NCI PO1-CA087971 (AK, KC, SS, SMA), NHLBI K23 HL116656, R03 HL135227 (EC).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Odgerel C-O, Takahashi K, Sorahan T, Driscoll T, Fitzmaurice C, Yoko-O M, Sawanyawisuth K, Furuya S, Tanaka F, Horie S, et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup Environ Med. 2017;74(12):851–858. doi: 10.1136/oemed-2017-104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stayner L, Welch LS, Lemen R.. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health. 2013;34:205–216. doi: 10.1146/annurev-publhealth-031811-124704. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DB, Rice DC, Niu J, Atay SM, Vaporciyan AA, Antonoff MB, Hofstetter WL, Walsh GL, Swisher SG, Roth JA, et al. Predictors of trimodality therapy and trends in therapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2018;53(5):960–966. doi: 10.1093/ejcts/ezx427. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.Aerts J, de Goeje PL, Cornelissen R, Kaijen-Lambers MEH, Bezemer K, van der Leest CH, Mahaweni NM, Kunert A, Eskens FALM, Waasdorp C, et al. Autologous dendritic cells pulsed with allogeneic tumor cell lysate in mesothelioma: from mouse to human. Clin Cancer Res. 2018;24(4):766–776. doi: 10.1158/1078-0432.CCR-17-2522. [DOI] [PubMed] [Google Scholar]
- 6.Sterman DH, Alley E, Stevenson JP, Friedberg J, Metzger S, Recio A, Moon EK, Haas AR, Vachani A, Katz SI, et al. Pilot and feasibility trial evaluating immuno-gene therapy of malignant mesothelioma using intrapleural delivery of Adenovirus-IFNalpha combined with chemotherapy. Clin Cancer Res. 2016;22(15):3791–3800. doi: 10.1158/1078-0432.CCR-15-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowak AK, McDonnell A, Cook A.. Immune checkpoint inhibition for the treatment of mesothelioma. Expert Opin Biol Ther. 2019;1–10. doi: 10.1080/14712598.2019.1606209. [DOI] [PubMed] [Google Scholar]
- 8.Maio M, Scherpereel A, Calabrò L, Aerts J, Cedres Perez S, Bearz A, Nackaerts K, Fennell DA, Kowalski D, Tsao AS, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017;18(9):1261–1273. doi: 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 9.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, van Brummelen E. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):623–630. doi: 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 10.Singhal S, Stadanlick J, Annunziata MJ, Rao AS, Bhojnagarwala PS, O'Brien S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci Transl Med. 2019;11(479). doi: 10.1126/scitranslmed.aav5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien S, Thomas RM, Wertheim GB, Zhang F, Shen H, Wells AD. Ikaros imposes a barrier to CD8+ T cell differentiation by restricting autocrine IL-2 production. J Immunol. 2014;192(11):5118–5129. doi: 10.4049/jimmunol.1301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan A-P, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, Samaniego-Castruita D, Singh D, Seumois G, Alzetani A, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017;18(8):940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194(7):3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 14.Edwards J, Wilmott JS, Madore J, Gide TN, Quek C, Tasker A, Ferguson A, Chen J, Hewavisenti R, Hersey P, et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during Anti-PD-1 treatment. Clin Cancer Res. 2018;24(13):3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien S, Klampatsa A, Thompson JC, Martinez MC, Hwang W-T, Rao AS, Standalick JE, Kim S, Cantu E, Litzky LA, et al. Function of human tumor infiltrating lymphocytes in early stage non-small cell lung cancer. Cancer Immunol Res. 2019;7:896–909. doi: 10.1158/2326-6066.CIR-18-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ujiie H, Kadota K, Nitadori JI, Aerts JG, Woo KM, Sima CS, Travis WD, Jones DR, Krug LM, Adusumilli PS. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology. 2015;4(6):e1009285. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burt BM, Rodig SJ, Tilleman TR, Elbardissi AW, Bueno R, Sugarbaker DJ. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer. 2011;117(22):5234–5244. doi: 10.1002/cncr.26143. [DOI] [PubMed] [Google Scholar]
- 18.Marcq E, Siozopoulou V, De Waele J, van Audenaerde J, Zwaenepoel K, Santermans E, Hens N, Pauwels P, van Meerbeeck JP, Smits ELJ. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology. 2017;6(1):e1261241. doi: 10.1080/2162402X.2016.1261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awad MM, Jones RE, Liu H, Lizotte PH, Ivanova EV, Kulkarni M, Herter-Sprie GS, Liao X, Santos AA, Bittinger MA, et al. Cytotoxic T cells in PD-L1-positive malignant pleural mesotheliomas are counterbalanced by distinct immunosuppressive factors. Cancer Immunol Res. 2016;4(12):1038–1048. doi: 10.1158/2326-6066.CIR-16-0171. [DOI] [PubMed] [Google Scholar]
- 20.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissick HT, Ireland DJ, Beilharz MW. Combined intratumoral regulatory T-cell depletion and transforming growth factor-beta neutralization induces regression of established AE17 murine mesothelioma tumors. J Interferon Cytokine Res. 2009;29(4):209–215. doi: 10.1089/jir.2008.0055. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Yun Z, Tagawa T, Rey-McIntyre K, Anraku M, de Perrot M. Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol. 2011;6(9):1578–1586. doi: 10.1097/JTO.0b013e3182208ee0. [DOI] [PubMed] [Google Scholar]
- 24.Ireland DJ, Kissick HT, Beilharz MW. The role of regulatory T cells in mesothelioma. Cancer Microenviron. 2012;5(2):165–172. doi: 10.1007/s12307-012-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Zha Y, Gajewski TF. Molecular regulation of T-cell anergy. EMBO Rep. 2008;9(1):50–55. doi: 10.1038/sj.embor.7401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25(2):214–221. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabins NC, Chornoguz O, Leander K, Kaplan F, Carter R, Kinder M, Bachman K, Verona R, Shen S, Bhargava V, et al. TIM-3 engagement promotes effector memory T cell differentiation of human antigen-specific CD8 T cells by activating mTORC1. J Immunol. 2017;199(12):4091–4102. doi: 10.4049/jimmunol.1701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnorfeil FM, Lichtenegger FS, Emmerig K, Schlueter M, Neitz JS, Draenert R, Hiddemann W, Subklewe M. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93. doi: 10.1186/s13045-015-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than “exhaustion” of human CD8 T cells. Front Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124(5):2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inozume T, Hanada K-I, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, Yang JC. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33(9):956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC, et al. CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog. 2015;11(10):e1005177. doi: 10.1371/journal.ppat.1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougall WC, Kurtulus S, Smyth MJ, Anderson AC. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276(1):112–120. doi: 10.1111/imr.12518. [DOI] [PubMed] [Google Scholar]
- 34.Blake SJ, Dougall WC, Miles JJ, Teng MWL, Smyth MJ. Molecular Pathways: targeting CD96 and TIGIT for Cancer Immunotherapy. Clin Cancer Res. 2016;22(21):5183–5188. doi: 10.1158/1078-0432.CCR-16-0933. [DOI] [PubMed] [Google Scholar]
- 35.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y-H, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen B-S, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517(7534):386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avery L, Filderman J, Szymczak-Workman AL, Kane LP. Tim-3 co-stimulation promotes short-lived effector T cells, restricts memory precursors, and is dispensable for T cell exhaustion. Proc Natl Acad Sci U S A. 2018;115(10):2455–2460. doi: 10.1073/pnas.1712107115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. 2018;24(7):986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 40.Koh J, Kim S, Kim M-Y, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8(8):13762–13769. doi: 10.18632/oncotarget.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20(2):434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Xu J, Niu Y, Bromberg JS, Ding Y. T-bet and eomesodermin play critical roles in directing T cell differentiation to Th1 versus Th17. J Immunol. 2008;181(12):8700–8710. doi: 10.4049/jimmunol.181.12.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mognol GP, Spreafico R, Wong V, Scott-Browne JP, Togher S, Hoffmann A, Hogan PG, Rao A, Trifari S. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc Natl Acad Sci U S A. 2017;114(13):E2776–E2785. doi: 10.1073/pnas.1620498114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity. 2015;43(6):1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Quatromoni JG, Singhal S, Bhojnagarwala P, Hancock WW, Albelda SM, Eruslanov E. An optimized disaggregation method for human lung tumors that preserves the phenotype and function of the immune cells. J Leukoc Biol. 2015;97(1):201–209. doi: 10.1189/jlb.5TA0814-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


