Abstract
Objective
To compare referral patterns, genetic testing and pathogenic variant rates in Black women (BW) and White women (WW) in a large academic Gynecologic Cancer Risk Assessment Clinic (GCRAC).
Methods
Cross sectional study of an IRB-approved prospective, cohort study from a GCRAC. Data evaluated included: age, race, referral provider specialty and indication, genetic testing frequency, as well as frequency and types of pathogenic variants.
Results
588 WW and 57 BW were evaluated from ½010–12/2015. Although approximately one-third of BW and WW were referred for family history alone, referral indications varied. BW were more likely referred for a known pathogenic variant (20.0% vs. 6.2%) although less likely referred for a personal history of ovarian cancer (24.0% vs. 46.8%; p = 0.0023). While gynecologic oncologists referred most patients (BW 43.6% vs. WW 63.0%), BW were more likely to be referred by surgical oncologist (23.0% vs. 12.8%) or genetic counselor (12.8% vs. 5.9%) than WW (p=0.0234). Referral from non-OBGYN primary care providers was less than 3% in both groups. Genetic testing rates were similar in both races (82.4% vs. 85.5%). Rates of BRCA1 mutations (12.7% vs. 11.5%) were similar; however, BW had more BRCA2 mutations (21.3% vs. 9.5%; p =0.0194).
Conclusions
Since BW are more likely to be referred by surgical oncology or genetics counselor, breast clinics might be an entry point to ensure genetic counseling and testing. Continued efforts to increase awareness regarding the importance of patient referral at the primary care level may help identify the subset of women not currently undergoing counseling and testing.
Keywords: Genetic testing, Genetic predisposition, Ovarian cancer, African Americans
Introduction
It is estimated that greater than 22,000 American women will be diagnosed with ovarian cancer in 2018. With a predicted 14,070 deaths, ovarian cancer is the most deadly gynecology malignancy in the United States and fifth for cancer deaths among women(1). Half of ovarian cancer diagnoses are attributed to somatic mutations in the TP53 gene, and approximately 20% of ovarian cancer cases are attributed to pathogenic germline variants (mutations) (2). Pathogenic germline variants are most often in the following genes: BRCA1, BRCA2, BRIP1, RAD51C, RAD51D, and Lynch syndrome genes (MLH1, MSH2, MSH6, PMS2, EPCAM), among others (3). Genetic counseling and testing for a germline pathogenic variant in ovarian cancer patients offers prognostic information with early treatment and may assist in tailoring treatment options. The need for this information is highlighted by a clear demonstration of the benefit of olaparib for primary maintenance therapy in patients who carry a pathogenic BRCA variant (4). Additionally, screening relatives via cascade testing allows the possibility for interventions to reduce cancer risk (5). For these reasons, the National Comprehensive Cancer Network now recommends genetic evaluation and testing for all women who have an epithelial ovarian cancer.
While black women (BW) have a lower incidence of ovarian cancer than white women (WW) (10.1/100,000 vs. 14.1/100,000), BW have worse five-year survival across all ages when compared in WW (6). This is a common trend as BW generally have the highest death rate and shortest survival in most cancer groups, with a 6% lower risk of diagnosis but 14% higher risk of death (1). There is a paucity of data regarding ovarian cancer in BW, and they are underrepresented in published literature (7). Previous studies have suggested that BW are underrepresented in Gynecologic Cancer Risk Assessment Clinics (GCRAC)(8). The objective of this study was to compare referral patterns including referring provider specialty type, genetic testing, and pathogenic variant rates between BW and WW in a large southeastern GCRAC.
Materials and Methods
We performed a cross sectional study of an IRB-approved prospective, cohort study of patients from a GCRAC embedded within our NCI designated Comprehensive Cancer Center. This multidisciplinary clinic is composed of a board-certified gynecologic oncologist, a nurse practitioner, and cancer genetic counselors. Detailed genetic evaluation, including counseling and testing, is performed for high-risk individuals. Patients are referred to this clinic for four general indications: (1) women with a personal history of ovarian or other gynecologic cancers, (2) unaffected women with a strong family history of cancer, (3) women with a first, or less commonly, a second degree relative with a positive germline test who have not themselves undergone germline testing and (4) women who have undergone germline testing at an outside institution, were found to have a pathogenic germline variant, and need either surveillance and/or prophylactic surgical recommendations. This study was conducted in a state where African Americans comprise about a quarter of the population and Caucasians comprise approximately two-thirds. For the current study, we limited our analysis to WW and BW evaluated from ½010–12/2015. Data evaluated included: age, race, frequency of genetic testing, referral provider specialty and indication, as well as frequency and types of pathogenic variants. When questions arose regarding genetic test results, ClinVar (www.clinvar.com) was utilized for categorizing genetic test results as pathogenic or not. Women reporting Hispanic or Jewish ethnicity as well as Asian, Native American or Pacific Islander race were excluded. Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary NC) to compare means with standard deviations and frequencies of categorical variables between the two groups.
Results
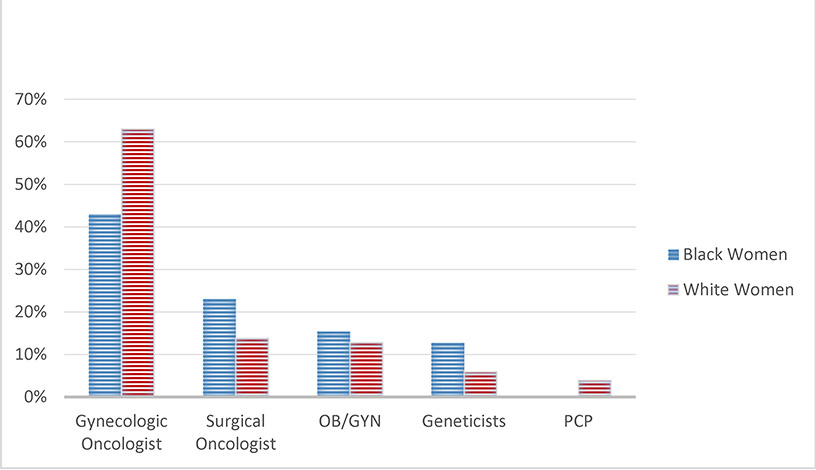
588 (91.2%) WW and 57 (8.8%) BW were evaluated in our GCRAC. The mean age of BW was younger (49.3 ± 12.5 years) than WW (54.2 ± 14.8 years) (p=0.016). Although approximately one third of both BW (36.0%) and WW (32.6%) were referred for family history alone, BW were more likely to be referred for a known pre-existing pathogenic variant (20.0% vs. 6.2%) and less likely to be referred for a personal history of ovarian cancer (24.0% vs. 46.8%; p = 0.0023). Other referral indications are depicted in Table 1. While both groups were most likely referred by a gynecologic oncologist (BW 43.6% vs. WW 63.0%), BW were more likely to be referred by surgical oncologist (23.0% vs. 12.8%) or genetic counselor (12.8% vs. 5.9%) than WW (p=0.02) (Figure 1). Referral rates from primary OBGYNs were similar (BW 15.3% vs. WW 12.8%), while referral from other primary care providers was low in both groups (BW 0% vs. WW 2.9%).
Table 1.
Characteristics of White and Black Women Evaluated at a Gynecologic Cancer Risk Assessment Clinic
| Race | White | Black | P value |
|---|---|---|---|
| N, (%) | 588 (91.2) | 57 (8.8) | |
| Age (years) – mean ± St. Dev | 54.2 ± 14.8 | 49.3 ± 12.5 | 0.016 |
| Referral Indication | N (%) | N (%) | |
| BRCA + relative | 33 (5.9) | 4 (8.0) | |
| Personal history of breast cancer* | 27 (4.8) | 4 (8.0) | |
| Personal history of ovarian cancer | 263 (46.8) | 12 (24.0) | 0.0023 |
| Family history of cancer | 183 (36.0) | 18 (32.6) | |
| Known pathologic mutation | 35 (6.2) | 10 (20.0) | |
| Referring Provider Specialty | N (%) | N (%) | |
| Gynecologic Oncologist | 310 (63.0) | 17 (43.6) | |
| Surgical Oncologist | 68 (13.8) | 9 (23.1) | |
| OBGYN | 63 (12.8) | 6 (15.4) | |
| Genetic Counselor / Medical Geneticist | 29 (5.9) | 5 (12.8) | 0.0234 |
| Internal Medicine | 14 (2.9) | 0 (0.0) | |
| Medical oncologist | 3 (0.6) | 2 (5.1) | |
| Other | 5 (1.0) | 0 (0.0) | |
with other high-risk feature (relative with ovarian cancer, age <40, founder population, etc)
Fig. 1.
Type of referring provider for women evaluated in GCRAC*.
*GCRAC – Gynecologic Cancer Risk Assessment Clinic
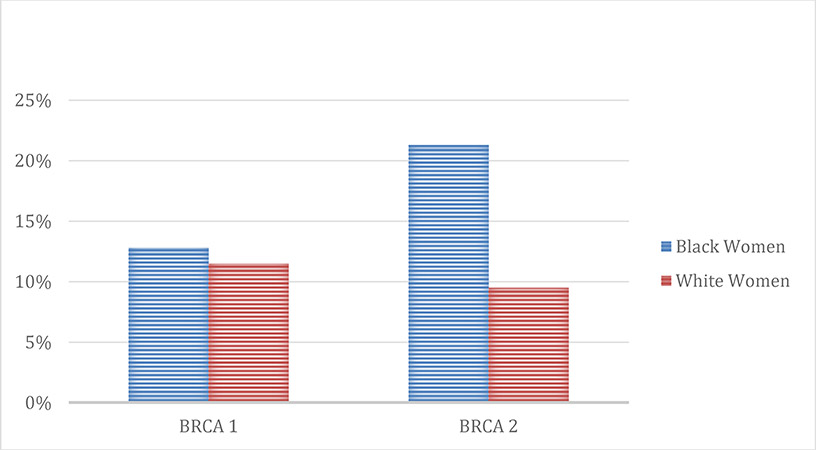
Overall 556 of 645 (86.2%) of WW and BW evaluated underwent genetic testing following genetic counseling. The rates of genetic testing were similar for both groups (BW 82.4% vs. WW 85.5%; p=0.5563). WW and BW had similar rates of pathogenic BRCA1 variants (12.7% vs. 11.5%); however, BW were more likely to have pathogenic BRCA2 variants (21.3% vs. 9.5%; p =0.02) (Figure 2). Findings of other pathogenic variants and variants of uncertain significance results were similar and are depicted in Table 2.
Fig. 2.
Rates of BRCA mutations in women tested at GCRAC*
*GCRAC – Gynecologic Cancer Risk Assessment Clinic
Table 2.
Genetic Testing Results for Women Evaluated in Gynecologic Cancer Risk Assessment Clinic by Race
| Race | White N (%) | Black N (%) | p value |
|---|---|---|---|
| Underwent genetic testing | 503 (85.5) | 47 (82.4) | 0.5563 |
| Results | |||
| Negative | 368 (73.2) | 26 (55.3) | |
| BRCA1 pathogenic variant | 58 (11.5) | 6 (12.8) | |
| BRCA2 pathogenic variant | 48 (9.5) | 10 (21.3) | 0.0194 |
| Other pathologic variant | 4 (0.8) | 2 (4.3) | |
| Variant of unknown significance | 18 (3.6) | 3 (6.4) | |
| Hereditary nonpolyposis colorectal cancer | 7 (1.4) | 0 (0.0) | |
Discussion
Our prior study in gynecologic risk assessment demonstrated that the proportion of BW represented in risk assessment clinics is low and was similar to what was seen in the current study (8). Most data regarding BW and BRCA genetic testing has been studied in breast cancer patients (9–11) and has noted that BW women with a history of breast or ovarian cancer were less likely than WW to undergo genetic testing (12). Overall, the number of BW women represented in our study was low. BW were more likely than WW to be referred for a known pathogenic variant and were more likely to have pathogenic BRCA2 variants than WW (Table 1). Previous research has shown that ethnically diverse populations have a higher proportion of pathogenic BRCA2 variants compared to more homogenously white samples (13).
Given ovarian cancer is the leading cause of death from gynecologic cancers and has an associated hereditary risk, it is imperative that at-risk patients be appropriately evaluated via genetic counseling, and when appropriate, with genetic testing. Genetic risk assessments provide patients with the ability to understand hereditary risks and aid in management. Our current research shows that women are most likely to be referred based on family history (BW 36.0% vs. WW 32.6%) or a personal history of ovarian cancer (BW 24.0% vs. WW 46.8%). Interestingly, 20% of BW were referred to our clinic for a known pathogenic variant. It has also been presented that BW with greater perceived benefit of genetic testing, higher income, and higher risk of pathogenic BRCA variants are more likely to undergo testing (14). It has been recommended that all women with high-grade epithelial tumors should have genetic testing, and current NCCN guidelines endorse that all women with ovarian cancer be offered genetic testing (15–17). The Society of Gynecologic Oncology (SGO) also put forth recommendations for referral for genetic testing including (but not limited to): high-grade epithelial ovarian or peritoneal cancer, breast cancer less than or equal to 45 years old, breast cancer with greater than or equal to 2 close relatives with breast cancer, a close relative with a pathogenic BRCA 1 or 2 variant (18).
Our study demonstrated that BW and WW were most likely to be referred by a gynecologist oncologist and least likely by a primary care providers (PCP). Referral rates from OBGYNs were similar (BW 15.3% vs. WW 12.8%), while referral from other primary care providers was low in both groups (BW 0% vs. WW 2.9%). In fact, no BW were referred by a PCP to the genetic clinic (Table 1). Primary care providers can be instrumental in discovering patients who need genetic evaluation. Hamilton et al. performed a literature review evaluating breast and colorectal cancer genetic referral patterns (19). Their results suggested that PCPs have limited knowledge about genetic testing options, and furthermore, PCPs expressed ethical, legal, and social concerns about testing. ACOG recommends that all women with a familial or personal history of ovarian or breast cancer be referred for genetic counseling to evaluate if genetic testing is warranted. It is apparent that further research, resources, and interventions need to be utilized in order to increase PCP knowledge and communication with gynecologic genetic referral centers.
When BW are actually referred to genetic counseling, the rates of genetic testing were comparable with WW (Table 2). This testing may allow for potential preventive treatment options that are available to be discussed both for the patient and even the patient’s family. Patients with pathogenic variants in the BRCA1 or BRCA2 genes may undergo risk reducing or prophylactic bilateral total mastectomy and bilateral salpingo-oophorectomy and/or hysterectomy. These prophylactic surgery options are known to dramatically reduce the risk of development of breast or ovarian cancer. Undergoing a risk-reducing salpingo-oophorectomy (RRSO) for women with a pathologic variant has been associated with lower all-cause mortality along with lower breast and ovarian cancer-specific mortality (5, 17, 20–23). Similarly, bilateral mastectomies have been shown to decrease the risk of developing breast cancer in at least 90% of women with known pathogenic BRCA1 or BRCA2 variants (24). Another option for these women is high-risk breast cancer assessments which include an annual breast MRI starting at age 25 with annual mammograms added at age 30 for those who do not wish to undergo a mastectomy (17).
Limitations in our study include its single institutional design and limitation to two racial groups. We are also potentially missing some patients given there are three cancer institutes in our state, however, ours is the largest and only designated Comprehensive Cancer Center. In addition to the described clinic which is embedded within our Gynecologic Oncology Division, there are two similar cancer genetic counseling clinics on campus. Patients with a risk of gynecologic cancer, such as those described in our manuscript are preferentially referred to our clinic, even if they were initially referred to one of the other two clinics. In addition, a comparatively small proportion of BW were evaluated, which is largely secondary to the limited referral patterns. Nonetheless, we were able to determine referral indications based on review of referral documentation from the patient’s referring provider when the patient’s genetic counseling visit was scheduled. Evaluating medical providers’ understanding of referral indications may assist in addressing be sub-optimal referral patterns.
The current study continues to aid in the investigation of BW with hereditary cancer syndromes given the limited literature at this time. While barriers to genetic referral remain poorly defined, the strength in guidelines should assist progression in clinical translation. Given that many guidelines require knowledge regarding the patient’s family history, it is important that providers elicit this information in order to evaluate if the patient needs referral. Potential future strategies for improving awareness to address poor referral patterns include: outreach services such as presentations at conferences given for primary care physicians, targeted mailing questionnaires evaluating referral patterns and providing educational information regarding indications for referrals, and working in concert with support groups to raise awareness in the general populations. Studies addressing providers’ understanding of referral indications will continue to aid in addressing the gap in identifying minorities at risk for hereditary cancer syndromes. Initiatives targeting increasing primary care providers’ knowledge of genetic referral indications may help to improve the identification of at-risk individuals of all races.
Highlights.
Black women were more likely referred for genetic assessment by surgical oncology or a genetic counselor than white women.
Black women evaluated in our clinic were more than twice as likely as white women to have a BRCA2 pathogenic variant.
No black women were referred by primary care physicians to the genetic assessment clinic.
Opportunities exist for improving minority referral patterns for genetic evaluation.
Acknowledgments
Grant Support: CAL was supported in part by the NIH 5K12HD001258-14, 3P30CA013148-43S3 and U10CA180855.
Funding Support: Funding was also provided by both the Lynne Cohen Foundation as well as the Norma Livingston Ovarian Cancer Foundation
Footnotes
Conflict of Interest Report: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Walsh T, Casadei S, Lee MK, Pennil CC, Nord AS, Thornton AM, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(44):18032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA oncology. 2016;2(4):482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. The New England journal of medicine. 2018;379(26):2495–505. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti C, De Felice F, Palaia I, Perniola G, Musella A, Musio D, et al. Risk-reducing salpingo-oophorectomy: a meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC women’s health. 2014;14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HK, Ruterbusch JJ, Cote ML. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(10):1511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecologic oncology. 2013;129(1):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrington DA, Champion ML, Boitano TKL, Walters-Haygood CL, Farmer MB, Alvarez RD, et al. Characteristics of African American women at high-risk for ovarian cancer in the southeast: Results from a Gynecologic Cancer Risk Assessment Clinic. Gynecologic oncology. 2018;149(2):337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal T, Bonner D, Cragun D, Johnson S, Akbari M, Servais L, et al. BRCA sequencing and large rearrangement testing in young Black women with breast cancer. Journal of community genetics. 2014;5(2):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pal T, Bonner D, Kim J, Monteiro AN, Kessler L, Royer R, et al. Early onset breast cancer in a registry-based sample of African-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. The breast journal. 2013;19(2):189–92. [DOI] [PubMed] [Google Scholar]
- 11.Greenup R, Buchanan A, Lorizio W, Rhoads K, Chan S, Leedom T, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Annals of surgical oncology. 2013;20(10):3254–8. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA½ testing among women with a family history of breast or ovarian cancer. Jama. 2005;293(14):1729–36. [DOI] [PubMed] [Google Scholar]
- 13.Lee R, Beattie M, Crawford B, Mak J, Stewart N, Komaromy M, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genetic testing. 2005;9(4):306–12. [DOI] [PubMed] [Google Scholar]
- 14.Jones T, McCarthy AM, Kim Y, Armstrong K. Predictors of BRCA½ genetic testing among Black women with breast cancer: a population-based study. Cancer medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. [DOI] [PubMed] [Google Scholar]
- 16.Trainer AH, Meiser B, Watts K, Mitchell G, Tucker K, Friedlander M. Moving toward personalized medicine: treatment-focused genetic testing of women newly diagnosed with ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20(5):704–16. [DOI] [PubMed] [Google Scholar]
- 17.Daly MB, Pilarski R, Berry M, Buys SS, Farmer M, Friedman S, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. Journal of the National Comprehensive Cancer Network : JNCCN. 2017;15(1):9–20. [DOI] [PubMed] [Google Scholar]
- 18.Lancaster JM, Powell CB, Chen LM, Richardson DL. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic oncology. 2015;136(1):3–7. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton JG, Abdiwahab E, Edwards HM, Fang ML, Jdayani A, Breslau ES. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: A systematic review and research agenda. Journal of general internal medicine. 2017;32(3):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finch A, Beiner M, Lubinski J, Lynch HT, Moller P, Rosen B, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. Jama. 2006;296(2):185–92. [DOI] [PubMed] [Google Scholar]
- 21.Domchek SM, Friebel TM, Singer CF, Evans DG, Lynch HT, Isaacs C, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. Jama. 2010;304(9):967–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(8):1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eleje GU, Eke AC, Ezebialu IU, Ikechebelu JI, Ugwu EO, Okonkwo OO. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. The Cochrane database of systematic reviews. 2018;8:Cd012464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig KK, Neuner J, Butler A, Geurts JL, Kong AL. Risk reduction and survival benefit of prophylactic surgery in BRCA mutation carriers, a systematic review. American journal of surgery. 2016;212(4):660–9. [DOI] [PubMed] [Google Scholar]