Abstract
Chronic hyperglycemia (HG) promotes pancreatic islet dysfunction which leads to the onset of T2DM. This study is aimed at defining regulatory roles of Rac1, a small G-protein, in the activation of p53 and ATM kinase in pancreatic β-cells, under the duress of HG conditions. We report significant stimulatory effects of HG (20 mM; 24 h) on p53 activation in INS-1 832/13 cells, normal rodent and human islets. Pharmacological inhibition of Rac1 (EHT1864 or NSC23766) significantly suppressed HG-induced p53 activation in INS-1 832/13 cells and rat islets, suggesting novel roles for this small G-protein in the activation of p53. Inhibition of Rac1 geranylgeranylation with simvastatin or GGTI-2147, significantly attenuated HG-induced p53 activation, suggesting requisite roles for this signaling step in HG-mediated effects on β-cells. HG-induced p53 activation was also suppressed by SB203580, a known inhibitor of p38MAPK. Additionally, we observed increased activation of ATM kinase under HG conditions, which was blocked in presence of EHT1864. Furthermore, pharmacological inhibition of ATM kinase (KU55933) reduced activation of ATM kinase, but not p53, suggesting that HG-mediated activation of p53 and ATM could represent independent pro-apoptotic events. In conclusion, these data indicate that sustained activation of Rac1-p38MAPK signaling axis leads to activation of p53 leading to β-cell dysfunction under the duress of chronic hyperglycemic conditions.
Keywords: Pancreatic islet, Glucotoxicity, Rac1, p38MAPK, p53, ATM kinase
Introduction
Glucose-stimulated insulin secretion (GSIS) from the pancreatic β-cells involves interplay between metabolic and cationic events leading to translocation of insulin granules to the plasma membrane for fusion and secretion of the hormone [1–5]. Recent studies have demonstrated essential roles of small G-proteins (Rac1, Cdc42 and Arf6) in cytoskeletal remodeling and GSIS [3–7]. Activation-deactivation of G-proteins (e.g., Rac1) requires intermediacy of various regulatory factors, including guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) which catalyze binding and hydrolysis of GTP, respectively [4, 7]. The majority of small G-proteins also undergo post-translational modifications at their C-terminal cysteines (e.g., prenylation) for targeting to appropriate cellular compartments for optimal cell function [4, 8]. Previous studies have demonstrated the requisite roles of Rac1 in promoting actin cytoskeletal remodeling and GSIS [9–11].
Rac1 is also involved in the functional assembly and activation of phagocyte-like NADPH oxidase (Nox2) enzyme complex, a known source of extra-mitochondrial reactive oxygen species (ROS) [7, 12, 13]. The Nox2 holoenzyme catalyzes one electron reduction of oxygen to generate superoxide species and is comprised of membranous and cytosolic components. Upon stimulation, the cytosolic components translocate and interact with the membranous core, resulting in Nox2 activation. Although published evidence suggests positive modulatory roles of ROS in GSIS [14–16], recent findings indicate that β-cell dysfunction under hyperglycemic conditions might be due to sustained activation of Rac1-Nox2 enzyme complex and excess generation of ROS [7, 12, 17]. We recently implicated abnormal Rac1-Nox2 activity in β-cell dysfunction under the duress of glucolipotoxicity and exposure to pro-inflammatory cytokines [12, 18–20]. However, the downstream signaling steps that underlie the accelerated Rac1-Nox2 pathway leading to cell dysregulation remain largely unknown.
We previously reported key regulatory roles for Rac1-Nox2 signaling cascade in the activation of pro-apoptotic p38MAPK, resulting in β-cell dysfunction under glucotoxic conditions [20]. p38MAPK has been implicated in the activation of p53, a tumor suppressor and a transcriptional factor [21–23]. p53 is comprised of several functional domains that regulate its stability and function [24, 25]. Following activation, p53 binds to specific sites in the DNA and promotes the transcription of genes including p21, PUMA and Noxa [26]. Published evidence also links intracellular oxidative stress and DNA damage to the activation of the p53 pathway to promote cell cycle arrest, DNA repair and apoptosis [27]. However, role of p53 in the onset of β-cell dysfunction under glucotoxic conditions is less understood.
Post-translational phosphorylation, acetylation and ubiquitination are involved in the activation, stabilization and degradation of p53 [28]. Studies in multiple cell types have shown that phosphorylation of p53 at multiple residues is required for its stability, DNA binding and transcriptional activation [28]. In particular, multiple studies have demonstrated a crucial role of p53 phosphorylation at serine-15 residue in its functional activation, by regulating its interaction with modulatory factors, including MDM2 and CBP/p300 [29, 30]. Further, ATM kinase has been implicated in the phosphorylation events leading to p53 activation [28, 31]. Yoshida and associates have shown the involvement of ATM kinase and p53 in doxorubicin-induced cardiotoxicity [32]. They also implicated Rac1 in ATM kinase and p53 activation. Oleson et al. have reported activation of ATM kinase in pancreatic β-cells exposed to cytokines. Further, they suggested that upon extensive dsDNA breaks ATM kinase may be responsible for activating apoptotic pathways primarily through p53-dependent mechanisms [33].
Herein, we studied potential mechanisms underlying HG-induced metabolic dysfunction of the islet β-cell. Specifically, we investigated putative regulatory mechanisms involved in the activation of p53 in islet β-cells exposed to glucotoxic (HG; 20 mM for 24 h) conditions. Our findings suggest novel roles of Rac1 in the activation and nuclear targeting of p53 leading to activation of nuclear events terminating in cell death.
Materials and methods
Materials
Antibodies directed against total (Catalog# 2524) and phosphorylated (Serine-15) p53 (Catalog# 9284) were from Cell Signaling Technology (Danvers, MA). Antisera against total (Catalog# ab78) and phosphorylated (Serine-1981) ATM kinase (Catalog# ab36810) were obtained from Abcam (Cambridge, MA). Published evidence from several laboratories have confirmed the specificity of antibodies utilized in this study [30, 33]. IRDye® 800CW anti-rabbit and anti-mouse were obtained from LICOR (Lincoln, NE). KU-55933, Simvastatin and Cell Death Detection ELISA® kit were from Sigma (St. Louis, MO). EHT1864 and SB203580 were from R&D Systems (Minneapolis, MN). GGTI-2147 was purchased from VDM Biochemicals (Bedford, OH). NE-PER® Nuclear and Cytoplasmic Extraction Reagents were from Thermo Scientific (Waltham, MA).
Insulin secreting cells
Pancreatic islets were isolated [18, 20] from male Sprague–Dawley rats (6–8 weeks; ENVIGO, Indianapolis, IN), Zucker Diabetic fatty (ZDF) and Zucker lean control rats (ZLC; 13 weeks; Charles River, Wilmington, MA). All protocols were approved by the Institutional Animal Care and Use Committee at Wayne State University. INS-1 832/13 cells or rat islets were incubated with low glucose (LG; 2.5 mM) and high glucose (HG; 20 mM) for 24 h in the absence or presence of EHT1864 (10 μM), NSC23766 (20 μM), KU-55933 (10–20 μM), SB203580 (10–20 μM), Simvastatin (15 μM) or GGTI-2147 (10 μM). Human pancreatic islets from a Caucasian male donor (63 years; BMI = 21.8; HbA1c = 5.8%) were from Prodo Laboratories, Inc. (Irvine, CA) and incubated in the supplier’s human islet medium with 5.8 mM glucose or supplemented with 30 mM glucose for 24 h.
Isolation of nuclear fraction from INS-1 832/13 cells
Following incubation of cells with LG or HG in the absence or presence of EHT1864, cell fractionation was carried out using NE-PER® Nuclear and Cytoplasmic Extraction Kit according to the manufacturer’s instructions.
Western blotting
Cell lysates were separated by SDS–PAGE and transferred onto nitrocellulose membrane and probed for total and phosphorylated p53 and ATM kinase. For detection, the membranes were probed with appropriate IRDye® 800CW secondary antibody and developed using Odyssey® Imaging Systems. Band Intensities were then quantified by densitometry and ratios between phosphorylated and total protein were calculated and expressed as fold change over LG. For nuclear and non-nuclear fractions, ratios of phosphorylated and total p53 were calculated over Lamin B.
Cell death detection
Cell death detection was determined using Annexin V/Propidium Iodide kit as well as the Cell Death Detection ELISAplus kit. Assays were carried out according to the manufacturer’s instructions.
Statistical analysis of experimental data
Results are expressed as means with their standard errors as indicated. The statistical significance of differences between the control and experimental groups were evaluated by ANOVA followed by SNK Post Hoc test where appropriate. p values <0.05 were considered as statistically significant.
Results
Glucotoxic conditions promote cell death in INS-1 832/13 cells
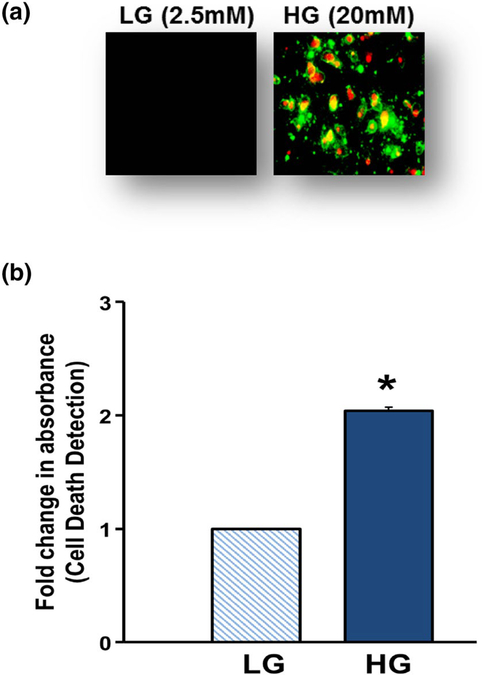
We recently established a glucotoxicity (HG conditions) model in our laboratory in which chronic exposure of INS-1 832/13 cells to HG (20 mM for 24 h) resulted in significant inhibition of GSIS [20]. Under the same conditions, we now report accelerated β-cell apoptosis as evidenced by increased Annexin V/Propidium Iodide staining (Fig. 1a). These findings were further confirmed in a cell death quantification assay using Cell Death Detection® kit, which indicated a significant increase in cell death signal under HG exposure conditions (Fig. 1b). Together, these findings indicate that long-term exposure of pancreatic β-cells to HG conditions leads to cell dysfunction (i.e., loss in ability to secrete insulin in response to physiological glucose concentrations) and demise. We used this model system for studies described below.
Fig. 1.
Glucotoxic conditions promote cell death in INS-1 832/13 cells. INS-1 832/13 cells (a) were incubated with LG (2.5 mM) or HG (20 mM) for 24 h and stained with Annexin V/propidium iodide. Cells were then visualized under Olympus IX71 inverted fluorescence microscope. Following incubation with LG (2.5 mM) or HG (20 mM) for 24 h, INS-1 832/13 cells (b) were analyzed for cell death using cell death detection ELISAPlus kit. Absorbance was measured at 405 nm and expressed as fold change over basal LG. Data shown are representative of three independent studies (*p < 0.05 vs. 2.5 mM glucose alone)
HG conditions induce p53 activation in INS-1 832/13 cells, rodent islets and human islets: such effects are replicated in islets from the ZDF rat
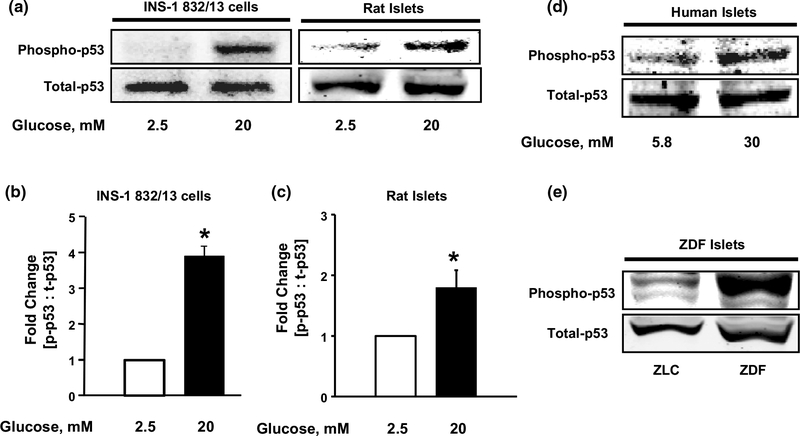
Experimental evidence indicates that phosphorylation of p53 at serine-15 residue is critical for its stabilization and transcriptional activation [30]. Therefore, at the outset, we examined the regulatory effects of HG exposure on p53 phosphorylation in a variety of β-cells. Data depicted in Fig. 2 (Fig. 2a, b) demonstrate significant increase (~4 fold) in p53 phosphorylation in INS-1 832/13 cells exposed to HG. Such stimulatory effects of HG on p53 phosphorylation were also demonstrable in primary rat islets (Fig. 2a, c) and human islets (Fig. 2d). We observed no effect on p53 phosphorylation with equimolar concentrations of mannitol, an osmotic control, and 3–0-methylglucose, a non-metabolizable glucose analog, indicating that these effects are consequential to glucose metabolism. In further support of these findings, we also noticed marked increase in levels of phosphorylated p53 in islets from the Zucker diabetic fatty (ZDF) rats compared to their counterparts from age-matched non-diabetic lean controls (Fig. 2e). Together, these findings suggest that long-term exposure of pancreatic β-cells to HG or diabetic conditions results in increased p53 phosphorylation in pancreatic β-cells.
Fig. 2.
Activation of p53 in INS-1 832/13 cells, rodent islets and human islets upon exposure to HG conditions and in ZDF pancreatic islets. INS-1 832/13 cells and normal rat islets (a) were incubated with LG (2.5 mM) or HG (20 mM) for 24 h and lysates were analyzed by western blotting for total and phosphorylated p53. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 in INS-1 832/13 cells (b). *p < 0.05 vs. 2.5 mM glucose alone (n = 3). Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 in rodent islets (c). *p < 0.05 vs. 2.5 mM glucose alone (n = 3). Total and phosphorylated p53 levels were determined in human islets (d) following exposure to LG or HG as indicated. Total and phosphorylated p53 levels were determined in islet lysates from ZDF and ZLC rats (e) as indicated
Activation and post-translational prenylation of Rac1 are critical for HG-induced p53 activation in pancreatic β-cells
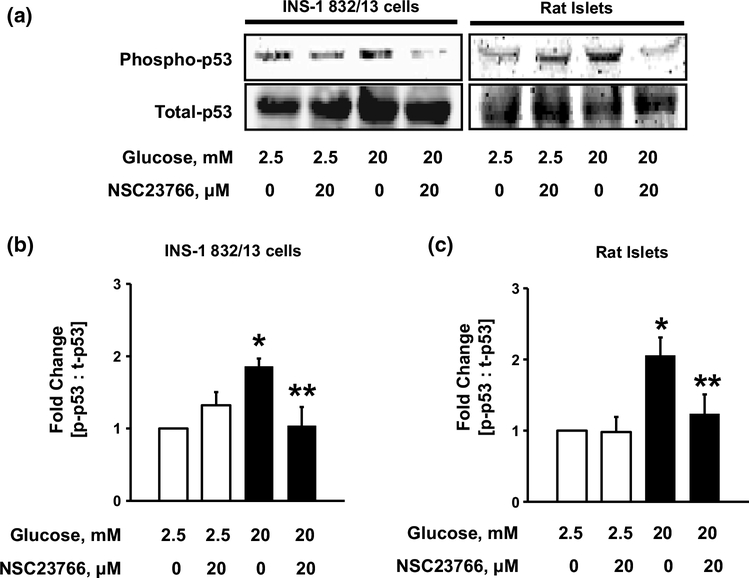
We and others have demonstrated novel regulatory roles for Rac1, in islet β-cell dysregulation under the duress of metabolic stress [7, 18, 34]. When stimulated, Rac1 is activated by its dissociation with GDP and concomitant GTP binding, which is mediated by GEFs namely, Tiam1 and Vav2. Previous experiments in our laboratory have employed NSC23766, a specific inhibitor of Tiam1-mediated Rac1 activation, to demonstrate the role of Rac1 in islet dysfunction in models of diabetes [20, 35]. Therefore, to assess the role of Rac1 in the activation of p53, we quantified HG-induced p53 phosphorylation in INS-1 832/13 cells treated with NSC23766. As shown in Fig. 3, HG-mediated p53 phosphorylation was blocked in presence of NSC23766 in INS-1 832/13 cells and rat islets. Data pooled from multiple studies in INS-1 832/13 cells and rat islets is provided in Fig. 3b, c, respectively.
Fig. 3.
NSC23766, an inhibitor of Rac1, prevents HG-induced p53 phosphorylation. INS-1 832/13 cells and rat islets (a) were incubated with LG (2.5 mM) or HG (20 mM) in the absence or presence of NSC23766 for 24 h. Lysates proteins were then separated and analyzed by western blotting for phosphorylated and total p53. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 in the presence of NSC23766 (b n = 4 in INS-1 832/13 cells and c n = 3 in rat islets). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone
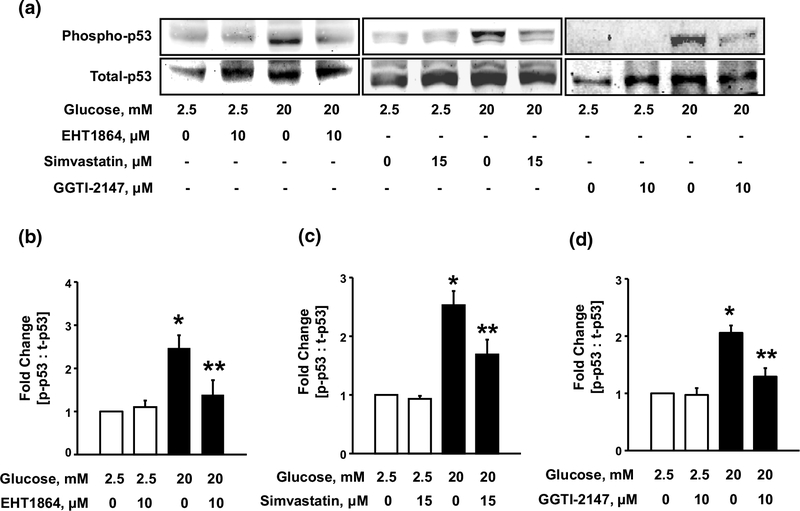
To further confirm these findings, we utilized EHT1864, a known inhibitor of Rac1, which blocks Rac1 activation by preventing its association with GDP/GTP, thereby retaining this G-protein in its inactive conformation [9]. Data depicted in Fig. 4 (Fig. 4a, b) demonstrate significant inhibition of HG-induced activation of p53 by EHT1864 suggesting that Rac1 activation is requisite for HG-induced activation of p53 in INS-1 832/13 cells. Similar inhibitory effects of EHT1864 on HG-induced p53 phosphorylation were observed in rat islets. Furthermore, as depicted in Fig. 1b, we noticed a significant increase in cell death signal under HG conditions. Our studies also indicated a significant protection against HG-induced cell death in presence of EHT1864 (~35%; n = 3; additional data not shown), thus suggesting that Rac1-p53 signaling module plays novel regulatory roles in HG-induced cell dysfunction (loss in GSIS; 7) and apoptosis.
Fig. 4.
Glucose-induced p53 activation requires activation and post translational geranylgeranylation of Rac1. INS-1 832/13 cells (a) were incubated with LG and HG in the absence or presence of EHT1864, Simvastatin or GGTI-2147 for 24 h. Lysate proteins were separated and analyzed by western blotting for total and phosphorylated p53. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 in the presence of EHT1864 (b n = 4), Simvastatin (c n = 3) or GGTI-2147 (d n = 3). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone
In addition, post-translational prenylation (i.e., incorporation of isoprenoid groups into the C-terminal cysteine residues) is necessary for optimal function of G-proteins [8, 36]. Therefore, we next examined the role of prenylation in HG-induced activation of p53 by assessing the effects of Simvastatin, which inhibits biosynthesis of mevalonic acid and downstream intermediates of cholesterol biosynthetic pathway, including isoprenoids (farnesyl and geranylgeranyl pyrophosphates). Data from these studies (Fig. 4a; middle) indicated significant reduction in HG-induced p53 phosphorylation by co-provision of Simvastatin. Pooled data from multiple studies demonstrating inhibitory effects of Simvastatin are provided in Fig. 4c.
We next determined the roles of geranylgeranylation of Rac1 in the activation of p53 under glucotoxic conditions. This was accomplished by pharmacological inhibition of geranylgeranylation of Rac1 using GGTI-2147, a known inhibitor of geranylgeranyltransferase-1 [37]. Data from these studies (Fig. 4a; right) indicated marked attenuation, by GGTI-2147, of HG-induced phosphorylation of p53. Pooled data from multiple studies demonstrating inhibitory effects of GGTI-2147 are provided in Fig. 4d. Taken together, data in Figs. 3 and 4 suggest novel regulatory roles of Rac1 in mediating HG-induced phosphorylation of p53, and that post-translation prenylation, specifically geranylgeranylation is necessary for such a regulation.
Glucotoxic conditions promote translocation of p53 to the nuclear fraction in β-cells
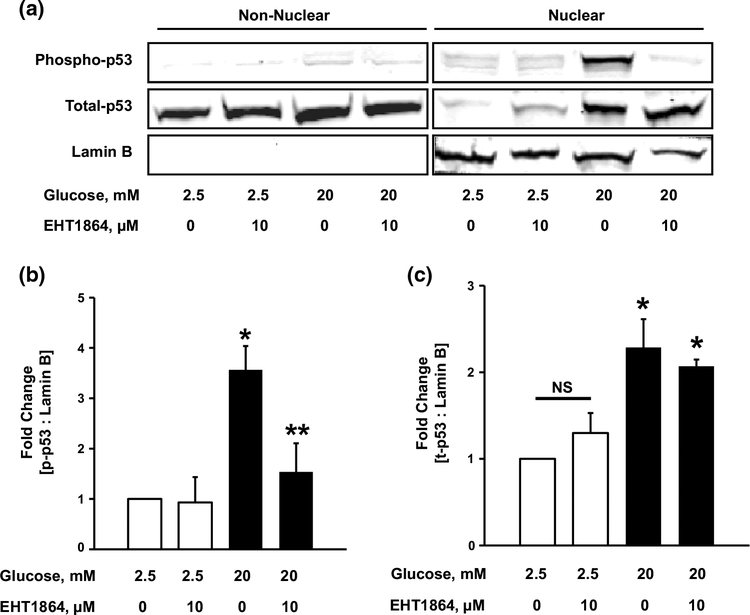
p53 has been implicated in several cellular functions both in the nuclear and non-nuclear compartments [38]. However, accumulation in the nuclear fraction is critical for p53 stabilization and optimal transcriptional activation of apoptotic genes [39, 40]. To further elucidate the mechanisms underlying HG-mediated regulation of p53, we questioned if p53 undergoes translocation to the nuclear fraction under HG conditions. To accomplish this, p53 localization in nuclear fractions from INS-1 832/13 cells exposed to LG or HG was determined. The purity of the nuclear fractions was assessed by the enrichment of Lamin B. As depicted in Fig. 5 (Fig. 5a), under HG conditions, we observed significant translocation of total p53 in the nuclear fraction in cells exposed to HG conditions. Furthermore, in line with our observations in Fig. 3, inhibition of Rac1 activation (with EHT1864) markedly attenuated HG-induced p53 phosphorylation (Fig. 5a, b). However, inhibition of Rac1 had no effect on total p53 in the nuclear fraction (Fig. 5a, c) indicating nuclear translocation of p53 independent of Rac1-sensitive phosphorylation of p53 at Serine-15. Taken together, these observations indicate that exposure of INS-1 832/13 cells to HG conditions leads to accumulation of p53 in the nuclear fraction, and that phosphorylation of p53 is mediated via active (i.e., GTP-bound) Rac1.
Fig. 5.
Exposure to HG induces nuclear translocation of total p53. Nuclear and non-nuclear fractions were isolated from INS-1 832/13 cells (a) incubated with LG or HG in the absence or presence of EHT1864. Lysates were analyzed for total and phosphop-53. The purity of the nuclear fractions was verified by probing for Lamin B. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over Lamin B (b n = 3). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone. Total-p53 band intensities were quantified by densitometry and ratios were calculated over Lamin B (c n = 3). *p < 0.05 vs. 2.5 mM glucose alone; NS not significant
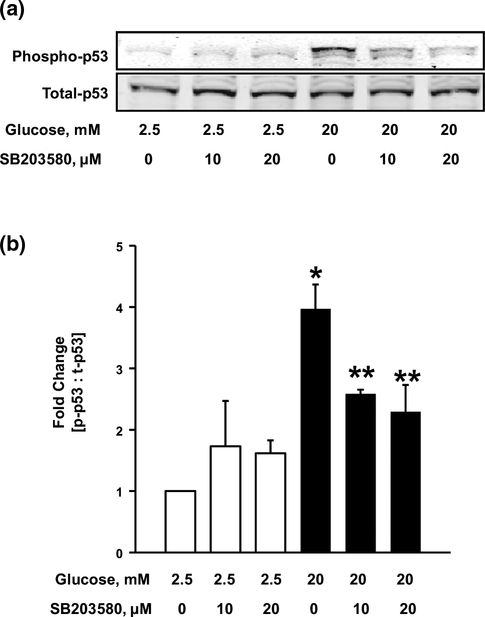
Inhibition of p38MAPK attenuates HG-induced p53 phosphorylation in INS-1 832/13 cells
Published evidence suggests the involvement of p38MAPK in the activation of p53 by phosphorylation in response to stress stimuli [21–23]. In this context, we recently reported activation of p38MAPK in INS-1 832/13 cells, rodent islets [20] and human islets (unpublished) exposed to HG conditions. Therein, we also demonstrated a regulatory role for Rac1 in HG-induced p38MAPK activation. Therefore, as a logical extension to our current findings, we asked if p38MAPK activation is upstream to p53 activation under HG exposure conditions. To address this, we quantified HG-induced p53 phosphorylation in the absence or presence of SB203580, a specific inhibitor of p38MAPK activity [41]. As shown in Fig. 6 (Fig. 6a), SB203580 significantly reduced HG-induced p53 phosphorylation at both 10 and 20 μM, indicating the involvement of p38MAPK in the activation of p53. Based on these data (Fig. 6a, b) we conclude that Rac1-p38MAPK module might be upstream to p53 activation in pancreatic β-cells exposed to HG conditions.
Fig. 6.
SB203580, a specific inhibitor of p38MAPK, attenuates HG-induced p53 phosphorylation. INS-1 832/13 cells (a) were incubated with LG (2.5 mM) or HG (20 mM) in the absence or presence of SB203580 for 24 h. Cell lysates were analyzed for total and phospho-p53. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 (b n = 4). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone
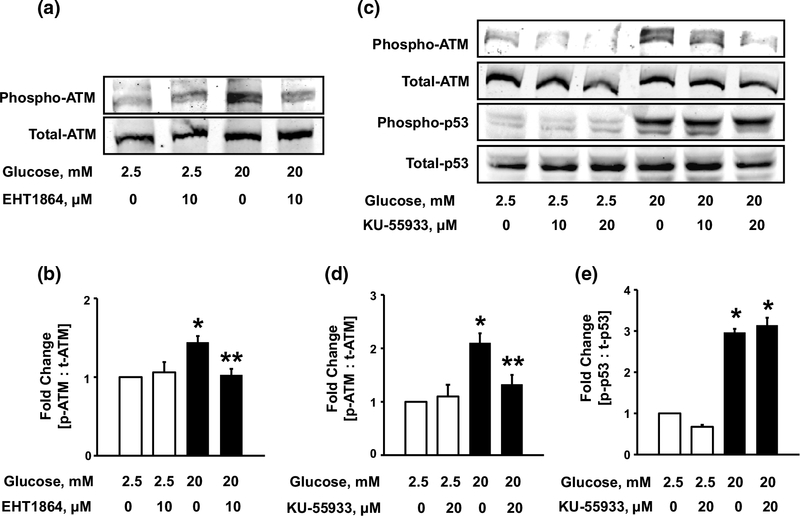
Rac1 mediates HG-induced ATM kinase activation in pancreatic β-cells
Earlier studies have demonstrated that ATM kinase is activated by auto-phosphorylation at serine-1981 residue [42]. Studies by Yoshida et al. have also implicated the role of ATM kinase in the activation of p53 resulting in cardiotoxicity caused by doxorubicin [32]. We, therefore, asked if HG conditions promote activation of ATM kinase, and if so, whether such a regulatory step involves the intermediacy of Rac1. Data depicted in Fig. 7 (Fig. 7a, b), demonstrate significant increase in ATM kinase phosphorylation under HG conditions, which was blocked by EHT1864, an inhibitor of Rac1 (above). These findings demonstrate Rac1-dependent activation of ATM kinase under HG conditions.
Fig. 7.
Glucotoxic conditions induce Rac1-dependent phosphorylation of ATM kinase, which is independent of p53 activation. INS-1 832/13 cells (a) were incubated with LG (2.5 mM) or HG (20 mM) in the absence or presence of EHT1864 for 24 h. Lysate proteins were probed for total and phospho-ATM kinase. Phospho-ATM band intensities were quantified by densitometry and ratios were calculated over total-ATM (b n = 4). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone. INS-1 832/13 cells (c) were incubated with LG (2.5 mM) or HG (20 mM) in the absence or presence of KU-55933 for 24 h. Cell lysates were analyzed for total and phosphorylated ATM and p53. Phospho-ATM band intensities were quantified by densitometry and ratios were calculated over total-ATM (d n = 3). *p < 0.05 vs. 2.5 mM glucose alone, **p < 0.05 vs. 20 mM glucose alone. Phospho-p53 band intensities were quantified by densitometry and ratios were calculated over total-p53 (e n = 3). *p < 0.05 vs. 2.5 mM glucose alone
HG-induced ATM kinase activation is not upstream to p53 activation
Recent pharmacological (KU55933) evidence implicates ATM kinase in the activation of p53 in human tumor cell lines exposed to ionizing radiations [31]. To determine if ATM kinase mediates activation of p53 in INS-1 832/13 cells exposed to HG conditions, we quantified HG-induced phosphorylation of ATM kinase and p53 following co-provision of either diluent or KU55933 (20 μmol/l). Data depicted in Fig. 7 (Fig. 7c, d) demonstrate significant increase in the auto-phosphorylation of ATM kinase under HG conditions, which was markedly suppressed by KU55933 at 20 μM. Interestingly, however, we observed no significant effect of KU55933 on HG-induced p53 phosphorylation (Fig. 7c, e). Not shown here are our data to indicate no effect of KU55993 on HG-induced phosphorylation of p53 in normal rat pancreatic islets exposed to HG conditions (n = 2). Based on these findings, we conclude that the signaling step of HG-induced p53 phosphorylation is independent of ATM kinase, and that p53 and ATM kinase, although regulated by Rac1, might be involved in the regulation of independent downstream pathways.
Discussion
The overall objective of our study was to investigate cellular mechanisms underlying dysfunction of the pancreatic β-cell following exposure to chronic hyperglycemic conditions. We report that: (a) HG conditions promote activation of p53 in INS-1 832/13 cells, normal rodent islets and human islets; (b) p53 phosphorylation is significantly higher in islets derived from the ZDF rat, a model for T2DM, compared to their counterparts from non-diabetic ZLC rats; (c) HG-induced phosphorylation of p53 is mediated by geranylgeranylated Rac1; (d) HG conditions promote nuclear translocation and accumulation of p53, and that phosphorylation, but not translocation of p53, is sensitive to activation of Rac1; (e) HG-induced phosphorylation and activation of p53 is downstream to HG-induced Rac1-p38MAPK module; (f) HG conditions also promote activation of ATM kinase, a DNA repair enzyme, in a Rac1-dependent manner; (g) p53 activation does not require the intermediacy of ATM kinase; and (h) pharmacological inhibition of Rac1 attenuates cell death induced by HG conditions.
It is well established that exposure to HG results in β-cell dysfunction in diabetes [42, 43]. Using an in vitro model, we have demonstrated significant reduction in GSIS [20] and accelerated cell death in INS-1 832/13 cells (Fig. 1) exposed to HG. Furthermore, using this model system, we have recently demonstrated key regulatory roles for Nox2 in the excessive generation of ROS under HG conditions, resulting in mitochondrial (caspase activation) and nuclear (lamin degradation) dysregulation and loss of islet β-cell function [20]. We were able to replicate these findings in normal rodent islets and human islets and islets derived from ZDF rat and T2DM human donors [18]. Findings from the current study provided additional insights into the signaling pathways that control cell demise under HG and diabetic conditions. For example, we have demonstrated that HG-induced activation of p53 is mediated via Rac1-p38MAPK pathway [20]. In this context we have reported that specific inhibition of Nox2 with gp91-ds-tat, a specific inhibitor of Nox2, but not its inactive analog, significantly attenuated HG-induced Nox2 activation, ROS generation and p38MAPK activation, thus suggesting that Nox2 activation couples with p38MAPK activation. We also reported that pharmacological inhibition of Rac1 (EHT 1864, NSC23766 and Ehop-016) markedly suppressed HG-induced p38MAPK activation in isolated β-cells. Based on these data, we proposed key roles for Rac1 in promoting the Nox2-p38MAPK signaling axis in the β-cell under the duress of HG. The current studies identify p53 as one of the downstream signaling steps to Rac1-p38MAPK activation in the sequence of events leading to HG-induced dysfunction of the islet. Our findings are further supported by a recent report by Vitale and associates that demonstrated phosphorylation of p53 by p38MAPK, specifically p38MAPKα isoform, in the cascade of events leading to activation of p53 in tetraploid cancer cells consequential to depletion of checkpoint kinase 1 [44]. siRNA-p38MAPKα or SB203580 significantly attenuated phosphorylation of p53 (at serines 15 and 46) induced by Chk1 knockdown. Therefore, it is likely that HG-induced Rac1-p38MAPK-module mediates phosphorylation of p53 (at serine 15) in the islet β-cells. Additional studies, including siRNA-p38MAPK may be needed to further validate this postulation.
We also observed that HG conditions significantly augmented the auto-phosphorylation of ATM kinase in our model system in a Rac1-dependent fashion. Interestingly, however, in contrast to several reports [28, 31–33], our findings appear to support ATM kinase-independent activation of p53 in pancreatic β-cells under the duress of HG conditions since KU-55933, a known inhibitor of ATM kinase, failed to inhibit HG-induced p53 activation (Fig. 7e). Together, these findings implicate independent signaling mechanisms might underlie HG-mediated dysfunction of the islets. Further, as we proposed earlier, it may be beneficial to target Rac1 or factors that regulate its activation (GEFs and GAPs) as a means to suppress the activation of pro-apoptotic kinases/factors, including Nox2, p38MAPK, p53 and ATM kinase [7].
Based on available evidence, it seems likely that an increase in intracellular oxidative stress could contribute to the activation of stress kinases including p38MAPK [20], p53 and ATM kinase (current study) and JNK1/2 [18]. Such a hypothesis would be worth validating since several extant studies have demonstrated relatively low anti-oxidant capacity of the islet β-cell [45] and any alterations or imbalance in the anti-oxidant capacity at the height of increased activation of Nox2-like enzymes would create more oxidative environment leading to activation of stress kinases and other pro-apoptotic proteins, culminating in cell dysfunction. Indeed, our recent observations of marked suppression of HG-induced p38MAPK by gp91-ds-tat, a specific inhibitor of Nox2, but not its inactive analog, affords support to such a hypothesis. Recent investigations by Kim and associates have demonstrated significant protection of human mesenchymal stem cells against oxidative stress induced apoptosis [46]. These investigators were able to demonstrate significant restoration of H2O2-induced cell dysfunction following pretreatment with lycopene, a known antioxidant. Lycopene pretreatment not only attenuated increased levels of ROS and activation of p38MAPK, JNK1/2, ATM kinase and p53, but also increased the expression of manganese-dependent superoxide dismutase, an anti-oxidant enzyme in these cells under conditions of increased oxidative stress induced by H2O2.
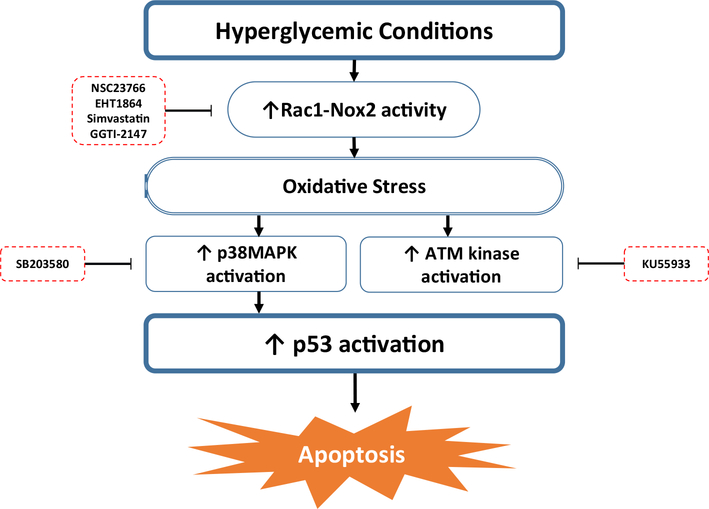
In conclusion, we present evidence demonstrating the role of Rac1-Nox2 in HG-induced activation of apoptotic p38MAPK-p53 signaling axis, resulting in pancreatic β-cell dysfunction. We propose (Fig. 8) that HG conditions promote activation of p53 in INS-1 832/13 β-cells, rodent and human islets. Inhibition of Rac1 results in inhibition of HG-induced phosphorylation of p53 and ATM kinase, demonstrating that activation of Rac1 or p38MAPK are upstream to HG-induced p53 phosphorylation. Our studies have also indicated that HG-induced ATM kinase activation is not requisite for p53 activation. Based on these observations, we propose that exposure of pancreatic β-cells to glucotoxic conditions causes activation of Rac1-Nox2-p38MAPK-p53 signaling cascade, leading to activation of downstream pro-apoptotic target proteins resulting in islet β-cell death.
Fig. 8.
Proposed model for the activation of p53 in pancreatic β-cells exposed to glucotoxic conditions. Based on our findings accrued in our study, we propose that exposure to glucotoxic conditions leads to activation of Rac1-Nox2-p38MAPK signaling cascade, which promotes activation of p53 and its downstream pro-apoptotic target proteins resulting in islet β-cell death
Acknowledgements
This work was supported by grants to A.K from the Department of Veterans Affairs (Merit Review Program) and National Institutes of Health, and a Research Stimulation Award from Wayne State University. A.K is the recipient of a Senior Research Career Scientist Award from the Department of Veterans Affairs (13S-RCS-006). VS received a Pre-Doctoral Fellowship from Graduate School at Wayne State University. The authors thank Drs. Anil K. Chekuri and Anil Poudel for contributions and support throughout these studies.
Abbreviations
- ATM
kinase Ataxia telangiectasia mutated kinase
- GAPs
GTPase-activating proteins
- GEFs
Guanine nucleotide exchange factors
- GTP
Guanosine triphosphate
- GGTI
Geranylgeranyl transferase inhibitor
- GSIS
Glucose-stimulated insulin secretion
- HG
High glucose
- LG
Low glucose
- Nox2
NADPH oxidase 2
- p38MAPK
p38 mitogen activated protein kinase
- Rac1
Ras-related C3 botulinum toxin substrate 1
- ROS
Reactive oxygen species
- T2DM
Type 2 diabetes mellitus
- ZDF
Zucker diabetic fatty rat
- ZLC
Zucker lean control
Footnotes
Conflict of interest None.
References
- 1.Hedeskov CJ (1980) Mechanism of glucose-induced insulin secretion. Physiol Rev 60(2):442–509 [DOI] [PubMed] [Google Scholar]
- 2.Komatsu M, Takei M, Ishii H, Sato Y (2013) Glucose-stimulated insulin secretion: a newer perspective. J Diabetes Investig 4(6):511–516. doi: 10.1111/jdi.12094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara S, Shibutani Y, Teruyama K, Inoue HY, Kawada Y, Etoh H, Matsuda T, Kimura-Koyanagi M, Hashimoto N, Sakahara M, Fujimoto W, Takahashi H, Ueda S, Hosooka T, Satoh T, Inoue H, Matsumoto M, Aiba A, Kasuga M, Kido Y (2013) Ras-related C3 botulinum toxin substrate 1 (RAC1) regulates glucose-stimulated insulin secretion via modulation of F-actin. Diabetologia 56(5):1088–1097. doi: 10.1007/s00125-013-2849-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowluru A (2010) Small G proteins in islet beta-cell function. Endocr Rev 31(1):52–78. doi: 10.1210/er.2009-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Thurmond DC (2009) Mechanisms of biphasic insulin-granule exocytosis: roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci 122(Pt 7):893–903. doi: 10.1242/jcs.034355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayaram B, Syed I, Kyathanahalli CN, Rhodes CJ, Kowluru A (2011) Arf nucleotide binding site opener [ARNO] promotes sequential activation of Arf6, Cdc42 and Rac1 and insulin secretion in INS 832/13 beta-cells and rat islets. Biochem Pharmacol 81(8):1016–1027. doi: 10.1016/j.bcp.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kowluru A (2011) Friendly, and not so friendly, roles of Rac1 in islet beta-cell function: lessons learnt from pharmacological and molecular biological approaches. Biochem Pharmacol 81(8):965–975. doi: 10.1016/j.bcp.2011.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowluru A, Kowluru RA (2015) Protein prenylation in islet beta-cell function in health and diabetes: putting the pieces of the puzzle together. Biochem Pharmacol 98(3):363–370. doi: 10.1016/j.bcp.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sidarala V, Veluthakal R, Syeda K, Kowluru A (2015) EHT 1864, a small molecule inhibitor of Ras-related C3 botulinum toxin substrate 1 (Rac1), attenuates glucose-stimulated insulin secretion in pancreatic beta-cells. Cell Signal 27(6):1159–1167. doi: 10.1016/j.cellsig.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veluthakal R, Madathilparambil SV, McDonald P, Olson LK, Kowluru A (2009) Regulatory roles for Tiam1, a guanine nucleotide exchange factor for Rac1, in glucose-stimulated insulin secretion in pancreatic beta-cells. Biochem Pharmacol 77(1):101–113. doi: 10.1016/j.bcp.2008.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veluthakal R, Tunduguru R, Arora DK, Sidarala V, Syeda K, Vlaar CP, Thurmond DC, Kowluru A (2015) VAV2, a guanine nucleotide exchange factor for Rac1, regulates glucose-stimulated insulin secretion in pancreatic beta cells. Diabetologia. doi: 10.1007/s00125-015-3707-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowluru A, Kowluru RA (2014) Phagocyte-like NADPH oxidase [Nox2] in cellular dysfunction in models of glucolipotoxicity and diabetes. Biochem Pharmacol 88(3):275–283. doi: 10.1016/j.bcp.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pick E (2014) Role of the Rho GTPase Rac in the activation of the phagocyte NADPH oxidase: outsourcing a key task. Small GTPases 5:e27952. doi: 10.4161/sgtp.27952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pi J, Collins S (2010) Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab 12(Suppl 2):141–148. doi: 10.1111/j.1463-1326.2010.01269.x [DOI] [PubMed] [Google Scholar]
- 15.Morgan D, Rebelato E, Abdulkader F, Graciano MF, Oliveira-Emilio HR, Hirata AE, Rocha MS, Bordin S, Curi R, Carpinelli AR (2009) Association of NAD(P)H oxidase with glucose-induced insulin secretion by pancreatic beta-cells. Endocrinology 150(5):2197–2201. doi: 10.1210/en.2008-1149 [DOI] [PubMed] [Google Scholar]
- 16.Syed I, Kyathanahalli CN, Kowluru A (2011) Phagocyte-like NADPH oxidase generates ROS in INS 832/13 cells and rat islets: role of protein prenylation. Am J Physiol Regul Integr Comp Physiol 300(3):R756–R762. doi: 10.1152/ajpregu.00786.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newsholme P, Morgan D, Rebelato E, Oliveira-Emilio HC, Procopio J, Curi R, Carpinelli A (2009) Insights into the critical role of NADPH oxidase(s) in the normal and dysregulated pancreatic beta cell. Diabetologia 52(12):2489–2498. doi: 10.1007/s00125-009-1536-z [DOI] [PubMed] [Google Scholar]
- 18.Syed I, Kyathanahalli CN, Jayaram B, Govind S, Rhodes CJ, Kowluru RA, Kowluru A (2011) Increased phagocyte-like NADPH oxidase and ROS generation in type 2 diabetic ZDF rat and human islets: role of Rac1-JNK1/2 signaling pathway in mitochondrial dysregulation in the diabetic islet. Diabetes 60(11):2843–2852. doi: 10.2337/db11-0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subasinghe W, Syed I, Kowluru A (2011) Phagocyte-like NADPH oxidase promotes cytokine-induced mitochondrial dysfunction in pancreatic beta-cells: evidence for regulation by Rac1. Am J Physiol Regul Integr Comp Physiol 300(1):R12–R20. doi: 10.1152/ajpregu.00421.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sidarala V, Veluthakal R, Syeda K, Vlaar C, Newsholme P, Kowluru A (2015) Phagocyte-like NADPH oxidase (Nox2) promotes activation of p38MAPK in pancreatic beta-cells under glucotoxic conditions: evidence for a requisite role of Ras-related C3 botulinum toxin substrate 1 (Rac1). Biochem Pharmacol 95(4):301–310. doi: 10.1016/j.bcp.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulavin DV, Saito S, Hollander MC, Sakaguchi K, Anderson CW, Appella E, Fornace AJ Jr (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J 18(23):6845–6854. doi: 10.1093/emboj/18.23.6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karunakaran S, Saeed U, Mishra M, Valli RK, Joshi SD, Meka DP, Seth P, Ravindranath V (2008) Selective activation of p38 mitogen-activated protein kinase in dopaminergic neurons of substantia nigra leads to nuclear translocation of p53 in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice. J Neurosci 28(47):12500–12509. doi: 10.1523/JNEUROSCI.4511-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Prieto R, Rojas JM, Taya Y, Gutkind JS (2000) A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res 60(9):2464–2472 [PubMed] [Google Scholar]
- 24.Joerger AC, Fersht AR (2008) Structural biology of the tumor suppressor p53. Annu Rev Biochem 77:557–582. doi: 10.1146/annurev.biochem.77.060806.091238 [DOI] [PubMed] [Google Scholar]
- 25.Joerger AC, Fersht AR (2010) The tumor suppressor p53: from structures to drug discovery. Cold Spring Harb Perspect Biol 2(6):a000919. doi: 10.1101/cshperspect.a000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Zhang L (2005) The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331(3):851–858. doi: 10.1016/j.bbrc.2005.03.189 [DOI] [PubMed] [Google Scholar]
- 27.Liu D, Xu Y (2011) p53, oxidative stress, and aging. Antioxid Redox Signal 15(6):1669–1678. doi: 10.1089/ars.2010.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bode AM, Dong Z (2004) Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer 4(10):793–805. doi: 10.1038/nrc1455 [DOI] [PubMed] [Google Scholar]
- 29.Lambert PF, Kashanchi F, Radonovich MF, Shiekhattar R, Brady JN (1998) Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem 273(49):33048–33053 [DOI] [PubMed] [Google Scholar]
- 30.Loughery J, Cox M, Smith LM, Meek DW (2014) Critical role for p53-serine 15 phosphorylation in stimulating transactivation at p53-responsive promoters. Nucleic Acids Res 42(12):7666–7680. doi: 10.1093/nar/gku501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hickson I, Zhao Y, Richardson CJ, Green SJ, Martin NM, Orr AI, Reaper PM, Jackson SP, Curtin NJ, Smith GC (2004) Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res 64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727 [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Shiojima I, Ikeda H, Komuro I (2009) Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol 47(5):698–705. doi: 10.1016/j.yjmcc.2009.07.024 [DOI] [PubMed] [Google Scholar]
- 33.Oleson BJ, Broniowska KA, Schreiber KH, Tarakanova VL, Corbett JA (2014) Nitric oxide induces ataxia telangiectasia mutated (ATM) protein-dependent gammaH2AX protein formation in pancreatic beta cells. J Biol Chem 289(16):11454–11464. doi: 10.1074/jbc.M113.531228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syed I, Jayaram B, Subasinghe W, Kowluru A (2010) Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol 80(6):874–883. doi: 10.1016/j.bcp.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veluthakal R, Sidarala V, Kowluru A (2016) NSC23766, a known inhibitor of Tiam1-Rac1 signaling module, prevents the onset of Type 1 diabetes in the NOD mouse model. Cell Physiol Biochem 39(2):760–767. doi: 10.1159/000445666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S, Kim ES, Moon A (2009) Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol Rep 21(5):1317–1322 [DOI] [PubMed] [Google Scholar]
- 37.Veluthakal R, Kaur H, Goalstone M, Kowluru A (2007) Dominant-negative alpha-subunit of farnesyl- and geranyltransferase inhibits glucose-stimulated, but not KCl-stimulated, insulin secretion in INS 832/13 cells. Diabetes 56(1):204–210. doi: 10.2337/db06-0668 [DOI] [PubMed] [Google Scholar]
- 38.Green DR, Kroemer G (2009) Cytoplasmic functions of the tumour suppressor p53. Nature 458(7242):1127–1130. doi: 10.1038/nature07986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang SH, Clarke MF (1999) The nuclear import of p53 is determined by the presence of a basic domain and its relative position to the nuclear localization signal. Oncogene 18(12):2163–2166. doi: 10.1038/sj.onc.1202350 [DOI] [PubMed] [Google Scholar]
- 40.O’Brate A, Giannakakou P (2003) The importance of p53 location: nuclear or cytoplasmic zip code? Drug Resist Updat 6(6):313–322 [DOI] [PubMed] [Google Scholar]
- 41.Young PR, McLaughlin MM, Kumar S, Kassis S, Doyle ML, McNulty D, Gallagher TF, Fisher S, McDonnell PC, Carr SA, Huddleston MJ, Seibel G, Porter TG, Livi GP, Adams JL, Lee JC (1997) Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem 272(18):12116–12121 [DOI] [PubMed] [Google Scholar]
- 42.Bakkenist CJ, Kastan MB (2003) DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421(6922):499–506. doi: 10.1038/nature01368 [DOI] [PubMed] [Google Scholar]
- 43.Poitout V, Robertson RP (2008) Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29(3):351–366. doi: 10.1210/er.2007-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale I, Senovilla L, Galluzzi L, Criollo A, Vivet S, Castedo M, Kroemer G (2008) Chk1 inhibition activates p53 through p38 MAPK in tetraploid cancer cells. Cell Cycle 7(13):1956–1961. doi: 10.4161/cc.7.13.6073 [DOI] [PubMed] [Google Scholar]
- 45.Lenzen S, Drinkgern J, Tiedge M (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20(3):463–466 [DOI] [PubMed] [Google Scholar]
- 46.Kim JY, Lee JS, Han YS, Lee JH, Bae I, Yoon YM, Kwon SM, Lee SH (2015) Pretreatment with lycopene attenuates oxidative stress-induced apoptosis in human mesenchymal stem cells. Biomol Ther (Seoul) 23(6):517–524. doi: 10.4062/biomolther.2015.085 [DOI] [PMC free article] [PubMed] [Google Scholar]