Abstract
Aim:
Weight gain appears to accelerate age-related ventricular-arterial stiffening, which has been implicated in the development of heart failure (HF), but it is unclear whether body fat accumulation underpins this association. We evaluated the relationship of adiposity, using measures of body composition, with ventricular-arterial stiffness among elderly in the community.
Methods and Results:
Adiposity was accessed through body mass index (BMI), waist circumference, and body fat percentage. We studied the association of these measures with carotid-femoral pulse wave velocity (cfPWV), arterial elastance index (EaI), left ventricle (LV) end-systolic elastance index (EesI) and LV end-diastolic elastance index (EedI) in 5520 community-based, elderly Atherosclerosis Risk in Communities (ARIC) Study participants, who underwent echocardiography between 2011 and 2013. BMI and waist circumference were directly associated with EaI, EedI and EesI even after adjusting for age, sex, race, hypertension, diabetes mellitus, heart rate, prevalent coronary heart disease and HF. After further adjustment for BMI, body fat percentage demonstrated significant independent linear relationships with EaI [Standardized beta coefficient (β)=0.17, p<0.001], EesI (β=0.08, p=0.003) and EedI (β=0.20, p<0.001), and significant non-linear relationships with cfPWV (p=0.033).
Conclusion:
In this biracial community-based cohort, increased adiposity was associated with increased ventricular-arterial stiffness among elderly and suggests a potential mechanism by which obesity might contribute to the development of heart failure.
Keywords: Obesity, Adiposity, Arterial Stiffness, Ventricular Stiffness, Aging
INTRODUCTION
Obesity independently increases the risk of heart failure (HF)1. Although obese individuals have higher prevalence of cardiovascular risk factors, including hypertension, diabetes mellitus and dyslipidemia, these factors cannot fully explain why they are at higher risk of developing HF. In fact, obese individuals without criteria for metabolic syndrome, recently called metabolic healthy, do not display increased risk of myocardial infarction (MI), but still have higher incidence of HF, compared with normal-weight metabolic healthy individuals.2, 3 Thus far, mechanisms linking obesity to increased risk of HF remain incompletely understood.
Left ventricular (LV) and arterial stiffness have been implicated in the pathophysiology of HF, particularly HF with preserved ejection fraction (HFPEF).4 Ventricular and arterial stiffness increase with age, causing augmented blood pressure sensitivity to volume shifts and higher myocardial oxygen consumption for a given stroke volume, which increases the predisposition to develop HF.5–8 Patients with HFPEF display increased ventricular stiffness, and they are more likely to be women and more obese than those with HF with reduced ejection fraction.8, 9 In parallel, LV stiffness increases with weight gain, and central obesity is associated with increased age-related LV stiffening among women.10 However, it is uncertain if the association between obesity and ventricular stiffness still holds in the elderly, among whom obesity has been suggested to be protective. Moreover, this association has not been previously evaluated using measures of body fat percentage, such as bioelectrical impedance. Aging is accompanied by changes in body composition and reduction in body height, limiting the use of some anthropometric measures, as body mass index (BMI), to estimate the degree of adiposity in this population.11
Therefore, we assessed the association of obesity, using both anthropometric measures and body impedance, with measures of LV and arterial stiffness among elderly. We hypothesized that increased adiposity would be associated with higher LV and arterial stiffness in a community-based study.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing, community-based cohort study. Detailed description of population characteristics, sampling, design, procedures and rationale have been previously published.12 Originally, 15,792 individuals from 45 to 64 years old were recruited in four communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD) between 1987 and 1989. Subsequently, the participants underwent to four follow-up visits: visit 2 during 1990–1992, visit 3 during 1993–1995, and visit 4 during 1996–1998, and the latest visit (visit 5) during 2011–2013. At each visit, the participants provided information on demographic variables and medical history to a trained interviewer, and a physical assessment and blood sample collection were performed. An echocardiogram was performed only at the time of Visit 5. From 10,740 participants who were alive at the moment of Visit 5 (aging from 66 to 90 years old), 6118 had an echocardiogram performed.
Race was self-reported by a questionnaire. Hypertension was defined as a systolic blood pressure (BP - average between 2nd and 3rd of 3 measures) ≥140 mmHg, diastolic BP (average between 2nd and 3rd of 3 measures) ≥90 mmHg or if taking anti-hypertensive medication in any of the ARIC visits. Participants were on use of anti-hypertensive medications at visit 5 if they were taking any BP lowering medication in the past 4 weeks. Diabetes mellitus was defined by self-reporting a physician diagnosis of diabetes, taking medication for diabetes, fasting blood glucose level ≥126 mg/dL, or non-fasting blood glucose level ≥ 200 mg/dL in any of the ARIC visits.
From the 6118 participants, we excluded those with missing required echocardiographic parameters to estimate arterial or ventricular elastance measures (n=244), missing weight or height (n=30), with moderate or severe aortic valve disease (stenosis or insufficiency, n = 62), atrial fibrillation during the echocardiography examination (n = 256) and those whose race was neither white nor black (n=6), resulting in 5520 individuals for the present analysis. Institutional review boards from each center approved the study protocol and all participants provided written informed consent.
Body composition, weight, height and waist circumference were measured by trained technicians according to standardized protocols. Body surface area (BSA) was estimated with the Du Bois method (BSA= 0.007184 × height in cm0.725 × weight in kg0.425).13 Body composition analysis was performed using the Tanita Body Composition Analyzer, TBF-300A (Tanita corporation), which uses bioelectrical impedance method to provide the body fat percentage. Body mass index (BMI) was calculated as weight in kilograms (Kg) divided by height in squared meters (m2).
We defined metabolic health based on the definition of metabolic syndrome, by the International Diabetes Federation, except for the criteria for waist circumference.14 Therefore, participants were defined as metabolically unhealthy if they had 2 or more of the following four criteria: BP ≥ 130/85 mm Hg or use of anti-hypertensive medication; fasting glucose ≥ 100 mg/dL or diagnosis of diabetes; triglycerides ≥ 150 mg/dL; and high-density lipoprotein cholesterol < 40 mg/dL for men or < 50 mg/dL for women. Participants with fewer than 2 criteria were considered metabolic healthy.
C- reactive protein (hs-CRP) blood levels were measured by the immunoturbidimetric CRP-Latex (II) high sensitivity assay (Denka Seiken - Tokyo, Japan), with a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis). We also estimated the total plasma volume noninvasively as previously described using the equation (1–hematocrit) × (a + [b×weight in Kg]), where a=1.530 in men and 864 in women, and b=41 in men and 47.9 in women.15
We calculated the homeostatic model assessment of insulin resistance (HOMA-IR) index using the equation: fasting glucose [mg/dl] × fasting insulin [uIU/ml])/405.16, 17
Echocardiography
All studies were obtained using a dedicated Philips IE33 Ultrasound system by trained sonographers according to a specific echocardiographic protocol.18 Measurements were performed according to American Society of Echocardiography (ASE) guidelines in a echocardiography core laboratory.19 Details about the design and protocol of the ARIC visit 5 echo study, including reproducibility data, have been previously published18.
LV dimensions and wall thickness were obtained from the parasternal long-axis view according to the recommendations of the ASE19. LV mass was calculated by the linear method and indexed to BSA and to height2.7, due to potential advantages over indexing to BSA in obese patients.20 LV outflow tract (LVOT) diameter was obtained from the parasternal long-axis view, and stroke volume (SV) was calculated by the product of LVOT area and LVOT velocity-time integral. Stroke volume index (SVI) was the SV indexed by BSA. LV volumes and the LV ejection fraction (LVEF) were assessed by the modified Simpson’s rule. Speckle tracking analysis was performed using TomTec Cardiac Performance Analysis package. Global longitudinal strain was obtained from apical 4-chamber and 2-chamber views.
Pulse wave Doppler of mitral inflow was used to measure peak E wave, and LV early diastolic relaxation velocity (e’) was determined by the average between peak septal and lateral mitral annular relaxation tissue Doppler velocities. End-diastolic pressure (EDP) was estimated as (EDP = 11.96 + 0.596 × E/e’) as previously described.21
Assessment of arterial and ventricular stiffness
Because measures of arterial and ventricular stiffness are correlated with body size, we indexed these measures to BSA. This approach provides similar results to allometric indexation, which has been shown to eliminate the relationships between measures of arterial load and body size in normal subjects.22
Arterial elastance index (EaI) was calculated as end-systolic pressure (ESP) divided by SVI.23 ESP was estimated by systolic BP at the time of echocardiographic examination multiplied by 0.9.24 Total arterial compliance index (TACI) was calculated as SVI divided by pulse pressure, and systemic vascular resistance index (SVRI) as mean arterial pressure multiplied by 80 and divided by cardiac index.25, 26
Carotid-femoral pulse wave velocity (cfPWV) was measured by technicians using an automated vascular screening device Omron VP-1000 plus system (Omron Healthcare, Kyoto, Japan), and following a standardized protocol. After 5 to 10 minutes in supine position, carotid and femoral arterial pressure waveforms were obtained for 30 seconds from participants using applanation tonometry sensors attached to the left common carotid artery and left common femoral artery.27 Distance for cfPWV was calculated as the straight distance between the carotid and femoral recording sites minus the straight distance between the suprasternal notch and the carotid recording site, using a customized segmometer for PWV measurements that can measure a straight distance even in obese individuals (Rosscraft, Surray, Canada).28, 29 This method provides the most accurate aortic length compared with the aortic length determined by magnetic resonance image.28 At least two measurements were obtained per participant and the last two measurements averaged. Quality assurance for cfPWV included central training and recertification, periodic equipment calibration, and ongoing quality control reviews by one of the authors (H.T.). The intra-class correlation coefficient for cfPWV was 0.70 (95% Confidence Interval 0.59, 0.81).27 Higher level of cfPWV, EaI and lower TACI indicate higher level of arterial stiffness.
Ventricular stiffness was assessed by LV end-systolic and end-diastolic elastance indexes. LV end-systolic elastance index (EesI) was determined by the single beat method, which uses LVEF, brachial BP, SVI, pre-ejection period, and total ejection period.24 The later two were obtained from pulse-wave Doppler of LVOT flow using the peak of the R wave as the fiducial point. LV end-diastolic elastance index (EedI) was estimated as EDP divided by end diastolic volume index (EDVI).10, 30 Higher levels of EesI and EedI indicate higher level of LV end-systolic and end-diastolic ventricular stiffness, respectively.
Statistical analysis
BMI categories were defined as: “normal” (lower than 25.0), overweight (25.0 to lower than 30.0) and obese (30.0 or higher). We compared the clinical and echocardiographic characteristics between BMI categories using analysis of variance (ANOVA) or Chi-squared test accordingly. To test for linear trends across BMI categories, we performed a Univariate Linear Regression and a Mantel-Haenszel Trend test for continuous and discrete variables, respectively. Pulse wave velocity was log-transformed due to skewness. Then, we performed linear regression analysis using two models to assess the associations of BMI, waist circumference and body fat with measures of ventricular-arterial stiffness. Model 1 was adjusted for age, sex and race, and model 2 also included hypertension, use of anti-hypertensive medications at visit 5, diabetes mellitus, heart rate and history of coronary heart disease (CHD) or HF. Models evaluating cfPWV were also adjusted for mean arterial pressure, calculated as (systolic BP + 2xdiastolic BP)/3. Comparisons of standardized regression coefficients were conducted by calculating the pairwise difference between regression parameter estimates over 500 bootstrap samples, and estimating confidence intervals for the difference via normal approximation.31 We then tested the association between body fat percentage and ventricular-arterial stiffness adjusting for model 2 variables and BMI. We assessed the linearity of each association between the obesity measure and stiffness measures by using adjusted restricted cubic spline models with the lowest Akaike information criterion. We assumed non-linear associations when there was a p-value < 0.05 for the test for non-linearity. Also, we performed a sensitivity analysis to address potential non-linear relationships between BMI and ventricular-arterial stiffness by further adjusting for BMI squared. (Supplemental Figure 1) Subsequently, we tested for effect modification by age categories (lower than 70, 70 to lower than 80 and 80 or higher), sex and race for the association between body fat percentage and ventricular-arterial stiffness adjusted for BMI and model 2 covariates using interaction terms with age categories, sex and race, respectively. We created interaction terms using all the restricted cubic spline variables, and the overall interaction test is conducted by likelihood ratio test comparing models with vs without the interaction terms. (Supplemental Figures 2 to 7). Additionally, a 4-level race/sex categorical variable was added to the models testing for effect modification by race and sex. To test the association between obesity and ventricular-arterial stiffness independently of metabolic features of the metabolic syndrome, we built an additional model with systolic and diastolic BP, fasting glucose blood levels, triglycerides and high-density lipoprotein cholesterol blood levels to model 2 variables (Model 3). Then, we restricted the analysis among metabolic healthy participants without history of CHD or HF and tested for the association between body fat percentage and elastance measures using linear regression model adjusted for age, sex, race and BMI. Finally, we added potential mediators based upon previously hypothesized mechanisms by which obesity might cause myocardial dysfunction, examining inflammation (hs-CRP blood levels), HOMA-IR, preload (total plasma volume), and concentric remodeling (LV mass index and relative wall thickness) as potential mediators for the association of body fat with ventricular stiffness. (Supplemental Tables 2 and 3) We used structural equation models to assess the direct and indirect effects of obesity measures, which allows for the estimation of the percent of the total effect that is mediated through these potential mediators. Analyses were performed using Stata version 14 (Stata Corp, College Station, TX). All statistics were two-sided and the level of significance was set as 0.05.
RESULTS
Study participants
The 5520 study participants were aged 66 to 90 years old, 59% women, 22% black and 34% obese, which reflects the prevalence of obesity in the United States among elderly in 2011–2012.32 Less than 1% of the participants were underweight, and 4% were severely obese (BMI over 40 Kg/m2). Characteristics of participants in each of these sub-categories are presented in Supplemental Table 1. Obese participants were more frequently blacks and were younger, as compared with those with normal weight and overweight (Table 1). Participants categorized as overweight were less likely to be women, as compared with obese and those with normal weight. Overall, body mass index was similar between sexes (28.7±6.1 vs 28.4±4.6 Kg/m2, p=0.12), but body fat percentage was higher among women, compared to men (39.1±7.3 vs 28.3±6.8%, p<0.001). BMI, waist circumference and body fat were all positively correlated with one another (r=0.45 to 0.81, Supplemental Figure 8). As expected, obesity was associated with higher prevalence of hypertension and diabetes mellitus. The prevalence of HF, but not CHD, was higher among obese individuals, compared with those with normal weight. During the echocardiogram, systolic BP was similar across BMI categories, but diastolic BP was progressively higher, with consequent lower pulse pressure among obese individuals.
Table 1:
Sample characteristics according to body mass index categories
| BMI categories | Below 25.0 | 25.0 to < 30.0 | 30.0 or higher | p | p |
|---|---|---|---|---|---|
| n=1458 | n=2204 | n=1858 | overall* | trend† | |
| Clinical characteristics | |||||
| Age, y | 76.6 ± 5.3 | 76.1 ± 5.1 | 74.8 ± 4.6 | < 0.001 | < 0.001 |
| Women, n(%) | 939 (64) | 1173 (53) | 1130 (61) | < 0.001 | 0.12 |
| Blacks, n(%) | 215 (15) | 445 (20) | 553 (30) | < 0.001 | < 0.001 |
| Weight, Kg | 62 ± 9 | 76 ± 10 | 94 ± 14 | < 0.001 | < 0.001 |
| BMI, Kg/m2 | 22.6 ± 1.9 | 27.4 ± 1.4 | 34.6 ± 4.5 | < 0.001 | < 0.001 |
| BSA, m2 | 1.68 ± 0.17 | 1.85 ± 0.17 | 2.01 ± 0.19 | < 0.001 | < 0.001 |
| Hypertension, n(%) | 1059 (73) | 1827 (83) | 1678 (90) | < 0.001 | < 0.001 |
| Diabetes, n(%) | 313 (21) | 789 (36) | 921 (50) | < 0.001 | < 0.001 |
| HF, n(%) | 33 (2) | 84 (4) | 106 (6) | < 0.001 | < 0.001 |
| CHD, n(%) | 179 (13) | 343 (16) | 235 (13) | 0.006 | 0.95 |
| Systolic BP, mmHg | 131 ± 19 | 130 ± 17 | 130 ± 18 | 0.75 | 0.45 |
| Diastolic BP, mmHg | 64 ± 11 | 66 ± 10 | 67 ± 10 | < 0.001 | < 0.001 |
| Pulse pressure, mmHg | 66 ± 15 | 64 ± 14 | 63 ± 15 | < 0.001 | < 0.001 |
| Anti-hypertensive meds, n(%) | 863 (59) | 630 (74) | 1569 (85) | < 0.001 | < 0.001 |
| EGFR, mL/min | 70.8 ± 16.3 | 69.3 ± 16.7 | 69.8 ± 18.3 | 0.047 | 0.13 |
| hs-CRP, mg/L | 3.1 ± 6.6 | 3.6 ± 6.8 | 5.1 ± 8.3 | < 0.001 | < 0.001 |
| HOMA-IR | 2.3 ± 4.3 | 3.8 ± 4.5 | 5.7 ± 5.1 | < 0.001 | < 0.001 |
| e-Plasma vol, mL | 2333 ± 288 | 2699 ± 289 | 3199 ± 423 | < 0.001 | < 0.001 |
| Cardiac structure and function | |||||
| LV mass/BSA, g/m2 | 75.3 ± 19.9 | 79.5 ± 19.9 | 81.8 ± 20.1 | < 0.001 | < 0.001 |
| LV mass/height2.7, g/m2.7 | 32.8 ± 9.0 | 37.2 ± 9.4 | 42.5 ± 10.9 | < 0.001 | < 0.001 |
| EDVI, mL/m2 | 44 ± 11 | 44 ± 12 | 44 ± 11 | 0.53 | 0.33 |
| Relative wall thickness | 0.419 ± 0.077 | 0.427 ± 0.075 | 0.431 ± 0.077 | < 0.001 | < 0.001 |
| LVEF, % | 65.7 ± 6.3 | 65.4 ± 6.6 | 65.3 ± 6.3 | 0.15 | 0.05 |
| Longitudinal strain | −18.27 ± 2.36 | −18.00 ± 2.46 | −17.83 ± 2.60 | < 0.001 | < 0.001 |
| Midwall fractional shortening, % | 17.1 ± 2.8 | 16.9 ± 2.8 | 16.8 ± 2.9 | 0.01 | 0.006 |
| SV, mL | 63 ± 14 | 67 ± 15 | 70 ± 15 | < 0.001 | < 0.001 |
| SVI, mL/m2 | 38 ± 8 | 37 ± 8 | 35 ± 7 | < 0.001 | < 0.001 |
| Heart Rate, bpm | 65 ± 10 | 65 ± 10 | 67 ± 11 | < 0.001 | < 0.001 |
| Cardiac index, L.min-1.m−2 | 2.42 ± 0.60 | 2.37 ± 0.58 | 2.30 ± 0.55 | < 0.001 | < 0.001 |
| e’, cm/s | 6.46 ± 1.55 | 6.22 ± 1.45 | 6.17 ± 1.42 | < 0.001 | < 0.001 |
| E/e’ ratio | 11.02 ± 3.98 | 11.20 ± 3.91 | 11.75 ± 3.97 | < 0.001 | < 0.001 |
| Ventricular-arterial coupling | |||||
| EaI, mmHg.mL-1.m2 | 3.26 ± 0.84 | 3.34 ± 0.82 | 3.51 ± 0.86 | < 0.001 | < 0.001 |
| TACI, mL.mmHg-1.m−2 | 0.591 ± 0.165 | 0.594 ± 0.164 | 0.578 ± 0.162 | 0.005 | 0.014 |
| cfPWV, cm/s | 1184 ± 341 | 1167 ± 376 | 1181 ± 429 | 0.39 | 0.85 |
| SVRI, dyne.s.cm−5 .m2 | 3022 ± 889 | 3129 ± 873 | 3234 ± 861 | < 0.001 | < 0.001 |
| EedI, mmHg.mL-1.m2 | 0.458 ± 0.124 | 0.463 ± 0.124 | 0.473 ± 0.127 | 0.002 | < 0.001 |
| EesI, mmHg.mL-1.m2 | 5.03 ± 1.38 | 5.18 ± 1.41 | 5.43 ± 1.45 | < 0.001 | < 0.001 |
| Ea/Ees ratio | 0.67 ± 0.18 | 0.67 ± 0.19 | 0.67 ± 0.17 | 0.93 | 0.79 |
Continuous data are presented as mean ± SD.
HF – heart failure, CHD – coronary heart disease, BP – blood pressure, EGFR – estimated glomerular filtration rate, Anti-hypertensive meds – use of anti-hypertensive medication at visit 5, hs-CRP – C-reactive protein blood levels, HOMA-IR – homeostatic model assessment of insulin resistance index, LVEF – left ventricle ejection fraction, SV – stroke volume (non-indexed); e-Plasma vol – estimated plasma volume, SVI – stroke volume index, LV – left ventricle, EDVI – end-diastolic volume index, EaI – Arterial elastance indexed by body surface area, TACI – total arterial compliance indexed by body surface area; cfPWV – carotid-femoral pulse wave velocity; EedI – left ventricle end-diastolic elastance indexed by body surface area, EesI - left ventricle end-systolic elastance indexed by body surface area
p overall: p values from Chi Square or ANOVA tests.
p trend: p values from Mantel-Haenszel trend test or univariate regression analysis using BMI categories as ordinal variable.
Obesity was associated with higher levels of inflammation and insulin resistance, as suggested by higher hs-CRP blood levels and HOMA-IR index in obese participants, compared to those with normal weight and overweight.(Table 1)
Cardiac structure and function
Obese participants presented higher LV mass, normalized by either BSA or height2.7, than those with normal weight, while end diastolic volume index was similar among BMI categories (Table 1). As a result, relative wall thickness, a measure of concentricity, was higher among obese individuals. Although LVEF was similar among BMI categories, systolic function was lower among obese participants, as reflected by lower absolute values of longitudinal strain and midwall fractional shortening. Cardiac index was also lower among obese participants, as a consequence of lower SVI, even though they displayed higher HR. Compared with other BMI categories, obese participants had worse diastolic function, as reflected by lower e’ and higher E/e’ ratio, indicating higher filling pressures.
Ventricular and arterial stiffness
Obese participants displayed higher EaI, lower TACI and higher SVRI. Although higher EaI may be related to higher HR, these results suggest both pulsatile and steady components of the arterial load may play a role on the higher EaI among obese participants (Table 1).
After adjusting for potential confounders, including age, sex, hypertension, diabetes mellitus, heart rate and history of CHD or HF, obesity was associated with high EaI, EedI and EesI. Except for BMI, which showed non-linear associations with measures of stiffness, all relationships did not significantly depart from linearity. BMI had significantly steeper relationships with ventricular-arterial stiffness for values of 30 Kg/m2 or below than for values above 30 kg/m2. These associations were stronger with body fat percentage than with BMI and waist circumference (Table 2). After further adjusting for BMI, increased body fat was associated with high arterial stiffness, as reflected by high EaI [Standardized beta coefficient (β)=0.17, p<0.001], and low TACI (β=−0.05, p=0.041), and with high ventricular stiffness, as reflected by high EesI (β=0.08, p=0.003) and EedI (β=0.20, p<0.001 - Figure 1). On the other hand, the associations between BMI and stiffness measures weakened when body fat was included in model.(Supplemental Table 4).
Table 2:
Association between measures of ventricular-arterial stiffness and individual measures of obesity adjusted for potential confounders
| Model 1 (n=5520) | Model 2 (n=5435) | Model 3 (n=5356) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Partial R2 | β ± SE | p | Partial R2 | β ± SE | p | Partial R2 | β ± SE | p | |||
| Pulse wave velocity | |||||||||||
| BMI (≤ 30 Kg/m2) † | −0.04 ± 0.03 | 0.028 | −0.12 ± 0.03 | < 0.001 | − 0.14 ± 0.03 | < 0.001 | |||||
| BMI (> 30 Kg/m2) | 0.07 ± 0.03 | 0.03 ± 0.03 | 0.04 ± 0.03 | ||||||||
| Waist circumference | 0.003 | 0.05 ± 0.01 | 0.001 | 0 | − 0.01 ± 0.01 | 0.50 | 0 | − 0.01 ± 0.02 | 0.68 | ||
| Body fat | 0.001 | 0.03 ± 0.02 | 0.078 | 0.001 | − 0.03 ± 0.02 | 0.059 | 0 | − 0.02 ± 0.02 | 0.20 | ||
| Arterial elastance index | |||||||||||
| BMI (≤ 30 Kg/m2) † | 0.14 ± 0.03 | < 0.001 | 0.12 ± 0.03 | < 0.001 | 0.06 ± 0.02 | 0.008 | |||||
| BMI (> 30 Kg/m2) | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.02 ± 0.02 | ||||||||
| Waist circumference | 0.012 | 0.11 ± 0.01 | < 0.001 | 0.008 | 0.09 ± 0.01 | < 0.001 | 0.004 | 0.06 ± 0.01 | < 0.001 | ||
| Body fat | 0.016 | 0.16 ± 0.02* | < 0.001 | 0.011 | 0.13 ± 0.02* | < 0.001 | 0.009 | 0.11 ± 0.02* | < 0.001 | ||
| Total arterial compliance index | |||||||||||
| BMI (≤ 30 Kg/m2) † | − 0.05 ± 0.03 | 0.013 | 0.00 ± 0.03 | 0.92 | − 0.07 ± 0.02 | 0.001 | |||||
| BMI (> 30 Kg/m2) | −0.03 ± 0.02 | −0.01 ± 0.02 | − 0.01 ± 0.02 | ||||||||
| Waist circumference | 0.004 | − 0.06 ± 0.01 | < 0.001 | 0.001 | − 0.03 ± 0.01 | 0.037 | 0.005 | − 0.06 ± 0.01 | <0.001 | ||
| Body fat | 0.003 | − 0.06 ± 0.02 | < 0.001 | 0.001 | − 0.03 ± 0.02 | 0.10 | 0.010 | − 0.11 ± 0.02* | <0.001 | ||
| End-diastolic elastance index | |||||||||||
| BMI (≤ 30 Kg/m2) † | 0.16 ± 0.02 | < 0.001 | 0.13 ± 0.02 | < 0.001 | 0.04 ± 0.06 | 0.30 | |||||
| BMI (> 30 Kg/m2) | 0.01 ± 0.02 | 0.00 ± 0.02 | − 0.01 ± 0.02 | ||||||||
| Waist circumference | 0.013 | 0.11 ± 0.01 | < 0.001 | 0.008 | 0.08 ± 0.01 | < 0.001 | 0.001 | 0.03 ± 0.01 | 0.012 | ||
| Body fat | 0.024 | 0.17 ± 0.02* | < 0.001 | 0.015 | 0.14 ± 0.02* | < 0.001 | 0.004 | 0.08 ± 0.02* | < 0.001 | ||
| End-systolic elastance index | |||||||||||
| BMI (≤ 30 Kg/m2) † | 0.15 ± 0.03 | < 0.001 | 0.12 ± 0.03 | < 0.001 | 0.13 ± 0.03 | < 0.001 | |||||
| BMI (> 30 Kg/m2) | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | ||||||||
| Waist circumference | 0.009 | 0.10 ± 0.01 | < 0.001 | 0.006 | 0.08 ± 0.01 | < 0.001 | 0.007 | 0.08 ± 0.01 | < 0.001 | ||
| Body fat | 0.011 | 0.13 ± 0.02* | < 0.001 | 0.007 | 0.11 ± 0.02 | < 0.001 | 0.011 | 0.13 ± 0.02 * | < 0.001 | ||
Beta coefficients represent 1 SD (Standard deviation) proportional change in the outcome for each 1 SD increase in adiposity. Pulse wave velocity SD = 3.8 m/s, Arterial elastance index SD = 0.84 mmHg.mL−1.m2, Total arterial compliance index SD = 0.164 mL.mmHg−1.m−2, End-diastolic elastance index SD = 0.125 mmHg.mL−1.m2, End-systolic elastance index SD = 1.43 mmHg.mL−1.m2, Body mass index SD = 5.5 Kg/m2, Waist circumference SD = 13.7 cm, Body fat SD = 8.9%.
BMI – body mass index,
Model 1: adjusted for age, sex and race
Model 2: adjusted for model 1 + hypertension, use of anti-hypertensive medications at visit 5, diabetes mellitus, heart rate and history of coronary heart disease or heart failure. For cfPWV, mean arterial pressure was also included.
Model 3: adjusted for all model 2 variables + systolic and diastolic blood pressure, fasting glucose blood levels, triglycerides and high-density lipoprotein cholesterol blood levels.
Beta coefficients for body fat significantly larger than for waist circumference, p<0.001
Because of non-linear relationships between body mass index (BMI) and measures of ventricular-arterial stiffness, beta coefficients are presented separately for BMI 30 Kg/m2 or below and for BMI above 30 kg/m2. The p values for these associations result from testing the null hypothesis that both beta coefficients are zero (i.e. if p value 0.05 or above, it means that there is no significant association between BMI and the respective measure of stiffness). For all other associations, the relationships did not significantly depart from linearity.
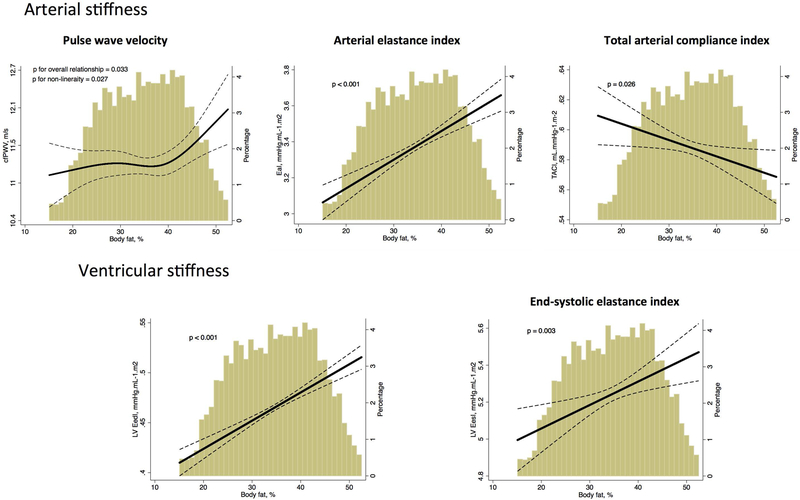
Figure 1: Association between body fat and ventricular-arterial stiffness, after adjusting for body mass index and other potential confounders in all participants.
cfPWV – carotid-femoral pulse wave velocity; EaI – Arterial elastance indexed by body surface area, TACI – total arterial compliance indexed by body surface area; LV EedI – left ventricle end-diastolic elastance indexed by body surface area, LV EesI - left ventricle end-systolic elastance indexed by body surface area
Adjusted plot for age, sex, race, body mass index, hypertension, use of anti-hypertensive medications at visit 5, diabetes mellitus, heart rate and prevalence of coronary heart disease or heart failure. For cfPWV, mean arterial pressure was included in the model.
Dashed lines show 95% prediction bands. The x-axis was truncated at the 1st and 99th percentiles of the empirical distribution of the exposure of interest. However, all data from the exposure of interest was used to fit the model. The truncation affected only the graphical display.
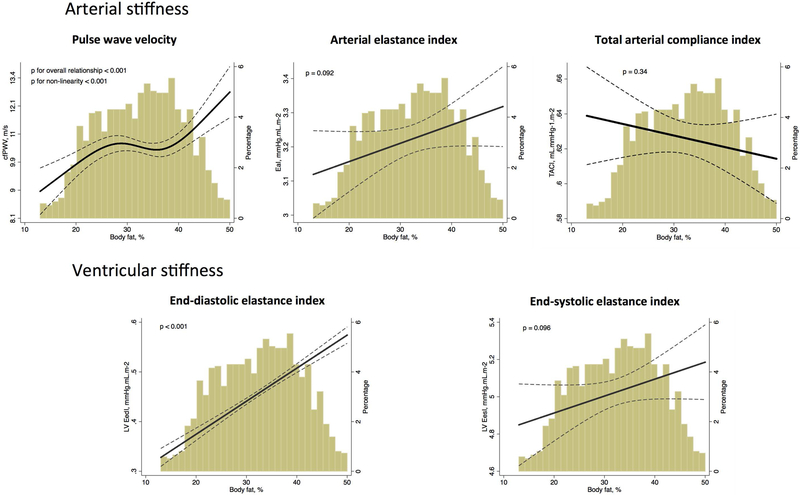
Body fat percentage showed significant non-linear relationships with cfPWV (Figure 1, p=0.033), where individuals with high body fat presented high cfPWV, indicating higher arterial stiffness. We observed a steep and direct relationship for body fat percentage above 40%, which is roughly the threshold for unhealthy obesity among women. Indeed, this association appeared to be stronger among women than men (p for interaction = 0.044; Supplemental Figure 4). Consistently, the association between increased body fat and high EaI were steeper among women (β=0.22, p<0.001) than among men (β=0.10, p=0.018, p for interaction = 0.044). For the associations with other ventricular and arterial stiffness measures, there was no effect modification by gender.(Supplemental Figures 4 and 5) Also, there was no effect modification by race (Supplemental Figures 6 and 7) or age categories (Supplemental Figure 2 and 3), suggesting that these associations persisted after 80 years of age. These associations remained similar when we further adjusted for BMI squared (Supplemental Figure 1). Moreover, these associations were consistent when we further adjusted for the metabolic features of the metabolic syndrome (Table 2, Model 3 column) and when we restricted the analysis to metabolic healthy participants without history of CHD or HF (Figure 2).
Figure 2: Association between body fat and ventricular-arterial stiffness, after adjusting for body mass index and other potential confounders among metabolic healthy participants.
cfPWV – carotid-femoral pulse wave velocity; EaI – Arterial elastance indexed by body surface area, TACI – total arterial compliance indexed by body surface area; LV EedI – left ventricle end-diastolic elastance indexed by body surface area, LV EesI - left ventricle end-systolic elastance indexed by body surface area
Adjusted plot for age, sex, race and body mass index. Participants with history of coronary heart disease or heart failure were excluded from this analysis. For cfPWV, mean arterial pressure was included in the model.
Dashed lines show 95% prediction bands. The x-axis was truncated at the 1st and 99th percentiles of the empirical distribution of the exposure of interest. However, all data from the exposure of interest was used to fit the model. The truncation affected only the graphical display.
Although body fat was directly associated with hs-CRP blood levels (Partial R2 = 0.002, p = 0.002) and with HOMA-IR index (Partial R2 = 0.011, p < 0.001), the effect estimates for the associations between adiposity and ventricular-arterial stiffness remained the same after including either hs-CRP blood levels or HOMA-IR in the models, suggesting that neither inflammation nor insulin resistance were important mediators for these associations. (Supplemental Table 2, Supplemental Figure 9 and 10)
Similarly, the associations between adiposity and ventricular stiffness did not change after adding plasma volume in the model. Although these associations attenuated when we included LV mass and relative wall thickness, the percent mediated by these potential mediators was small. (Supplemental Table 3)
DISCUSSION
The main finding of our study was that increased adiposity was associated with higher LV and arterial stiffness among elderly people in the community. The novel and important finding is that these relationships were observed with body fat percentage, assessed using an independent metric and device (bioelectrical impedance), without the limitations of BMI to estimate adiposity. The relationships of anthropometric measures with body composition may considerably change in the elderly, and the associations between adiposity and LV and arterial stiffness using these quantitative measurements of body fat have never been reported so far. These results were independent of the presence of cardiovascular risk factors, and they persisted among individuals with metabolic healthy obesity and among individuals over 80 years of age. Because ventricular and arterial stiffening have been implicated in the development of HF, especially HFpEF, these findings suggest that strategies to promote weight loss may help to prevent HF, even at advanced age.
Although arterial stiffness has historically been attributed to chronic systemic hypertension, data from the Framingham Offspring Study revealed that high arterial stiffness, as measured by cfPWV, actually precedes the development of hypertension.33 Arterial stiffness, in turn, plays an important role on the pathophysiology of HFpEF, as it was recently shown to be associated with abnormal hemodynamic responses to exercise in these patients.34 The association of body fat with arterial stiffness may, therefore, help explain why obese individuals have higher risk of developing HFpEF.35, 36 The association between increased body fat and high arterial stiffness has been found in other cross-sectional and longitudinal studies, in concordance with our findings.36, 37Although it has been suggested that metabolic and inflammatory processes are potential mechanisms by which increased adiposity leads to arterial stiffening, hs-CRP blood levels and HOMA-IR index do not appear to mediate this relationship in our study.36 Other mechanisms, such as those involving endothelial regulation, adipokines or unknown pathways, may also explain this association.37
Previous studies have shown the adverse effects of obesity on cardiac structure and function, and consistently indicate that obesity is associated with worse systolic and diastolic LV function. Abdominal obesity has been associated with subclinical LV systolic dysfunction, as defined by lower absolute values of longitudinal strain despite of normal LVEF, similar to what we found among obese individuals.38 Also, obese individuals display higher E/e’ ratio and lower e’, and higher prevalence of LV diastolic dysfunction, defined according to the ASE, compared with those with normal weight.19, 39, 40 These associations appears to be stronger for central obesity and visceral adiposity, and may involve multiple pathways, such as insulin resistance and increased oxidative stress leading to impaired myocardial relaxation, activation of the neuroendocrine and renin-angiotensin-aldosterone systems, chronic increase in preload and afterload leading to changes in LV mass and geometry, and cardiac lipotoxicity (see bellow).38, 39, 41–44 Moreover, Wohlfahrt et al demonstrated that weight gain was associated with significant increase in LV diastolic stiffness, and that central obesity was associated with increased age-related LV systolic stiffening, as reflected by LV end-systolic elastance.10 As subclinical systolic and diastolic LV dysfunction and ventricular-arterial stiffness are predecessors of HF, they underscore the clinical implications of the adverse effects of obesity.
Despite the strong evidence provided above, there has been little data available connecting ventricular-arterial stiffening with adiposity in the elderly. Indeed, it is well established in multiple chronic diseases that increased adiposity plays a protective role, a phenomenon termed the “obesity paradox”.11 The current study is the first to definitively show that the association between obesity and high LV stiffness still holds among the elderly, independent of other potentially confounding variables. These data provide strong support for the idea that body fat contributes importantly to arterial and LV stiffening across the lifetime. Body fat varies according to age, gender and race, making particularly important to account for body composition in this elderly population. Besides that, the aging-related compression of vertebral bodies and kyphosis makes BMI less accurate to estimate the degree of adiposity at advanced age. We found that body fat percentage was associated with LV and arterial stiffness, independently of BMI, which means that for any BMI value, an increased proportion of body fat is associated with higher ventricular-arterial stiffness.
Although fat mass elicits less increase in cardiac output than lean mass, obesity in general increases total blood volume, resulting in hemodynamic overload that can lead to LV dilation and hypertrophy.45 Coupled with a pathologic reduction in systemic vascular resistance, this is thought to contribute to the type of high output HF that develops among morbidly obese individuals.15 Although this is a plausible explanation for the association between obesity and high LV stiffness, our findings were not consistent with reduced vascular resistance among obese individuals and the effect estimates did not change after including plasma volume in the model (Supplemental table 3). On the other hand, these associations were partially attenuated after including LV mass and relative wall thickness, a measure of LV concentricity, in the model, suggesting that they are only partially explained by changes in LV geometry with obesity. Alternatively, obesity has been associated with excessive epicardial fat and fatty infiltration of the heart (lipotoxicity). Earlier reports have found in necropsy studies a substantial amount of adipose tissue in the heart of obese individuals, with the myocardial tissue infiltrated by adipocytes,46, 47 occasionally accompanied by a hemodynamic pattern of restrictive cardiomyopathy.48 This phenomenon, also called Adipositas Cordis, can help to explain the increased LV stiffness associated with obesity beyond to what can result from changes in cardiac geometry as a consequence of their characteristic hemodynamic overload. Besides, it was recently found that obese patients with HFPEF display increased epicardial fat thickness, compared to non-obese HFPEF patients and non-HFPEF non-obese controls, associated with greater pericardial restraint and ventricular interaction.49
Because the association between obesity and LV stiffness was independent from traditional cardiovascular risk factors, our results imply that obesity per se has cardiac repercussions that are involved in the development of HF. Such cardiac repercussions were also seen among individuals with metabolic healthy obesity, which can partially explain why this condition is associated with increased risk of HF but not MI.2 Moreover, LV stiffness was positively associated with measures of both central and general obesity among elderly, supporting the concept that there is no harmless pattern of increased weight.50 This underscores the importance of obesity treatment and, particularly, prevention to reduce the incidence of HF at advanced age.
Our study has limitations that deserve attention. First, this is a cross sectional study of individuals aging between 66 and 90 years, and the temporal sequence between obesity and ventricular-arterial stiffness cannot be established. Although reverse causality is unlikely, we cannot infer causal relationship between obesity and ventricular-arterial stiffness because this association may result from unmeasured/unknown confounders. Also, these results may have been influenced by survivor bias and not completely at random missing data – roughly 40% of alive participants did not attend visit 5. Nevertheless, the direction of the effect would likely remain unchanged if this bias had been removed, assuming that increased adiposity added to ventricular-arterial stiffness would shorten survival. Rather, survivor bias may have attenuated the true effect among women and explain why we did not find effect modification by gender for most of the relationships, in opposite to previous studies suggested.10 Although the magnitude of the relationships between body fat and stiffness measures were not strong, they were comparable to other factors known to influence ventricular-arterial stiffness such as age and heart rate (Supplemental Tables 5 to 11). Also, they are consistent with previous findings and biological plausible as discussed above. We used E/e’ to estimate LV EDP, which is the best single predictor of LV filling pressures, but it may be limited to estimate LV EDP in the individual patient if used alone.21 Also, hemodynamic parameters, including stroke volume, were measured by noninvasive methods, which have lower accuracy compared with invasive measurements, particularly among participants with severe obesity. On the other hand, these measures have been previously validated against gold-standard invasive methods and make large population-based studies feasible.24 Arterial and ventricular elastance measures are correlated with body size because of the relationships between arterial size, stroke volume and body size. We addressed this issue using allometric scaling, which was previously shown to eliminate the relationships between these measures and body size in normal subjects, allowing evaluating the abnormalities in arterial and ventricular stiffness associated with obesity, beyond to their expected relationship with body size.22, 51 The associations with body fat – an independent measure from BSA – persisted even after adjusting for BMI. Moreover, body fat percentage was related with cfPWV, which is measured independently of body size. Among individuals with similar BMI, those with high body fat percentage displayed high cfPWV, suggesting high arterial stiffness.
CONCLUSION
In this biracial community-based cohort, adiposity was associated with high ventricular and arterial stiffness. This association was stronger when adiposity was estimated by body impedance in this elderly population. This may help to explain why obesity, including metabolic healthy obesity, increases the risk HF. Further studies are needed to investigate whether strategies to promote weight loss can prevent the development of HF among individuals at advanced age.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the staff and participants of the ARIC study for their important contributions.
FUNDING
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported by NHLBI cooperative agreement NHLBI-HC-11–08[SDS], grants R00-HL-107642[SC] and K08-HL-116792[AMS]; American Heart Association grant 14CRP20380422[AMS]; grant from the Ellison Foundation[SC]; WN was supported by the Brazilian National Council for Scientific and Technological Development Grant 249481/2013–8; and MMF was supported by Lemann Foundation.
Footnotes
CONFLICTS OF INTEREST
None declared.
REFERENCES
- 1.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. The New England journal of medicine 2002;347(5):305–13. [DOI] [PubMed] [Google Scholar]
- 2.Morkedal B, Vatten LJ, Romundstad PR, Laugsand LE, Janszky I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. Journal of the American College of Cardiology 2014;63(11):1071–8. [DOI] [PubMed] [Google Scholar]
- 3.Stefan N, Fritsche A, Haring HU. Mechanisms explaining the relationship between metabolically healthy obesity and cardiovascular risk. Journal of the American College of Cardiology 2014;63(24):2748–9. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature reviews Cardiology 2014;11(9):507–15. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 2005;112(15):2254–62. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community-based study. Circulation Heart failure 2013;6(5):944–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. Journal of the American College of Cardiology 1998;32(5):1221–7. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 2003;107(5):714–20. [DOI] [PubMed] [Google Scholar]
- 9.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine 2006;355(3):251–9. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart failure 2014;2(5):489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorner TE, Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. International journal of cardiology 2012;155(1):56–65. [DOI] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129(4):687–702. [PubMed] [Google Scholar]
- 13.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989;5(5):303–11; [PubMed] [Google Scholar]
- 14.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr., International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, International Association for the Study of O. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 15.Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-Output Heart Failure: A 15-Year Experience. Journal of the American College of Cardiology 2016;68(5):473–82. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 17.Vardeny O, Gupta DK, Claggett B, Burke S, Shah A, Loehr L, Rasmussen-Torvik L, Selvin E, Chang PP, Aguilar D, Solomon SD. Insulin resistance and incident heart failure the ARIC study (Atherosclerosis Risk in Communities). JACC Heart failure 2013;1(6):531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circulation Cardiovascular imaging 2014;7(1):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picard MH, Adams D, Bierig SM, Dent JM, Douglas PS, Gillam LD, Keller AM, Malenka DJ, Masoudi FA, McCulloch M, Pellikka PA, Peters PJ, Stainback RF, Strachan GM, Zoghbi WA, American Society of E. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr 2011;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16(3):233–70. [DOI] [PubMed] [Google Scholar]
- 21.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 2000;102(15):1788–94. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 2009;54(3):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS, Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86(2):513–21. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. Journal of the American College of Cardiology 2001;38(7):2028–34. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. Journal of the American College of Cardiology 2013;61(1):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohlfahrt P, Redfield MM, Melenovsky V, Lopez-Jimenez F, Rodeheffer RJ, Borlaug BA. Impact of chronic changes in arterial compliance and resistance on left ventricular ageing in humans. European journal of heart failure 2015;17(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, Heiss G. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. American journal of hypertension 2016;29(1):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging 2008;1(6):739–48. [DOI] [PubMed] [Google Scholar]
- 29.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Carotid-Femoral Pulse Wave Velocity: Impact of Different Arterial Path Length Measurements. Artery Res 2010;4(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kass DA. Assessment of diastolic dysfunction. Invasive modalities. Cardiology clinics 2000;18(3):571–86. [DOI] [PubMed] [Google Scholar]
- 31.Mooney CZ, Duval RD, Duvall R. Bootstrapping: A Nonparametric Approach to Statistical Inference. 94–95 ed: SAGE; 1993. [Google Scholar]
- 32.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. Jama 2012;308(9):875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA. Arterial Stiffening With Exercise in Patients With Heart Failure and Preserved Ejection Fraction. Journal of the American College of Cardiology 2017;70(2):136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation 2007;115(20):2628–36. [DOI] [PubMed] [Google Scholar]
- 36.Brunner EJ, Shipley MJ, Ahmadi-Abhari S, Tabak AG, McEniery CM, Wilkinson IB, Marmot MG, Singh-Manoux A, Kivimaki M. Adiposity, obesity, and arterial aging: longitudinal study of aortic stiffness in the Whitehall II cohort. Hypertension 2015;66(2):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zachariah JP, Hwang S, Hamburg NM, Benjamin EJ, Larson MG, Levy D, Vita JA, Sullivan LM, Mitchell GF, Vasan RS. Circulating Adipokines and Vascular Function: Cross-Sectional Associations in a Community-Based Cohort. Hypertension 2016;67(2):294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. European journal of heart failure 2016;18(5):537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology 2011;57(12):1368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YC, Liang CS, Gopal DM, Ayalon N, Donohue C, Santhanakrishnan R, Sandhu H, Perez AJ, Downing J, Gokce N, Colucci WS, Ho JE. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circulation Heart failure 2015;8(5):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canepa M, Strait JB, Abramov D, Milaneschi Y, AlGhatrif M, Moni M, Ramachandran R, Najjar SS, Brunelli C, Abraham TP, Lakatta EG, Ferrucci L. Contribution of central adiposity to left ventricular diastolic function (from the Baltimore Longitudinal Study of Aging). The American journal of cardiology 2012;109(8):1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canepa M, Strait JB, Milaneschi Y, AlGhatrif M, Ramachandran R, Makrogiannis S, Moni M, David M, Brunelli C, Lakatta EG, Ferrucci L. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutr Metab Cardiovasc Dis 2013;23(12):1263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvaraj S, Martinez EE, Aguilar FG, Kim KY, Peng J, Sha J, Irvin MR, Lewis CE, Hunt SC, Arnett DK, Shah SJ. Association of Central Adiposity With Adverse Cardiac Mechanics: Findings From the Hypertension Genetic Epidemiology Network Study. Circulation Cardiovascular imaging 2016;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borlaug BA, Reddy YN. Getting at the Heart of Central Obesity and the Metabolic Syndrome. Circulation Cardiovascular imaging 2016;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart A, Obesity Committee of the Council on Nutrition PA, Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006;113(6):898–918. [DOI] [PubMed] [Google Scholar]
- 46.De Scheerder I, Cuvelier C, Verhaaren R, De Buyzere M, De Backer G, Clement D. Restrictive cardiomyopathy caused by adipositas cordis. European heart journal 1987;8(6):661–3. [DOI] [PubMed] [Google Scholar]
- 47.Roberts WC, Roberts JD. The floating heart or the heart too fat to sink: analysis of 55 necropsy patients. The American journal of cardiology 1983;52(10):1286–9. [DOI] [PubMed] [Google Scholar]
- 48.Dervan JP, Ilercil A, Kane PB, Anagnostopoulos C. Fatty infiltration: another restrictive cardiomyopathic pattern. Cathet Cardiovasc Diagn 1991;22(3):184–9. [DOI] [PubMed] [Google Scholar]
- 49.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure With Preserved Ejection Fraction. Circulation 2017;136(1):6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann Intern Med 2013;159(11):758–69. [DOI] [PubMed] [Google Scholar]
- 51.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circulation Heart failure 2012;5(6):710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.