Abstract
Aims:
Systolic time intervals change in the progress of cardiac dysfunction. The usefulness of left ventricular ejection time (LVET) to predict cardiovascular morbidity, however, is unknown.
Methods and Results:
We studied middle-aged African-Americans from one of four cohorts of the Atherosclerosis Risk in Communities study (Jackson cohort, n=1,980) who underwent echocardiography between 1993 and 1995. LVET was measured by pulsed-wave Doppler of the left ventricular outflow tract and related to outcomes.
A shorter LVET was associated with younger age, male sex, higher diastolic blood pressure (BP), higher proportion of diabetes, higher heart rate, higher blood glucose levels and worse fractional shortening (FS). During a median follow-up of 17.6 years, 384 (19%) had incident heart failure (HF), 158 (8%) had a myocardial infarction (MI), and 587 (30%) died. In univariable analysis, a lower LVET was significantly associated with increased risk of all events (p<0.05 for all). However, after multivariable adjustment for age, sex, hypertension, diabetes, body mass index, heart rate, systolic and diastolic BP, FS and left atrium diameter, LVET remained an independent predictor only of incident HF (HR1.07 (1.02–1.14), P=0.010, per 10ms decrease). In addition, LVET provided incremental prognostic information to the known risk factors included in the Framingham risk score, in regard to predicting all outcomes except for MI.
Conclusion:
LVET is an independent predictor of incident HF in a community-based cohort and provides incremental prognostic information on the risk of future HF and death when added to known risk prediction models.
Keywords: Incident heart failure, Echocardiography, Systolic Ejection Time, General population, Outcome
Introduction
The left ventricular ejection time (LVET) is defined by the opening and closing of the aortic valve, which in turn mainly is determined by the pressure differences across the valve. In the ailing heart, the LVET will change during disease progression as previously demonstrated in patients with ischemic heart disease, heart failure, hypertension and aortic stenosis1–3. As left ventricular (LV) function deteriorates, the ability of the heart to produce contractile force is attenuated and the rate of the LV pressure rise (LV dP/dt) during the isovolumic contraction decreases, resulting in a prolongation of isovolumic contraction time (IVCT)4–6. Furthermore, the ability of the ailing heart to maintain a high LV pressure during the ejection period decreases, resulting in reduction in the LVET4–6. Additionally, the LVET will also shorten with LV deterioration simply as the result of the prolonged IVCT which induces a delayed onset of ejection6.
Systolic time intervals, including LVET, were used frequently in the past because they were easily obtained by phonocardiography6–8, and are also easily obtained with Doppler echocardiography9. The LVET has been demonstrated to identify impaired cardiac function in patients with ischemic heart disease10, hypertension11, primary pulmonary hypertension12,13 and heart failure (HF)7,14,15. In addition, the LVET has previously been demonstrated to be a strong predictor of cardiovascular outcome in selected patient populations, especially in patients with ischemic heart disease8,10,16–19 and primary pulmonary hypertension12,13, but whether this measure has prognostic utility in a general population free of cardiovascular disease is unknown. In addition, it is not well understood which cardiovascular outcomes a shorter LVET is associated with.
Methods
Study Population:
The study population consists of the Jackson cohort of the Atherosclerosis Risk in Communities (ARIC) study. The ARIC study is an ongoing, prospective observational study of the natural history of atherosclerotic diseases and cardiovascular risk factors. Detailed study rationale, design, and procedures have been previously published20. The original cohort was recruited between 1987–1989 using probability sampling of middle aged (45–64 years old) men and women from 4 communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD). The Jackson field center enrolled an entirely African-American cohort, the Jackson cohort. Subsequent follow up visits occurred at approximately 3 year intervals up to Visit 4 (1996–1999), with annual telephone interviews conducted between visits. Institutional review boards approved the study and informed consent was obtained from all participants.
Transthoracic echocardiography was performed only in the Jackson cohort during visit 3 (1993–1996). Of 2,445 participants who underwent transthoracic echocardiography, 2,257 had LVET measured. After excluding participants with previous HF, missing HF data, and known coronary heart disease (n=277), our study population included 1980 participants.
Demographics:
Hypertension, diabetes mellitus, coronary heart disease, stroke, smoking status, and medication use was defined as previously described in ARIC21. Estimated glomerular filtration rate, hematologic parameters, lipids, and glucose were measured according to standardized protocols.
Echocardiography:
Echocardiograms were recorded by four trained sonographers and interpreted by experienced cardiologists in the Echocardiography Reading Center located at the University of Mississippi Medical Center. Two-dimensional, M-mode, and Doppler images were acquired with an Acuson 128XP/10c cardiac ultrasound machine with 2.5, 3.5, and 5.0 MHz transducers (Acuson, Malvern, PA). Measurements were performed by the interpreting physician who was blinded to the participants’ clinical data. LV end diastolic diameter (LVEDD), LV end systolic diameter, septal and posterior wall thickness, and left atrial dimension (LAD) were measured from 2-dimensional images according to American Society of Echocardiography criteria22. LVET was measured as the duration of the flow through the left ventricular outflow tract (LVOT) as assessed by pulsed-wave Doppler. The LVET corrected by the RR-interval obtained from the same cardiac cycle was calculated as LVET/RR.
Outcomes:
The follow up period was defined as the time elapsed from the date of echocardiography to the date of event, date of last contact for those lost to follow-up, or end of 2012. Incident HF was defined as the occurrence of a hospitalization with an ICD-9 discharge code 428 in any position or a death certificate with either an ICD-9 code 428 in any position or an ICD-10 code 150 in any position. Incident MI was defined as definite or probable hospitalization for MI based on committee adjudication of abstracted hospitalization records including chest pain symptoms, electrocardiograms (ECGs), and cardiac enzymes. Death was ascertained by annual phone call follow-up or through health department death certificate files23. The composite outcome of incident HF, incident MI or death was also assessed.
Statistical analysis:
Baseline characteristics across LVET quartiles were compared with trend tests (for continuous Gaussian distributed variables obtained by regression analysis, by an extension of the Wilcoxon rank-sum test24 for continuous non-Gaussian distributed variables and by a chi-square test for proportions (Cochran-Armitage trend test)). Rates of all events were calculated (number of events divided by person-time at risk) and stratified by quartiles of LVET. Hazard ratios (HR) were calculated by Cox proportional hazards regression analyses using time since visit 3. The association between LVET, LVET/RR and outcomes were assessed using the variables as linear predictors. In addition, departure from linearity for the association between LVET and outcomes were tested using restricted cubic splines with the number of knots selected according to the value associated with the lowest Akaike information criterion (AIC) value. The association was further assessed using a piecewise linear model, with separate linear relationships considered for SET values < 350ms and for SET values ≥ 350ms. The predictive capabilities of LVET was assessed in a univariable model, an age and gender adjusted model, a model including the important demographic determinants of LVET (model 2a), a model including the variables included in the Framingham risk score (2b) and a model including the important demographic determinants of LVET and echocardiographic measures of systolic and diastolic function. Harrell’s c-statistics25 obtained from Cox proportional hazards regression models including the variables from the Framingham Risk score26 (age, gender, total cholesterol, HDL cholesterol, smoking status and systolic blood pressure) and the SCORE risk chart27 (age, gender, cholesterol, smoking status and systolic blood pressure) were calculated and compared with the c-statistics obtained from Cox proportional hazards models also including the LVET in order to test the incremental prognostic performance of the models when adding LVET to the parameters from the conventional risk scores. C-statistics were compared using a transformation of the equivalent Somers’ D parameters28. Similarly, continuous and categorical net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were obtained when adding LVET to the parameters included in Framingham Risk score using Cox models and 15 years of follow-up (Supplemental Table 1). Colinearity and interaction was assessed for LVET and all covariates included in the final multivariable model (Model 3). A p-value ≤ 0.05 in 2-sided test was considered statistically significant. No corrections for multiple testing were performed. All analyses were performed with STATA Statistics/Data analysis, SE 12.0 (StataCorp, Texas,USA).
Results
The population demographics stratified by quartiles of LVET are shown in Table 1. Briefly, the participants had a mean age of 59 ± 6 years, 36% (n=718) were males, 57% (n=1128) had hypertension, 22% (n=718) had diabetes, the mean systolic and diastolic BP were 140 ± 20 mmHg and 82 ± 10 mmHg, respectively, the mean heart rate was 66 ± 10 beats per minute and the mean LVET was 342 ± 35 ms. Participants with a shorter LVET were younger, more likely male, had higher diastolic blood pressure, a higher proportion of diabetes, a higher heart rate, higher blood glucose levels, lower fractional shortening (FS), and a larger LV end-systolic dimension (Table 1).
Table 1.
Clinical and echocardiographic parameters by quartiles of left ventricular ejection time
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P-value | |
|---|---|---|---|---|---|
| LVET (ms) | <322 | 322–340 | 341–360 | >360 | for |
| n=495 | n=497 | n=506 | n=482 | trend | |
| Age | 57.9 ± 5.6 | 58.3 ± 5.7 | 58.7 ± 5.6 | 59.6 ± 5.7 | <0.001 |
| Male gender | 246 (50%) | 186 (37%) | 167 (33%) | 119 (25%) | <0.001 |
| Systolic Blood Pressure | 140 ± 21 | 139 ± 19 | 139 ± 20 | 142 ± 21 | 0.10 |
| Diastolic Blood Pressure | 85 ± 11 | 83 ± 10 | 82 ± 10 | 81 ± 10 | <0.001 |
| Hypertension | 313 (63%) | 263 (53%) | 268 (54%) | 284 (59%) | 0.21 |
| Diabetes | 143 (29%) | 92 (19%) | 95 (19%) | 98 (21%) | 0.002 |
| BMI | 30 ± 6 | 30 ± 6 | 30 ± 6 | 31 ± 6 | 0.33 |
| Heart Rate | 72 ± 11 | 66 ± 9 | 64 ± 9 | 60 ± 9 | <0.001 |
| Smoking status | |||||
| - Current | 120 (24%) | 102 (21%) | 87 (17%) | 82 (17%) | <0.001 |
| - Former | 181 (37%) | 148 (30%) | 151 (30%) | 135 (28%) | |
| - Never | 191 (39%) | 244 (49%) | 262 (52%) | 264 (55%) | |
| QRS interval (ms) | 95 ± 15 | 95 ± 14 | 95 ± 14 | 96 ± 16 | 0.49 |
| Total Cholesterol (mg/dL) | 207 ± 40 | 207 ± 39 | 208 ± 39 | 207 ± 39 | 0.80 |
| Total Glucose (mg/dL) | 105 (95–133) | 101 (93–113) | 102 (94–114) | 99 (92–113) | <0.001 |
| Echocardiographic Measures: | |||||
| Fractional Shortening (FS) (%) | 32.6 ± 9.4 | 34.9 ± 8.7 | 35.0 ± 8.4 | 36.0 ± 8.0 | <0.001 |
| LV end diastolic diameter (cm) | 4.7 ± 0.6 | 4.6 ± 0.6 | 4.6 ± 0.6 | 4.7 ± 0.6 | 0.79 |
| LV end systolic diameter (cm) | 3.0 ± 0.7 | 2.8 ± 0.6 | 2.8 ± 0.5 | 2.8 ± 0.5 | <0.001 |
| Left Atrium Diameter (LAD) (cm) | 3.4 ± 0.6 | 3.3 ± 0.5 | 3.3 ± 0.5 | 3.4 ± 0.5 | 0.22 |
LVET – Systolic Ejection Time; BMI – Body Mass Index; LV – Left Ventricle.
Systolic ejection time and outcome
During a median follow-up of 17.6 years, outcomes included 384 with incident HF (19%), 158 with MI (8%), and 587 were deceased (30%). Participants in the lowest LVET quartile had a 55% increased risk of the composite endpoint (HF, MI or death) compared to participants in the highest quartile (1st quartile vs. 4th quartile HR 1.55 (1.28–1.88), p<0.001)(Figure 1, Table 2 and 3). In comparison, the risk of incident HF was 66% higher in the 1st quartile (1st quartile vs. 4th quartile HR 1.66 (1.26–2.19), p<0.001) and risk of mortality 65% higher in the 1st quartile (1st quartile vs. 4th quartile HR 1.65 (1.31–2.07), p<0.001)(Figure 2 and Table 2) compared to participants in the highest quartile. The risk of MI did not differ significantly between participants in the lowest compared to the highest quartile (1st quartile vs. 4th quartile HR 1.46 (0.94–2.26), p=0.09)(Figure 2 and Table 2).
Figure 1: Association between Left Ventricular Ejection Time and risk of the Composite endpoint (HF, MI or death).
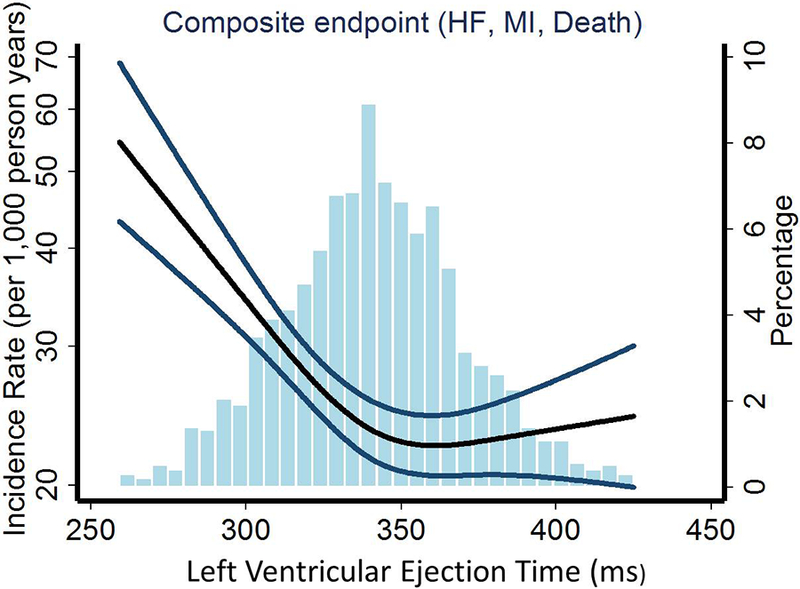
Depicting the unadjusted incidence rate of the composite endpoint (events per 1000 person years) on the left y-axis and systolic ejection time (LVET) on the x-axis. The percentage of the population corresponding to the histogram is displayed on the right y-axis. The black curve depicts the incidence, with 95% confidence intervals of the estimates (P for overall trend <0.001 and P for non-linearity <0.001). Also depicted is the histogram of the LVET in our population (n=1980).
LVET – Left Ventricular Ejection Time; HF – Heart Failure; MI – Myocardial Infarction.
Table 2.
Event rates by quartiles of left ventricular ejection time
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | P-value for | |
|---|---|---|---|---|---|
| Left Ventricular Ejection Time (ms) | <322 | 322–340 | 341–360 | >360 | trend |
| n=495 | n=497 | n=506 | n=482 | ||
| Event rate of composite endpoint (per 1000 person years) (95% CI) | 35 (31; 39) | 23 (20; 26) | 24 (21; 28) | 23 (20; 27) | <0.001 |
| Event rate of heart failure (per 1000 person years) (95% CI) | 17 (14; 21) | 11 (9; 14) | 12 (9; 14) | 11 (9; 13) | 0.001 |
| Event rate of myocardial infarction failure (per 1000 person years) (95% CI) | 6 (5; 9) | 4 (3; 6) | 6 (4; 7) | 4 (3; 6) | 0.25 |
| Event rate of mortality (per 1000 person years) (95% CI) | 25 (21; 28) | 17 (14; 20) | 12 (99; 14) | 11 (9; 13) | <0.001 |
Table 3.
Left ventricular ejection time and the association with outcomes
| Combined endpoint (782 events) Hazard Ratio (95% CI) |
P-value | HF (384 events) Hazard Ratio (95% CI) |
P-value | HF/Death (733 events) Hazard Ratio (95% CI) |
P-value | MI* (158 events) Hazard Ratio (95% CI) |
P-value | MI/Death* (664 events) Hazard Ratio (95% CI) |
P-value | Death (586 events) Hazard Ratio (95% CI) |
P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted model (n=1980): | ||||||||||||
| LVET per 10 ms decrease | 1.06 | 0.024 | 1.05 | <0.001 | ||||||||
| LVET per 10 ms decrease below 350 ms | 1.11 | <0.001 | 1.12 | <0.001 | 1.11 | <0.001 | 1.11 | <0.001 | ||||
| LVET/RR per 10 % increase | 1.24 | 0.003 | 1.38 | 0.001 | 1.32 | <0.001 | 0.93 | 0.67 | 1.21 | 0.015 | 1.29 | 0.002 |
| Multivariable Model 1 (n=1980): | ||||||||||||
| LVET per 10 ms decrease | 1.06 | 0.033 | 1.05 | <0.001 | ||||||||
| LVET per 10 ms decrease below 350 ms | 1.10 | <0.001 | 1.13 | <0.001 | 1.11 | <0.001 | 1.11 | <0.001 | ||||
| LVET/RR per 10% increase | 1.40 | <0.001 | 1.42 | 0.001 | 1.48 | <0.001 | 1.05 | 0.59 | 1.41 | <0.001 | 1.49 | <0.001 |
| Multivariable Model 2a (n=1887): | ||||||||||||
| LVET per 10 ms decrease | 1.09 | 0.007 | 1.04 | 0.012 | ||||||||
| LVET per 10 ms decrease below 350 ms | 1.08 | <0.001 | 1.10 | <0.001 | 1.08 | <0.001 | 1.07 | 0.006 | ||||
| LVET/RR per 10% increase | 1.06 | 0.54 | 0.85 | 0.23 | 1.08 | 0.43 | 0.86 | 0.49 | 1.13 | 0.21 | 1.15 | 0.20 |
| Multivariable Model 2b (n=1931): | ||||||||||||
| LVET per 10 ms decrease | 1.04 | 0.12 | 1.05 | <0.001 | ||||||||
| LVET per 10 ms decrease below 350 ms | 1.08 | <0.001 | 1.11 | <0.001 | 1.09 | <0.001 | 1.08 | <0.001 | ||||
| LVET/RR per 10% increase | 1.38 | <0.001 | 1.39 | 0.002 | 1.46 | <0.001 | 0.98 | 0.91 | 1.39 | <0.001 | 1.50 | <0.001 |
| Multivariable Model 3 (n=1653): | ||||||||||||
| LVET per 10 ms decrease | 1.06 | 0.06 | 1.03 | 0.06 | ||||||||
| LVET per 10 ms decrease below 350 ms | 1.07 | 0.003 | 1.07 | 0.028 | 1.07 | 0.009 | 1.05 | 0.07 | ||||
| LVET/RR per 10% increase | 1.13 | 0.23 | 0.89 | 0.43 | 1.14 | 0.21 | 0.91 | 0.68 | 1.18 | 0.14 | 1.20 | 0.12 |
Using LVET as linear predictor; all other outcomes use LVET as piece-wise linear above/below 350. Model 1 - adjusted for age and gender. Model 2a – Model 1 + hypertension, diabetes, BMI, heart rate, systolic and diastolic BP. Model 2b (Framingham risk score) – Model 1 + total cholesterol, HDL cholesterol, smoking status and systolic blood pressure. Model 3 – Model 2a + FS and LAD. The HR’s are not shown for LVET where the associations have been found to be significantly nonlinear. The Harrel’s c-statistic is displayed for all models below the hazard ratio.
LVET – Left Ventricular Ejection Time; HF – Heart Failure; MI – Myocardial Infarction; BMI – Body Mass Index; FS – Fractional Shortening; LAD – Left Atrium Dimension.
Figure 2: Association between Left Ventricular Ejection Time and risk of incident HF, MI or death, respectively.
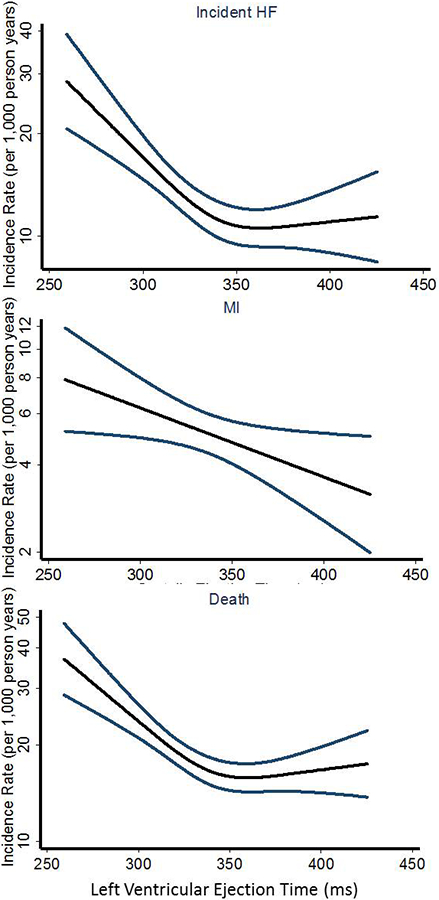
Depicting the unadjusted incidence rate of the incident HF (P for overall trend <0.001 and P for non-linearity = 0.002), MI (P for linear and overall trend = 0.024 and P for non-linearity = 0.69) or death (P for overall trend < 0.001 and P for non-linearity < 0.001), respectively (events per 1000 person years) on the y-axis and systolic ejection time (LVET) on the x-axis. The black curve depicts the incidence, with 95% confidence intervals of the estimates.
LVET – Left Ventricular Ejection Time; HF – Heart Failure; MI – Myocardial Infarction.
When assessing LVET as a continuous variable, decreasing LVET was significantly associated with increased risk of all outcomes, particularly for predicting incident HF (Figure 1, 2 and Table 3). After multivariable adjustment for age, gender, hypertension, diabetes, BMI, heart rate, systolic and diastolic BP, LVET remained an independent predictor of all outcomes (Table 3). When adjusting for our conventional measure of systolic and diastolic function, FS, and LAD, LVET remained an independent predictor only of incident HF and the composite outcome (Table 3).
Incremental prognostic information obtained from LVET
The primary risk stratification models used for assessing risk of cardiovascular morbidity and mortality in the general population are currently the Framingham Risk score26 and the SCORE risk chart27. We assessed if adding LVET to the known risk factors obtained from these risk models (SCORE: Age, gender, cholesterol, smoking status and systolic blood pressure and Framingham Risk score: Age, gender, total cholesterol, HDL cholesterol, smoking status and systolic blood pressure) would improve model performance. When adding LVET to the factors from the Framingham Risk score26 or the SCORE risk chart27 the risk models were significantly improved with regard to predicting the composite endpoint (SCORE: c-statistics difference 0.690 (0.671–0.708) vs. 0.678 (0.659–0.696), p<0.001; Framingham Risk score: c-statistics difference 0.695 (0.677–0.713) vs. 0.683 (0.665–0.702), p<0.001), incident HF (SCORE: c-statistics difference 0.713 (0.687–0.738) vs. 0.682 (0.656–0.709), p=0.001; Framingham Risk score: c-statistics difference 0.713 (0.687–0.738) vs. 0.693 (0.667–0.720), p=0.001), and death (SCORE: c-statistics difference 0.706 (0.685–0.727) vs. 0.695 (0.674–0.716), p=0.002; Framingham Risk score: c-statistics difference 0.706 (0.685–0.727) vs. 0.697 (0.676–0.718), p=0.002). Adding LVET to the Framingham Risk score26 or the SCORE risk chart27 did not improve the conventional models for predicting risk of MI (SCORE: c-statistics difference 0.690 (0.645–0.734) vs. 0.682 (0.637–0.727), p=0.11; Framingham Risk score: c-statistics difference 0.712 (0.671–0.753) vs. 0.706 (0.665–0.747), p=0.10).
Similar results were found when adding LVET to the Framingham Risk score as assessed by continuous NRI and IDI. The addition of LVET to models using Framingham risk factors produced significant increases in IDI of approximately +1% and in continuous NRI of approximately +13% with respect to the primary outcome, death, heart failure. Categorical NRI was also significantly improved with respect to the primary outcome and heart failure (+3.0% and +4.8%, respectively; Supplemental Table 1).
Discussion
In the present report we demonstrate the prognostic utility of LVET in a large general population free of cardiovascular disease using long term follow-up. LVET is a significant echocardiographic predictor of cardiovascular morbidity and mortality in a general population with no known cardiovascular disease. Additionally, the LVET is primarily a strong predictor of incident HF, but not MI and to a lesser extent all-cause mortality. LVET provided incremental prognostic information regarding risk of HF and all-cause mortality, but not MI, when added to the Framingham Risk score or the SCORE risk chart.
LVET and cardiovascular outcome
The LVET has previously been demonstrated to be a strong predictor of outcome, especially in patients with ischemic heart disease8,10,16–19 and primary pulmonary hypertension12,13. In a previous report, LVET obtained by tissue Doppler imaging (TDI) M-mode was a univariable predictor of a combined outcome of ischemic heart disease, HF, and cardiovascular mortality in a general population29, some of whom had a history of cardiovascular disease. With longer follow-up and therefore more events and higher statistical power, we were able to show that this relationship is mostly due to hospitalization for heart failure.
Weissler and colleagues demonstrated in 1964 that patients with HF had significantly shorter LVET and longer pre-ejection period (PEP = defined as the Q-wave of the ECG – the LVET from the central artery pulse) compared to normal individuals7. PEP and LVET are mainly measures of systolic function4, and measures of systolic and diastolic function have previously been demonstrated to predict different outcomes30. In the general population, early systolic dysfunction determined by reduced myocardial systolic velocity (TDI s’) or global longitudinal strain have been shown to be a strong predictor of HF30,31 whereas a reduced diastolic function as determined by a reduced early diastolic relaxation velocity (TDI e’) was a strong predictor of MI30. This is in accordance with our results where our measure of systolic function primarily predicted HF events and not of MI or death (Table 3).
In particular, we found that it was in the low range of LVET that the risk of HF, all-cause mortality, and the combined endpoint increased, whereas a decreasing LVET in the high range wasn’t associated with an increased risk (Table 2 and 3, Figure 1 and 2). This phenomenon has been seen in previous studies16,29. Furthermore, judging from our results it seems that incident HF primarily drives this range specific pattern of risk obtained from LVET. This is very important because it indicates that there might be a specific cutoff where a decreasing LVET no longer is within the physiological normal range and is a marker of an ailing heart, which if left untreated, might lead to HF. In the present report, the cutoff seems to be somewhere below 350 ms (Figure 1 and 2). However, since there are many methods of obtaining LVET (pulsed wave Doppler of the LVOT32, TDI velocity curves33, or M-mode TDI16,29,34–37) that lead to different absolute values, further studies are needed to assess the clinically useful cutoffs for each method.
Usefulness of LVET in clinical trials
The LVET is a very easy and fast measure to obtain with high reproducibility38,39. In contrast to TDI and speckle tracking, LVET is measurable with all conventional echocardiographic machines and software and improves our current risk prediction models. The LVET has therefore been used for several decades to monitor LV contractility and function when testing new medical therapies6. In clinical trials, the LVET has previously been demonstrated to improve with medical therapy in patients with ischemic heart disease10, hypertension11, and HF14,15. Recently, LVET was used as the primary echocardiographic outcome in a large, multicenter randomized trial40 testing a novel drug (omecamtiv mecarbil) that specifically increases the duration of LVET in patients with HF14,15. Furthermore, the effect of intravenous administration of this drug on LVET was consistent in healthy volunteers41, chronic HF patients14, and acute HF patients42, as well as the oral study40. Even though the LVET has been around for several decades, the usefulness of this simple measure might therefore be revived within the near future.
Limitations
Some limitations have to be noted. First, all participants included were African-Americans, with high BMI and high prevalence of both hypertension and diabetes, which limits the generalizability of our findings to other general populations with another composition. Nevertheless, previous studies comprised primarily of Caucasians have shown that LVET is also predictive of cardiovascular outcomes in general populations with other compositions. However, in these previous studies, the association of LVET with the individual endpoints was not assessed as only the association with composite endpoints was evaluated29,35. In addition, changes in therapies for hypertension and diabetes mellitus during the last two decades may influence the relevance of the results presented in this report to contemporary populations. Unfortunately, we were not able to assess whether the association between LVET and incident HF was derived from an association with HFpEF or HFrEF, since the ICD-9 codes do not discriminate between the two types of HF admissions. In addition, natriuretic peptides were not measured in the Jackson cohort of the ARIC study. Incident HF occurred more often than MI and less often than death, hence, LVET had greatest power for detecting the association between LVET, HF and death. Furthermore, it should be noted that the Framingham Risk score and the SCORE risk chart both are calibrated to predict risk of ischemic cardiovascular events, which might also explain the finding that LVET did not provide incremental prognostic information regarding risk of MI when added to the known risk factors. Interestingly, despite the fact that these two models were not originally calibrated for incident HF we find that the variables included in the Framingham risk model calibrated well to predict incident HF in the ARIC population as determined by a no-significant difference between predicted and observed number of outcomes (Grønnesby-Borgan X2 statistic of 6.214 and corresponding p= 0.72). We did not adjust for multiple comparisons. Like all other echocardiographic measures of systolic and diastolic function, LVET depends on heart rate43,44. Hence, physicians should always take heart rate into account when assessing LVET.
Unfortunately, volumetric measurements were not performed as part of the echocardiographic examination of the Jackson cohort of the ARIC study. All measures of cardiac structure and function are therefore based on dimensional measures. Likewise, the pre ejection period (PEP) and the time interval between the late transmitral flow (A wave) and the early transmitral flow (E wave) of the following heart cycle were not measured; hence, we were not able to calculate neither the myocardial performance index nor the PEP/LVET in the present study. The fact that the LVET is a strong predictor of incident HF using echocardiographic machines from 1993 demonstrates the generalizability and how easy it would be to implement in echocardiographic laboratories all around the world despite limited availability of the newest machines and software.
Conclusion
The LVET is a significant predictor of cardiovascular morbidity and mortality in a general population with no known cardiovascular disease. However, the LVET is primarily a strong predictor of incident HF and not MI or all-cause mortality. Furthermore, LVET provides incremental prognostic information regarding risk of HF and all-cause mortality, but not MI, when added to the Framingham Risk score or the SCORE risk chart.
Supplementary Material
Acknowledgement
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources:
TBS received a research grant from the P. Carl Petersen foundation. Additionally, TBS received a Sapere Aude research talent grant from The Danish Council for Independent Research (DFF – 4004–00248B). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). SMH receives funding from the National Institutes of Health grant T32 HL094301–06. The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Footnotes
Conflicts of interest: None.
References
- 1.Harris WS, Aytan N, Pouget JM. Effects of nitroglycerin on responses of the systolic time intervals to exercise. Circulation 1973;47:499–508. [DOI] [PubMed] [Google Scholar]
- 2.Kyle MC, Freis ED. Serial measurements of systolic time intervals: effects of propranolol alone and combined with other agents in hypertensive patients. Hypertension 1980;2:111–117. [DOI] [PubMed] [Google Scholar]
- 3.Kjaergaard J, Hassager C, Oh JK, Kristensen JH, Berning J, Sogaard P. Measurement of cardiac time intervals by Doppler tissue M-mode imaging of the anterior mitral leaflet. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2005;18:1058–1065. [DOI] [PubMed] [Google Scholar]
- 4.Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Biscottini E, Lauciello R, Pantano P, Gentile F, Nishimura RA, Ambrosio G. Improvement of myocardial performance (Tei) index closely reflects intrinsic improvement of cardiac function: assessment in revascularized hibernating myocardium. Echocardiogr Mt Kisco N 2012;29:298–306. [DOI] [PubMed] [Google Scholar]
- 5.Hodges M, Halpern BL, Friesinger GC, Dagenais GR. Left ventricular preejection period and ejection time in patients with acute myocardial infarction. Circulation 1972;45:933–942. [DOI] [PubMed] [Google Scholar]
- 6.Boudoulas H Systolic time intervals. Eur Heart J 1990;11 Suppl I:93–104. [DOI] [PubMed] [Google Scholar]
- 7.Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation 1968;37:149–159. [DOI] [PubMed] [Google Scholar]
- 8.Weissler AM, O’Neill WW, Sohn YH, Stack RS, Chew PC, Reed AH. Prognostic significance of systolic time intervals after recovery from myocardial infarction. Am J Cardiol 1981;48:995–1002. [DOI] [PubMed] [Google Scholar]
- 9.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol 1996;28:658–664. [DOI] [PubMed] [Google Scholar]
- 10.Lewis RP, Boudoulas H, Welch TG, Forester WF. Usefulness of systolic time intervals in coronary artery disease. Am J Cardiol 1976;37:787–796. [DOI] [PubMed] [Google Scholar]
- 11.Dodek A, Burg JR, Kloster FR. Systolic time intervals in chronic hypertension: Alterations and response to treatment. Chest 1975;68:51–55. [DOI] [PubMed] [Google Scholar]
- 12.Shigematsu Y, Hamada M, Kokubu T. Significance of systolic time intervals in predicting prognosis of primary pulmonary hypertension. J Cardiol 1988;18:1109–1114. [PubMed] [Google Scholar]
- 13.Sztrymf B, Günther S, Artaud-Macari E, Savale L, Jaïs X, Sitbon O, Simonneau G, Humbert M, Chemla D. Left ventricular ejection time in acute heart failure complicating precapillary pulmonary hypertension. Chest 2013;144:1512–1520. [DOI] [PubMed] [Google Scholar]
- 14.Cleland JGF, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJV, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet Lond Engl 2011;378:676–683. [DOI] [PubMed] [Google Scholar]
- 15.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 2011;331:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biering-Sørensen T, Mogelvang R, Søgaard P, Pedersen SH, Galatius S, Jørgensen PG, Jensen JS. Prognostic Value of Cardiac Time Intervals by Tissue Doppler Imaging M-Mode in Patients With Acute ST-Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Circ Cardiovasc Imaging 2013;6:457–465. [DOI] [PubMed] [Google Scholar]
- 17.Teodorescu P, Guţiu I, Predescu T, Frîncu P, Cucu N, Carp C. Prognosis of acute myocardial infarction using systolic time intervals recorded on the carotidogram. Médecine Interne 1981;19:131–136. [PubMed] [Google Scholar]
- 18.Northover BJ. Left ventricular systolic time intervals in patients with acute myocardial infarction. Br Heart J 1980;43:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northover BJ. Estimation of the risk of death during the first year after acute myocardial infarction from systolic time intervals during the first week. Br Heart J 1989;62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 21.Folsom AR, Yamagishi K, Hozawa A, Chambless LE, Atherosclerosis Risk in Communities Study Investigators. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail 2009;2:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MSJ, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 23.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 24.Cuzick J A Wilcoxon-type test for trend. Stat Med 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 27.Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM, SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 28.The Stata Journal - sjpdf.html.
- 29.Biering-Sørensen T, Mogelvang R, Jensen JS. Prognostic value of cardiac time intervals measured by tissue Doppler imaging M-mode in the general population. Heart Br Card Soc 2015;101:954–960. [DOI] [PubMed] [Google Scholar]
- 30.Mogelvang R, Biering-Sørensen T, Jensen JS. Tissue Doppler echocardiography predicts acute myocardial infarction, heart failure, and cardiovascular death in the general population. Eur Heart J Cardiovasc Imaging 2015;16:1331–1337. [DOI] [PubMed] [Google Scholar]
- 31.Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, Shah AM, Jensen JS. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol 1996;28:658–664. [DOI] [PubMed] [Google Scholar]
- 33.Tekten T, Onbasili AO, Ceyhan C, Unal S, Discigil B. Novel approach to measure myocardial performance index: pulsed-wave tissue Doppler echocardiography. Echocardiogr Mt Kisco N 2003;20:503–510. [DOI] [PubMed] [Google Scholar]
- 34.Biering-Sørensen T, Mogelvang R, Pedersen S, Schnohr P, Sogaard P, Jensen JS. Usefulness of the myocardial performance index determined by tissue Doppler imaging m-mode for predicting mortality in the general population. Am J Cardiol 2011;107:478–483. [DOI] [PubMed] [Google Scholar]
- 35.Biering-Sørensen T, Mogelvang R, Schnohr P, Jensen JS. Cardiac Time Intervals Measured by Tissue Doppler Imaging M-mode: Association With Hypertension, Left Ventricular Geometry, and Future Ischemic Cardiovascular Diseases. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biering-Sørensen T, Mogelvang R, Knegt MC, de Olsen FJ, Galatius S, Jensen JS. Cardiac Time Intervals by Tissue Doppler Imaging M-Mode: Normal Values and Association with Established Echocardiographic and Invasive Measures of Systolic and Diastolic Function. PloS One 2016;11:e0153636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biering-Sørensen T, Jensen JS, Andersen HU, Rossing P, Jensen MT. Cardiac time intervals and the association with 2D-speckle-tracking, tissue Doppler and conventional echocardiography: the Thousand&1 Study. Int J Cardiovasc Imaging 2016;32:789–798. [DOI] [PubMed] [Google Scholar]
- 38.Kupari M Reproducibility of the systolic time intervals: effect of the temporal range of measurements. Cardiovasc Res 1983;17:339–343. [DOI] [PubMed] [Google Scholar]
- 39.Lang-Jensen T Blood flow velocity and systolic time intervals measured by pulsed Doppler ultrasound: reproducibility of measurements. Cardiovasc Res 1987;21:582–586. [DOI] [PubMed] [Google Scholar]
- 40.Teerlink JR, Felker GM, McMurray JJV, Solomon SD, Adams KF, Cleland JGF, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsányi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N, COSMIC-HF Investigators. Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC-HF): a phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet Lond Engl 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 41.Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet Lond Engl 2011;378:667–675. [DOI] [PubMed] [Google Scholar]
- 42.Teerlink JR, Felker GM, McMurray JJV, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JGF, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM, ATOMIC-AHF Investigators. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: The ATOMIC-AHF Study. J Am Coll Cardiol 2016;67:1444–1455. [DOI] [PubMed] [Google Scholar]
- 43.Mertens HM, Mannebach H, Trieb G, Gleichmann U. Influence of heart rate on systolic time intervals: effects of atrial pacing versus dynamic exercise. Clin Cardiol 1981;4:22–27. [DOI] [PubMed] [Google Scholar]
- 44.Warrington SJ, Weerasuriya K, Burgess CD. Correction of systolic time intervals for heart rate: a comparison of individual with population derived regression equations. Br J Clin Pharmacol 1988;26:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


