Abstract.
Mucosal leishmaniasis (ML) is characterized by high production of inflammatory cytokines. Administration of pentoxifylline (PTX), an inhibitor of TNF-alpha, with pentavalent antimony (Sbv), has been successfully used as alternative treatment for refractory ML. Our study aims to investigate the in situ cellular response underlying the effectiveness of this therapy, by evaluating the intensity of the inflammatory infiltrate, cellular composition, and expression of cytokines and granzyme A in lesions from ML before and after treatment with Sbv alone or in combination with PTX. Our data showed no differences in the intensity of inflammatory infiltrate comparing before and after treatment, and comparing between different treatments. However, although the number and frequency of CD4+ and CD8+ cells were not different before and after treatments or comparing different treatments, frequency of CD68+ cells decreased after treatment with Sbv + PTX, but not with Sbv. This was due to a reduction in CD68+ TNF-alpha+ and not in CD68+ IL-10+ cells. The frequency of TNF-alpha+ cells was correlated with the intensity of the inflammatory infiltrate before treatment, but this correlation was lost after treatment with Sbv + PTX. Although the total expression of granzyme A did not significantly change after treatments, a clear trend of decrease was observed after treatment with Sbv + PTX. Interestingly, patients who took longer to heal, regardless of the treatment, displayed a higher frequency of granzyme A+ cells. Our data suggest that treatment with Sbv + PTX acts in CD68+ cells reducing the expression of TNF-alpha but not IL-10, resulting in more efficient modulation of the inflammatory response, accelerating the healing process.
INTRODUCTION
American tegumentary leishmaniasis is caused mainly by the infection with Leishmania braziliensis1,2 and leads to disseminated (disseminated leishmaniasis) or localized cutaneous (cutaneous leishmaniasis [CL]) and mucosal (mucosal leishmaniasis [ML]) clinical forms. The pathology associated with CL and ML is a result of an intense inflammatory reaction mediated by the host’s immune response.3,4 Mucosal leishmaniasis patients display a strong Th1 response, with high production of pro-inflammatory cytokines, such as gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha), and a low expression of the receptor for the immunoregulatory cytokine IL-10.5,6 Moreover, previous studies by our group and others have also shown that granzyme A, a cytotoxic molecule, is highly abundant in the lesions from patients with ML, and that CL lesion development is associated with the increased number of CD8+ T cells expressing granzyme A.7–11 These data suggest an important role for inflammatory cytokines and the cytotoxic molecule in inflammation and tissue destruction in American tegumentary leishmaniasis. Thus, not only the elimination of the parasite is important for the resolution of infection but also the control of the immune response is a critical aspect for the successful cure of leishmaniasis.
Conventional treatment for CL and ML involves the intravenous administration of pentavalent antimonial (Sbv). Although most patients respond relatively well to Sbv, resistant cases have been reported.12,13 We have shown that combination of Sbv with pentoxifylline (PTX), a TNF-alpha inhibitor,14 induces cure in resistant forms of CL and ML.12,15,16 However, the cellular mechanisms involved in the success of this treatment are not known. Understanding the effects of these drugs in the local immune response is critical and may help the search for new preventive or therapeutic strategies that guide the response to a profile of healing and resolution of the lesions. Our hypothesis is that successful therapy of PTX, as an adjunct to Sbv, induces cellular changes at the lesion site, which may contribute to its resolution. Thus, the purpose of this work was to study the effects of the treatment with Sbv + PTX on the cellular composition, cytokine, and granzyme A expression at the lesion site, to determine the cellular and molecular mechanisms associated with this successful treatment. Our data suggest that treatment with Sbv + PTX decreases the occurrence of inflammatory mechanisms associated with tissue destruction by acting in CD68+ cells, reducing their expression of TNF-alpha but not IL-10, favoring lesion healing.
MATERIALS AND METHODS
Patients.
This randomized and double-blinded study involved 10 patients with ML who were living in Corte de Pedra, an endemic area for L. braziliensis infection, located 280 km southwest of Salvador, in the state of Bahia, Brazil. All patients were volunteers, and informed consent was obtained from all individuals before collection of lesion material. Their age ranged between 21 and 65 years, comprising six males and four females, and they had severe ML, as defined as the presence of deep mucosal ulcers (Stage III) and/or perforation of the cartilaginous nasal septum (Stage IV). Clinical diagnosis was confirmed by at least two of the following laboratory methods: a positive intradermal skin test result with Leishmania antigen (Montenegro reaction), parasite isolation by culture, or histopathological findings characteristic of ML. No patient enrolled in this study received previous anti-leishmaniasis treatment. Patients who had mild ML with superficial mucosal ulcers (Stages I and II, according to ref. 17), prior therapy for ML, diabetes, or coinfection with HIV, or who were unavailable for follow-up, were excluded from the study. Two biopsies (one before and one after the beginning of treatment) were collected from each patient’s lesions for analysis. The time interval between the biopsy collections varied from 14 to 35 days. The parameters of the 10 patients of this study are shown in Table 1.
Table 1.
Clinical characteristics of patients with mucosal leishmaniasis
| Patient # | Gender | Age | Date of biopsy collection | Treatment | Healing time (months) | |
|---|---|---|---|---|---|---|
| First biopsy* | Second biopsy | |||||
| 1 | M | 47 | 20-Oct-09 | 20-Nov-09 | Sbv | 3 |
| 2 | M | 45 | 4-Dec-09 | 18-Dec-09 | Sbv | 2 |
| 3 | M | 60 | 18-Dec-09 | 8-Jan-10 | Sbv | 4 |
| 4 | M | 30 | 7-Aug-09 | 21-Aug-09 | Sbv | NA |
| 5 | F | 37 | 4-Jul-08 | 25-Jul-08 | Sbv | 1 |
| 6 | F | 65 | 26-Mar-10 | 9-Apr-10 | Sbv + PTX | 1 |
| 7 | F | 48 | 5-Oct-07 | 26-Oct-07 | Sbv + PTX | 2 |
| 8 | M | 27 | 19-Sep-08 | 10-Oct-08 | Sbv + PTX | 2 |
| 9 | F | 21 | 11-Sep-09 | 2-Oct-09 | Sbv + PTX | 6 |
| 10 | M | 51 | 11-Jan-08 | 15-Feb-08 | Sbv + PTX | 3 |
NA = information not available.
* Treatment was initiated immediately after collection of first biopsy.
The Ethical Committee from Federal University of Minas Gerais and Bahia approved all procedures involved in this study (ETIC 027/98 and 61.2007, respectively).
Treatment.
After the obtention of patients’ consent, they were randomized, through the use of a randomization table. Doctors and participants were blinded to treatment assignment during all the steps of the study, including the follow-up period. Five patients with ML received intravenous Sbv (meglumine antimony; Aventis), at a dosage of 20 mg/kg of body weight per day plus oral placebo three times daily for 30 days; five patients received the same dosage of Sbv, plus oral PTX (Pentox; Farmasa, São Paulo, SP, Brazil) at a dosage of 400 mg three times daily for 30 days. A data safety monitoring committee had access to patient assignment in the event of adverse events.
Sample collection.
Lesion biopsy samples were collected at the Corte de Pedra health-care facility or at the Immunology Service, Hospital Universitário Prof. Edgar Santos, Universidade Federal da Bahia in Salvador, Brazil. Samples were taken from lesions of the 10 patients with ML, 5 of which received Sbv treatment alone and 5 received PTX associated with Sbv, as described earlier. One biopsy was collected immediately before the beginning of the treatment and another biopsy was collected at variable intervals after the beginning of the treatment (see Table 1). Lesion biopsies were obtained by excision of a small fragment (approximately 3 mm, on average) using a scalpel, after local anesthetic application. Lesions were maintained in a 30% sucrose solution for approximately 30 minutes at 4°C; then, they were transferred to optimal cutting temperature Tissue Tek freezing medium and immediately placed in dry ice. The material was stored at −80°C until analysis.
Immunofluorescence staining.
Individual 4–5 µm cryosections were placed in silane–pre-coated slides and fixed for 10 minutes with acetone. Slides were incubated with phosphate-buffered saline for 30 minutes and subjected to hematoxylin–eosin (HE) staining or to immunofluorescence staining using specific monoclonal antibodies. Standard HE staining was performed solely to ensure tissue integrity, and after conventional HE staining, slides were observed under a light microscope. Sections with well-preserved morphology were submitted to immunofluorescence reaction. Immunofluorescence reactions were performed to determine the intensity and composition of the inflammatory infiltrate, through the incubation with fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled monoclonal antibodies (all from Biolegend, San Diego, CA) directed to surface receptors (CD4 clone S3.5, CD8 clone 3B5, or CD68 clone Ki-M7) and intracellular molecules (granzyme A clone CLB-GA28, IFN-gamma clone B27, IL-10 clone 9D7, or TNF-alpha clone 20A4). Sections were incubated with antibodies mixture overnight at 4°C. After staining, preparations were extensively washed with phosphate-buffered saline, counterstained with 4′,6′-diamidino-2-phenylindole (DAPI), and mounted using hydromount mounting medium (National diagnostics, Atlanta, GA). Slides were kept at 4°C and protected from light, until acquisition in a laser scanning confocal microscope (Zeiss, Thornwood, WV). Isotype controls were analyzed separately to confirm the lack of nonspecific staining. All the steps of the study were double-blinded, including the processing of samples and subsequent analysis.
Confocal analysis.
Confocal analysis was performed using a Meta-510 Zeiss laser scanning confocal system running LSM 510 software coupled to a Zeiss microscope (Axiovert 200M, Carl Zeiss, Munich, Germany) with an oil immersion Plan-Apochromat objective (40×, 1.2 numerical aperture) and Bio-Rad (Hercules, CA) MRC 1024 laser scanning confocal system running Laser Sharp 3.0 software coupled to a Zeiss microscope (Axiovert 100) with a water immersion objective (40×, 1.2 numerical aperture). A water-cooled argon UV laser (488 nm) or a krypton/argon laser was used to excite the preparation (through its 363-, 488-, or 568-nm line), and light emitted was selected with band-pass filters (522/35 for FITC or 598/40 for PE). For DAPI visualization, a mercury lamp was used to excite the preparation (through its 20/80 nm line), and light emitted was selected with band-pass filters (363/90 for DAPI). For each section, a minimum of six fields were acquired. Image analysis and processing were performed with Zeiss LSM Image Browser (Zeiss, Carl Zeiss, Munich, Germany), Adobe Photoshop CS4 (11,0, San Jose, CA) and Image Tool (3,00) software. Analyses were performed by counting the total number of cells in six to nine fields acquired and calculating the average number of cells for each sample (total number of cells/number of fields counted). This procedure was performed for each parameter analyzed, allowing determination of the total number of inflammatory cells (total number of DAPI+ cells within the inflammatory infiltrate), the number of FITC or PE single-positive cells, and the number of double-positive cells. The counts were performed blindly; the results were expressed as the average of cell number/field for each parameter for each patient. Percent frequency of each parameter (cell populations, cytokines, and granzyme) was also determined in relation to the inflammatory infiltrate. The results are representative of two experiments per patient.
Statistical analysis.
Statistical analysis of the data was performed using GraphPad/Prism 4 statistical software (San Diego, CA) and SPSS 12 Statistics (IBM Corporation, Armonk, NY). The comparisons of percentage for a given parameter between the groups of same patients (before and after treatment) were performed using the paired t-test. Comparison between averages of groups involving different patients (comparing groups submitted to different treatments) was performed using nonparametric test. The Spearman correlation analysis test was also performed. Differences were considered statistically significant at P-values equal or lower than 0.05.
RESULTS
Pentoxifylline associated with Sbv treatment reduces the frequency of CD68+ macrophages in ML patients, without changing the frequency of T cells.
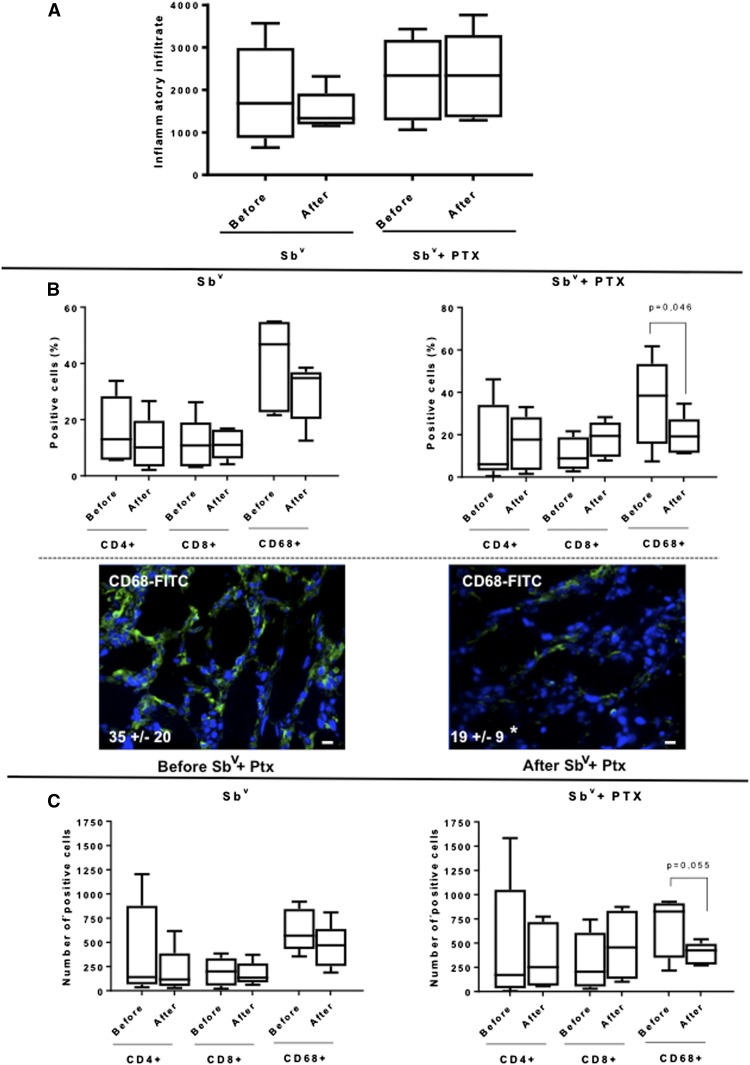
Leishmania braziliensis was the species identified in all lesions. The composition of the inflammatory infiltrate in lesions from ML patients was determined by counting the number of DAPI-positive cells per field using confocal microscopy, as described in Materials and Methods. The total number of cells composing the inflammatory infiltrate in lesions from ML patients submitted to treatments with Sbv alone or PTX associated with Sbv did not change before and after the treatments (Figure 1A). Moreover, the intensity of the inflammatory infiltrate was similar when comparing lesions from ML patients submitted with the different treatments. We then evaluated if there were qualitative differences in the inflammatory infiltrates from lesions of patients submitted to the different regimens, by determining the number and frequency of CD4+, CD8+, and CD68+ cells. We observed that the frequency (%, Figure 1B) and number (Figure 1C) of CD4+ and CD8+ did not change before and after treatment, or comparing after the different treatments. However, although the frequency (%, Figure 1B) and number (Figure 1C) of CD68+ macrophages did not significantly change after Sbv treatment, they were significantly lower in lesions from ML patients after treatment using PTX associated with Sbv, as compared with lesions before treatment.
Figure 1.
Comparative analysis of (A) total inflammatory infiltrate, (B) percent frequency, and (C) number of T CD4+, T CD8+, and CD68+ cells in lesions from mucosal leishmaniasis patients before and after treatment with Sbv alone (n = 5) or with Sbv + pentoxifylline (PTX) (n = 5). Data are expressed in box plots. The box extends from the 25th percentile to 75th percentile, with a horizontal line at the median (50th percentile). Whiskers extend from the lowest value to the 25th percentile and from the 75th percentile to the highest value, showing the range of data distribution. Statistical significance is indicated in each graph. Bottom panels in (B) show representative confocal images of lesions before and after treatment with Sbv + PTX, showing the staining for CD68 (green) and DAPI (blue). Values represent the average ± SD of each group following numeric determination of the percentage of CD68+ cells. Asterisks indicate statistically significant differences between groups at P < 0.05. Bar = 10 µm. This figure appears in color at www.ajtmh.org.
Pentoxifylline associated with Sbv reduces the number of CD68+ TNF-alpha+ cells in lesions from ML patients without reducing the number of CD68+ cells expressing IL-10.
The total expression of the cytokine TNF-alpha, IFN-gamma, and IL-10 was evaluated in lesions from ML patients submitted to the different treatment regimens. Our results showed that the number and percent frequency of cells expressing the different cytokines were not statistically different when comparing the different treatments, or comparing the groups before and after the treatments (Table 2).
Table 2.
Number and percentage of total IFN-γ, TNF-α, and IL-10-expressing cells, and ratio TNF/IL-10 expressing cells before and after treatment with pentavalent antimonial (Sbv) or pentavalent antimonial + pentoxifylline (Sbv + PTX)
| Sbv | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||||||||
| Patient | IFN-γ | % IFN-γ | TNF-α | % TNF-α | IL-10 | % IL-10 | Ratio TNF/IL-10 | IFN-γ | % IFN-γ | TNF-α | % TNF-α | IL-10 | % IL-10 | Ratio TNF/IL-10 |
| #1 | 238.5 | 65.8 | 553.8 | 50.9 | 481.5 | 132.9 | 1.15 | 291.00 | 57.90 | 87.0 | 5.8 | 352.0 | 70.0 | 0.25 |
| #2 | 745.0 | 62.7 | 247.0 | 6.9 | 436.0 | 36.7 | 0.57 | 123.50 | 32.10 | 597.0 | 51.8 | 38.0 | 9.9 | 15.71 |
| #3 | 35.3 | 6.3 | 1124.3 | 66.8 | 765.8 | 136.5 | 1.47 | 766.30 | 99.10 | 374.7 | 16.1 | 790.0 | 102.1 | 0.47 |
| #4 | 498.3 | 61.9 | 29.3 | 4.5 | 564.7 | 262.5 | 0.05 | 197.00 | 48.50 | 878.0 | 65.9 | 127.0 | 28.6 | 6.91 |
| #5 | 42.0 | 19.5 | 936.7 | 38.8 | 629.0 | 78.1 | 1.49 | 56.80 | 12.80 | 5.0 | 0.4 | 1097.7 | 270.1 | 0.00 |
| Average | 311.8 | 43.2 | 578.2 | 33.6 | 575.4 | 129.3 | 0.9 | 286.9 | 50.1 | 388.3 | 28.0 | 480.9 | 96.1 | 4.7 |
| SD | 307.0 | 28.1 | 457.8 | 27.3 | 129.9 | 85.2 | 0.6 | 281.8 | 32.3 | 361.0 | 29.1 | 451.1 | 103.6 | 6.8 |
| Sbv+PTX | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||||||||
| Patient | IFN-γ | % IFN-γ | TNF-α | % TNF-α | IL-10 | % IL-10 | Ratio TNF/IL-10 | IFN-γ | % IFN-γ | TNF-α | % TNF-α | IL-10 | % IL-10 | Ratio TNF/IL-10 |
| #6 | 0.0 | 0.0 | 37.5 | 3.5 | 468.0 | 132.1 | 0.08 | 355.2 | 25.0 | 28.0 | 2.0 | 493.0 | 104.2 | 0.06 |
| #7 | 313.0 | 10.7 | 216.0 | 7.4 | 59.0 | 6.0 | 3.66 | 186.5 | 5.0 | 140.0 | 3.7 | 852.0 | 67.9 | 0.16 |
| #8 | 74.0 | 4.9 | 1324.0 | 88.2 | 250.5 | 50.1 | 5.29 | 120.2 | 9.3 | 781.3 | 60.7 | 160.8 | 37.4 | 4.86 |
| #9 | 446.5 | 19.1 | 1178.3 | 50.4 | 578.5 | 74.2 | 2.04 | 535.0 | 19.0 | 1090.0 | 38.7 | 433.3 | 46.2 | 2.52 |
| #10 | 1597.7 | 46.6 | 933.0 | 27.2 | 619.3 | 54.2 | 1.51 | 838.7 | 35.9 | 90.0 | 3.8 | 55.7 | 7.1 | 1.62 |
| Average | 486.2 | 16.3 | 737.8 | 35.3 | 395.1 | 63.3 | 2.5 | 407.1 | 18.8 | 425.9 | 21.8 | 398.9 | 52.6 | 1.8 |
| SD | 646.8 | 18.4 | 578.5 | 34.9 | 236.2 | 45.8 | 2.0 | 290.0 | 12.4 | 479.6 | 26.6 | 312.2 | 36.2 | 2.0 |
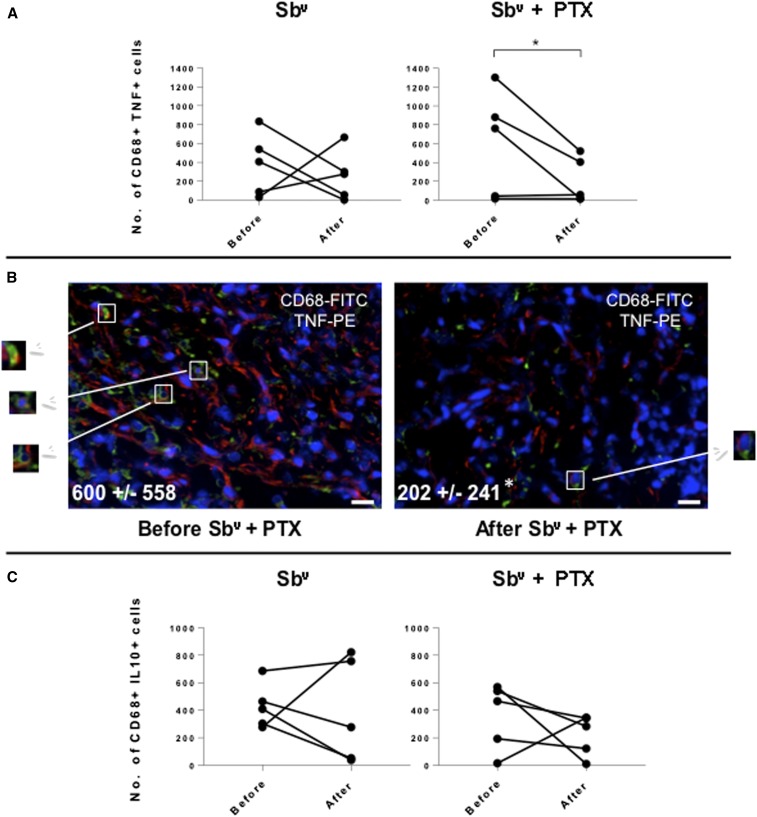
Given that PTX has known activity over TNF-alpha response,14 and that our previous studies showed that CD68+ cells are the main source of TNF-alpha in ML lesions,5 we determined the expression of TNF specifically by CD68+ cells in lesions before and after the different treatments. We observed that although treatment with SBv alone did not lead to changes in the number of CD68+ TNF-alpha+ cells, treatment with Sbv + PTX led to a reduction in the number of CD68+ TNF-alpha+ cells in lesions from ML patients (Figure 2A and B). The percent frequencies of CD68+ cells expressing TNF were 70 ± 30% and 59 ± 20% before and after treatment with SBv, respectively, and 63 ± 32% and 42 ± 17% before and after Sbv + PTX, respectively. These results show that CD68+ cells are responsible for the great majority of TNF expression. Treatment with Sbv + PTX did not significantly alter the frequency of CD68+IL-10+ cells (Figure 2C). Correlation analysis between the intensity of the inflammatory infiltrate and the number of TNF-alpha–expressing cells was also performed. Our data showed that there was a statistically significant positive correlation between the intensity of the inflammatory infiltrate and the number of TNF-alpha–expressing cells in lesions from ML patients before treatment with Sbv + PTX, which was lost after treatment (Figure 3). The opposite was observed after treatment with Sbv alone (Figure 3).
Figure 2.
(A) Comparative analysis of the number of CD68+TNF-alpha+ cells in lesions from mucosal leishmaniasis (ML) patients before and after treatment with Sbv alone or with Sbv + pentoxifylline (PTX), showing the variation for each patient. (B) Representative images from confocal microscopy analysis for determination of the frequencies of CD68+ cells in lesions from ML patients before and after treatment with Sbv + PTX. The overlay for CD68 (green), TNF (red), and DAPI (blue) is shown. Values represent the average ± SD of each group, following numeric determination of the percentage of CD68+TNF-alpha+ cells. Asterisks indicate statistically significant differences between groups at P < 0.05. Boxes and arrows indicate the detail of double-positive cells (slightly yellow, because of superimposing of green and red stains, for CD68 and TNF, respectively). Bar = 10 µm. (C) Comparative analysis of the number of CD68+IL-10+ cells in lesions from ML patients before and after treatment with Sbv alone or with Sbv + PTX, showing the variation for each patient. This figure appears in color at www.ajtmh.org.
Figure 3.
Correlation analysis between the inflammatory infiltrate and the number of TNF-alpha+ cells in lesions from mucosal leishmaniasis patients before and after treatment with Sbv alone or with Sbv + pentoxifylline (PTX). Statistical significance is indicated in the graphs.
Treatment with PTX associated with Sbv treatment does not alter the frequency of granzyme A expression, and high expression of granzyme A is correlated with longer healing time.
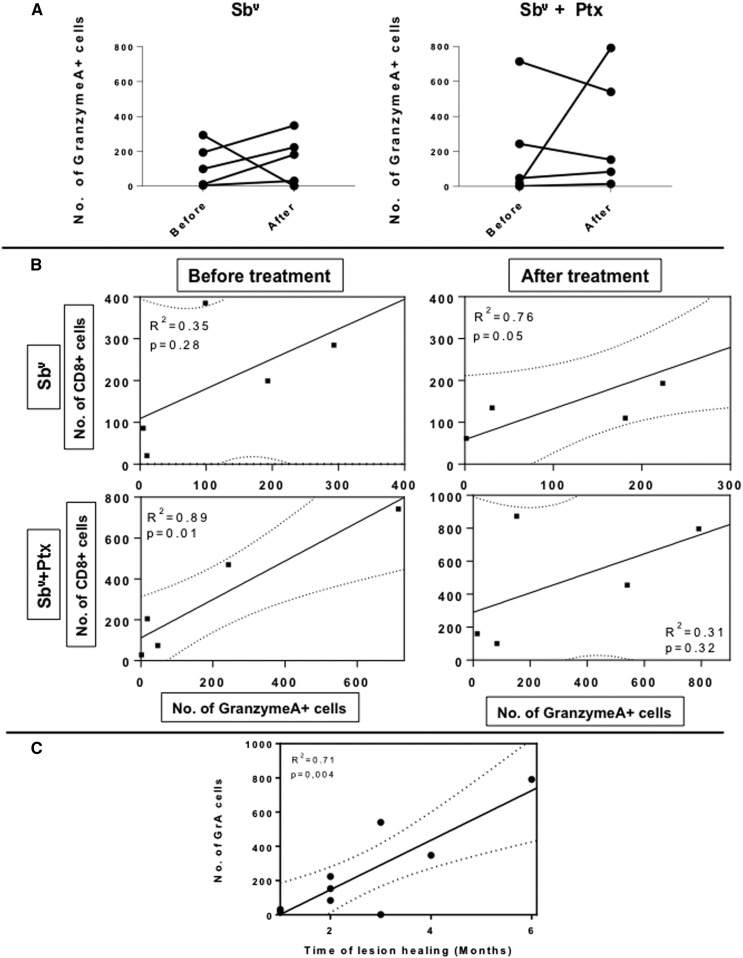
The number of cells expressing the granzyme A, a cytotoxic molecule associated with lesion progression in tegumentary leishmaniasis,5,7–9 was determined using confocal analysis. The results showed that the number of cells expressing the granzyme A was not statistically different when comparing the different treatments or before and after each treatment (Figure 4A). However, although a positive correlation between the number of granzyme A+ cells and CD8+ cells was observed after Sbv treatment, treatment with PTX + Sbv led to a loss of positive correlation between the frequency of CD8+ T cells and the expression of granzyme A, suggesting a functional control of this putative mechanism of tissue destruction (Figure 4B). Interestingly, high expression of granzyme was correlated with longer time of healing, corroborating the importance of this molecule in tissue pathology (Figure 4C).
Figure 4.
(A) Comparative analysis of the number of granzyme A+ cells in lesions from mucosal leishmaniasis (ML) patients before and after treatment with Sbv alone or with Sbv + pentoxifylline (PTX), showing the variation for each patient. (B) Correlation analysis between the number of CD8+ cells and the number of granzyme A+ cells in lesions from ML patients before and after treatment with Sbv alone or with Sbv + PTX. (C) Correlation analysis between the number of granzyme A+ cells and the healing time after treatment. The graph combines patients treated with the two different regimens. Statistical significance is indicated in the graphs.
DISCUSSION
Mucosal leishmaniasis is a destructive disease, caused predominantly by the infection with L. braziliensis. It results from an unregulated immune response, which is related to hyperactivation of T cells and consequent exacerbation of the inflammatory response.5,6 The drug of choice for treatment of ML is Sbv, but the high treatment failure rate or relapse observed with Sbv therapy indicates the need for an alternative drug intervention. Considering that the tissue damage observed in leishmaniasis is associated with the expression of pro-inflammatory mediators, immunomodulatory agents are potential candidates to be used as adjuvant therapy in patients with ML. Our group has studied the use of PTX for the treatment of infectious diseases in that the tissue damage is mediated by the inflammatory response induced by TNF-alpha. Those studies have shown that the combination of Sbv with PTX led to the healing of lesions of refractory cases of ML, as well as a higher cure rate when compared with Sbv and placebo in a controlled randomized trial.12,15,16,19 This was also observed here. Although the time of healing varied in the individuals submitted to the alternative therapy, all individuals cured. To better understand the mechanisms that led to this successful treatment, we evaluated the cellular composition of lesions from patients treated with Sbv alone and with the combination of PTX with Sbv, as well as cytokine and granzyme A expression, which are involved in immunoregulation and cytotoxic activity, respectively.
The composition of the inflammatory infiltrate in lesions from ML patients submitted to the different treatment regimen was determined using confocal microscopy analysis, and no differences were observed when we compared the intensity of the inflammatory infiltrate before and after each treatment and between the two treatments. We then asked whether the cell populations that composed the inflammatory infiltrates differed. Previous studies have demonstrated that the inflammatory infiltrate of ML lesions is primarily composed of T cells and macrophages.5,20,21 Our analysis of these cell populations showed that the frequency of CD4+ and CD8+ T cells did not differ when comparing between treatments, or before and after each treatment. However, we observed that treatment with Sbv + PTX led to a decrease in the frequency of CD68+ cells after treatment. Given that macrophages are cells that harbor the parasite,22 the lower frequency of these cells in the lesions may offer a less hospitable environment for the parasite, favoring its control. Treatment with Sbv alone did not decrease the frequency of these cells. This suggests that the combined treatment decreases the recruitment of CD68+ cells to lesion sites. Alternatively, the lower number of CD68+ cells after Sbv + PTX could be associated with the apoptosis of these cells. Treatment with Sbv + PTX reduced not only the macrophage population but also the expression of TNF-alpha by these cells. Previous study demonstrated that PTX had a significant suppressive effect in the spontaneous production of TNF-alpha in ML patients, preserving the viability of the cells.12 These data speak in favor of the decreased recruitment of CD68+ cells, given that PTX could, in fact, prevent apoptosis by decreasing TNF production.
Previous studies by our group have shown that monocytes are the main producers of TNF-alpha in lesions5 and peripheral blood23 of ML patients. Here, we also observed that most TNF-expressing cells were CD68+. We did not stain for CD4 and CD8 concomitantly with TNF, but it is possible to extrapolate from the total of TNF+ cells minus the CD68+TNF+ cells that non-CD68+ cells (mostly T cells) are responsible for approximately 40% of the TNF production in the lesions before and after treatment. Thus, although T-cell subpopulations may also contribute to TNF expression, the great majority of TNF comes from CD68+ cells. Although we did not observe a statistically significant decrease in total TNF-alpha expression, we did observe a clear tendency of a decrease in TNF expression in three of five patients treated with Sbv + PTX (Table 2), and a significantly lower frequency of CD68+ cells expressing TNF-alpha after Sbv + PTX treatment (Figure 2A and B). Moreover, we observed a positive correlation between the number of TNF-alpha+ cells and the intensity of the inflammatory infiltrate after Sbv treatment, but this correlation was not observed after treatment with Sbv + PTX (Figure 3), suggesting that Sbv + PTX treatment is acting in the modulation of the inflammatory response by interfering with the expression of TNF by CD68+ cells. The fact that PTX did not completely inhibit TNF expression is in agreement with previous studies showing that PTX was only able to decrease TNF-alpha expression by cells from HTLV-1 patients when used at a very high concentration.24,25 Clinical studies have shown that the major contribution of PTX in the therapeutic response in ML is likely due to the faster tissue reepithelialization, associated with decreased TNF-alpha secretion.12,15,18 It has been shown that TNF-alpha is capable of inducing apoptosis of intestinal mucosa epithelial cells in patients with inflammatory bowel disease and that the use of neutralizing anti–TNF-alpha antibodies led to a faster epithelialization due to a decrease in apoptosis of epithelial cells.26 The effect of TNF-alpha in inducing apoptosis of epithelial cells has also been shown in lung epithelial cells27 and normal human prostate epithelial cells.28 Thus, it is possible that the observed decrease in TNF-alpha expression by Sbv + PTX treatment has an effect in preventing apoptosis of epithelial cells in ML lesions, allowing for a faster healing.
Our group demonstrated that in peripheral blood and lesions from cutaneous leishmaniasis (CL) and ML patients, CD4+ T cells represented the majority of IFN-gamma–producing cells and the high expression of IFN-gamma was associated with intensity of inflammation in lesions.5,23 We have also shown that the intensity of inflammation is compatible with lesion progression in CL patients and associated with a striking recruitment of CD8+ T cells and higher IFN-gamma expression in patients with the late stage (L-CL) of CL compared with early stage (E-CL).7 Furthermore, IFN-gamma, an inflammatory cytokine, is a potent mediator of Leishmania killing in synergy with TNF-alpha and effector mediators, such as nitric oxide (NO).2,22,29 In addition to NO induction, IFN-gamma induces cytotoxic activity, which is directly correlated with granzyme A expression by CD8+ T cells.30,31 Cytotoxic T lymphocytes eliminate cells infected with intracellular pathogens by mechanisms involving the delivery of granzyme/perforin-rich vesicles, or by engaging cell surface death receptors, such as first apoptosis signal.32 Granzymes A and B are the most abundant and quickly induce cell death through alternative pathways without a clear overlapping.33–35 In addition, granzyme A can activate macrophages to secrete cytokines and act in the degradation of cellular matrix proteins causing tissue damage.35 Our previous studies demonstrated that L-CL patients express significantly higher levels of granzyme A than E-CL patients, and that granzyme A expression is more abundant in lesions of patients with ML, suggesting the key role of this cytotoxic molecule in lesion progression and tissue damage in human leishmaniasis.5,7 In this work, there was no change in expression of IFN-gamma and granzyme A after either treatments, or when compared between them. However, we observed a positive correlation between the number of CD8+ cells and granzyme A expression only after Sbv treatment. On the other hand, treatment with Sbv + PTX led to a loss of positive correlation between the number of CD8+ T cells and granzyme A observed before treatment in this group. These data suggest that the relative lower efficiency of the treatment with Sbv alone in these ML patients may be associated with the fact that they cannot control the immune response and, thus, cannot contain the activation of cytotoxic CD8+ cells, which have been associated with tissue destruction in CL and ML. Thus, the lack of association between the frequency of CD8+ T cells and granzyme A after treatment with Sbv + PTX suggests that the association of PTX with Sbv may assist in the establishment of tissue reconstruction by controlling activation of potentially cytotoxic cells. Adding to the role of granzyme A in tissue pathology, in this work, we show that the longer healing time is directly correlated with higher expression of granzyme A in lesions.
Although PTX does not completely inhibit TNF production, it is known to downregulate TNF-alpha production to some extent,14 and this mechanism can be responsible for the clinical improvement observed in ML patients. It is important to mention that the fact that TNF production is not completely inhibited may be beneficial for maintaining the other TNF-associated functions, such as triggering mechanisms of parasite control.36 Although PTX is very attractive therapeutically for the treatment of inflammatory complications associated with infectious diseases, and has been used, for example, in erythema nodosum leprosum,37 there is no evidence that PTX directly kills Leishmania or activates macrophages. Thus, it is likely that the effects of PTX are related to the control of the immune response, as supported by the data presented here. One interesting aspect is that the treatment with Sbv + PTX did not decrease the frequency of CD68+IL10+ cells, which can be helpful in controlling the inflammation during the treatment. The fact that the inflammatory infiltrate did not decrease even in the maintenance of high IL-10 expression can be related to the low expression of IL-10 receptor in lesion from ML previously described by us.5
Our data show the characteristics of the inflammatory infiltrate after treatment using Sbv + PTX, where we observed a lower number of CD68+TNF+ cells, as well as the loss of an association between the expression of granzyme A and TNF and pathology parameters. Thus, our results indicate a more efficient modulation of the immune response at the site of inflammation and consequently an accelerated healing in patients treated with Sbv + PTX. These findings strengthen the choice of this therapeutic approach for patients with refractory or severe ML.
Acknowledgments:
We are grateful to the funding agencies FAPEMIG, CAPES, INCT-DT, and NIH/TMCR. P. R. L. M., K. J. G., E. C., and W. O. D. are CNPq fellows. We are also indebted to Ednaldo Lago and the workers from the Corte de Pedra Health Post, where patients are received, examined, and all material collected, for their outstanding work.
REFERENCES
- 1.Desjeux P, 2004. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305–318. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira WN, Ribeiro LE, Schrieffer A, Machado P, Carvalho EM, Bacellar O, 2014. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of human tegumentary leishmaniasis. Cytokine 66: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souza AS, Giudice A, Pereira JM, Guimarães LH, de Jesus AR, de Moura TR, Wilson ME, Carvalho EM, Almeida RP, 2010. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-α production. BMC Infect Dis 10: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gollob KJ, Viana AG, Dutra WO, 2014. Immunoregulation in human American leishmaniasis: balancing pathology and protection. Parasite Immunol 36: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faria DR, et al. 2005. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun 73: 7853–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bacellar O, Lessa H, Schriefer A, Machado P, De Jesus AR, Dutra WO, Gollob KJ, Carvalho EM, 2002. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun 70: 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria DR, Souza PEA, Durães FV, Carvalho EM, Gollob KJ, MacHado PR, Dutra WO, 2009. Recruitment of CD8+ T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite Immunol 31: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Santos C, et al. 2013. CD8+GrazymeB+-mediated tissue injury vs. CD4+IFNgamma+-mediated killing in human leishmaniasis. J Invest Dermatol 133: 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardoso TM, Machado Á, Costa DL, Carvalho LP, Queiroz A, Machado P, Scott P, Carvalho EM, Bacellar O, 2015. Protective and pathological functions of CD8+ T cells in Leishmania braziliensis infection. Infect Immun 83: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novais FO, Scott P, 2015. CD8+ T cells in cutaneous leishmaniasis: the good, the bad and the ugly. Seim Immunopathol 37: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraz R, Cunha CF, Pimentel MIF, Lyra MR, Pereira-Da-Silva T, Schubach AO, Da-Cruz AM, Bertho AL, 2017. CD3+CD4negCD8neg (double negative) T lymphocytes and NKT cells as the main cytotoxic-related-CD107a+ cells in lesions of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. Parasit Vectors 10: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lessa HA, Machado P, Lima F, Cruz ÁA, Bacellar O, Guerreiro J, Carvalho EM, 2001. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg 65: 87–89. [DOI] [PubMed] [Google Scholar]
- 13.Cataldo JI, et al. 2018. Favorable responses to treatment with 5 mg Sbv/kg/day meglumine antimoniate in patients with American tegumentary leishmaniasis acquired in different Brazilian regions. Rev Soc Bras Med Trop 51: 769–780.Lessa HA, Lessa MM, Guimarães LH, Lima CMF, Arruda S, Machado PR, Carvalho EM, 2012. A proposed new clinical staging system for patients with mucosal leishmaniasis. Trans R Soc Trop Med Hyg 106: 376–381. [DOI] [PubMed] [Google Scholar]
- 14.Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U, 1999. Pentoxifylline inhibits TNF-α production from. Am J Respir Crit Care Med. 159: 508–511. [DOI] [PubMed] [Google Scholar]
- 15.Machado PRL, Lessa H, Lessa M, Guimaraes LH, Bang H, Ho JL, Carvalho EM, 2007. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for mucosal leishmaniasis. Clin Infect Dis 44: 788–793. [DOI] [PubMed] [Google Scholar]
- 16.Brito G, Dourado M, Guimarães LH, Merieles E, Schriefer A, Carvalho EM, Machado PRL, 2017. Oral pentoxifylline combined with pentavalent antimony: a randomized trial for cutaneoul leishmaniasis. Am J Trop Med Hyg 96: 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessa HA, Lessa MM, Guimarães LH, Lima CMF, Arruda S, Machado PR, Carvalho EM, 2012. A proposed new clinical staging system for patients with mucosal leishmaniasis. Trans R Soc Trop Med Hyg 106: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da-Cruz AM, De Oliveira MP, De Luca PM, Mendonça SC, Coutinho SG, 1996. Tumor necrosis factor-α in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz 91: 225–229. [DOI] [PubMed] [Google Scholar]
- 19.Brito G, Dourado M, Polari L, Celestino D, Carvalho LP, Queiroz A, Carvalho EM, Machado PRL, Passos S, 2014. Clinical and immunological outcome in cutaneous leishmaniasis patients treated with pentoxifylline. Am J Trop Med Hyg 90: 617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esterre P, Guerret S, Ravisse P, Dimier-David L, Dedet JP, Grimaud JA, 1994. Immunohistochemical analysis of the mucosal lesion in mucocutaneous leishmaniasis. Parasite 1: 305–309. [DOI] [PubMed] [Google Scholar]
- 21.da Costa DC, et al. 2014. Oral manifestations in the American tegumentary leishmaniasis. PLoS One 9: e109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunet LR, 2001. Nitric oxide in parasitic infections. Int Immunopharmacol 1: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 23.Bottrel RLA, et al. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble leishmania antigen in human cutaneous leishmaniasis. Infect Immun 69: 3232–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luna T, Santos SB, Nascimento M, Porto MAF, Muniz AL, Carvalho EM, Jesus AR, 2011. Effect of TNF-α production inhibitors on the production of pro-inflammatory cytokines by peripheral blood mononuclear cells from HTLV-1-infected individuals. Braz J Med Biol Res 44: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro de Jesus A, Luna T, Pacheco de Almeida R, Machado PRL, Carvalho EM, 2008. Pentoxifylline down modulate in vitro T cell responses and attenuate pathology in Leishmania and HTLV-I infections. Int Immunopharmacol 8: 1344–1353. [DOI] [PubMed] [Google Scholar]
- 26.Goretsky T, Dirisina R, Sinh P, Mittal N, Managlia E, Williams DB, Posca D, Ryu H, Katzman RB, Barrett TA, 2012. P53 mediates TNF-induced epithelial cell apoptosis in IBD. Am J Pathol 181: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, Alam G, Zagariya A, Gidea C, Pinillos H, Lalude O, Choudhary G, Oezatalay D, Uhal BD, 2000. Apoptosis of lung epithelial cells in response to TNF-α requires angiotensin II generation de novo. J Cell Physiol 185: 253–259. [DOI] [PubMed] [Google Scholar]
- 28.Chopra DP, Menard RE, Januszewski J, Mattingly RR, 2004. TNF-α-mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett 203:145–154. [DOI] [PubMed] [Google Scholar]
- 29.Miller RA, Britigan BE, 1997. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue ML, Schlossman SF, 1990. A monoclonal antibody reactive with a 15-kDa cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 144: 574–582. [PubMed] [Google Scholar]
- 31.Trapani JA, Smyth MJ, 2002. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol 2: 735–747. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman J, 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 3: 361–370. [DOI] [PubMed] [Google Scholar]
- 33.Fan Z, Zhang Q, 2005. Molecular mechanisms of lymphocyte-mediated cytotoxicity. Cell Mol Immunol 2: 259–264. [PubMed] [Google Scholar]
- 34.Sower LE, Froelich CJ, Allegretto N, Rose PM, Hanna WD, Klimpel GR, 1996. Extracellular activities of human granzyme A. monocyte activation by granzyme A versus alpha-thrombin. J Immunol 156: 2585–2590. [PubMed] [Google Scholar]
- 35.Dotiwala F, Mulik S, Polidoro RB, Ansara JA, Burleigh BA, Walch M, Gazzinelli RT, Lieberman J, 2016. Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites. Nat Med 22: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liew FY, Li Y, Millott S, 1990. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol 145: 4306–4310. [PubMed] [Google Scholar]
- 37.de Carsalade GY, Achirafi A, Flageul B, 2003. Pentoxifylline in the treatment of erythema nodosum leprosum. J Dermatol 30: 64–68. [PubMed] [Google Scholar]