Abstract.
In this study, we investigated the diagnostic utility of the cytokine profile of the cerebrospinal fluid (CSF) and enzyme-linked immunospot (ELISPOT) assays of patients with suspected tuberculous meningitis (TBM). We prospectively enrolled adult patients with suspected TBM, and CSF specimens were analyzed for 18 cytokines/chemokines and soluble programmed death protein 1 (PD-1) and programmed death ligand 1 (PD-L1). Enzyme-linked immunospot assays were performed on mononuclear cells from the CSF (CSF-MCs) and peripheral blood (PBMCs). A total of 87 patients with meningitis, including 42 TBM-suspected patients and 45 non-TBM patients, were enrolled. Excluding the 32 patients with possible TBM, 10 patients with TBM and 45 patients with non-TBM were finally analyzed. Levels of adenosine deaminase (ADA), interleukin 12 subunit β (IL-12p40), IL-13, macrophage inflammatory protein α (MIP-1α), and soluble PD-1 and PD-L1 in the CSF were significantly higher in the TBM group than in the non-TBM group (P < 0.05). The optimal cutoff values for the sensitivities and specificities of the test methods for diagnosing TBM with small samples of 10 cases of definite or probable TBM were as follows: ADA > 6.95 U/L, 70% and 81%; IL-12p40 > 52.04 pg/mL, 80% and 73%; IL-13 > 0.44 pg/mL, 90% and 47%; MIP-1α > 8.83 pg/mL, 80% and 62%; soluble PD-1 > 35.87 pg/mL, 80% and 63%; soluble PD-L1 > 24.19 pg/mL, 80% and 61%; CSF-MC ELISPOT > 13.5 spots/250,000 CSF-MC, 30% and 91%; and PBMC ELISPOT > 14 spots/250,000 PBMCs, 50% and 78%, respectively. Therefore, CSF IL-12p40, IL-13, MIP-1α, and soluble PD-1 and PD-L1 concentrations appear to be useful adjuncts for diagnosing TBM.
INTRODUCTION
Tuberculosis (TB) is the ninth leading cause of death worldwide, according to the WHO global tuberculosis report for 2017.1 Among various types of TB, tuberculous meningitis (TBM) is the most lethal and disabling form.2,3 Early initiation of treatment is the most significant factor determining outcome, but the diagnosis of TBM is difficult because the clinical features are nonspecific and the laboratory tests are insensitive. The gold standard for diagnosing TBM is culture of bacilli from the cerebrospinal fluid (CSF); however, this test is too slow to be clinically useful. Although the acid-fast bacilli (AFB) smear test is a rapid and inexpensive screen test for TBM, it has low sensitivity in routine clinical practice.4 The nucleic acid amplification test (NAAT) has been widely used to provide rapid diagnosis of TB5 compared with culture of bacilli from sample. However, the previous studies revealed that that commercial NAATs have high specificity but low sensitivity6,7 for diagnosis of TBM. The recently developed Xpert Mycobacterium tuberculosis (MTB)/RIF assay, the automated polymerase chain reaction (PCR) system endorsed by WHO, has shown the promising results in paucibacillary TB.8 However, it still showed low sensitivity in diagnosis of TBM,9,10 although the recent study11 showed that Xpert MTB/RIF Ultra assay reported improved sensitivity of the assay compared with Xpert MTB/RIF and MGIT culture. Recently, interferon-γ (IFN-γ) releasing assays that assess MTB-specific cell-mediated immune responses have yielded promising results in the diagnosis of active TB.12–14 Enzyme-linked immunosorbent spot (ELISPOT) assays using compartmentalized mononuclear cells at the site of infection, such as peritoneal fluid, bronchoalveolar fluid, and CSF, showed better diagnostic specificity than those using peripheral blood mononuclear cells (PBMCs).15 Also, there have been many attempts to investigate biomarkers to diagnose TBM.16–20
Although immunity against M. tuberculosis is not fully understood, immunological markers associated with the host-M. tuberculosis interaction are considered as potential biomarkers for diagnosis. Previous studies have suggested that some cytokines and chemokines could be such biomarkers.21–25 Moreover, recent studies reported that the expression of programmed death protein 1 (PD-1) and programmed death ligand 1 (PD-L1), which play a role in terminating immune responses and lead to incomplete elimination of pathogens in several persistent infections,26–28 is associated with decreased numbers of effector T cells and blockade of PD-1 pathway restored T-cell functions during active TB.29–33 However, studies on cytokines and chemokines in CSF as biomarkers for the diagnosis of TBM are limited.34–37 We, therefore, investigated the diagnostic utility of various cytokines in CSF and cell-mediated immune responses from CSF in patients with suspected TBM.
METHODS
Patients.
Adult patients with suspected TBM admitted to Asan Medical Center, a 2,700-bed tertiary hospital in Seoul, South Korea, were prospectively enrolled from March 2015 to August 2017. We enrolled adult patients of more than 16 years old with suspected TBM who agreed to additional sampling of CSF and blood for ELISPOT assays. Cerebrospinal fluid and blood samples were collected before starting anti-TB treatment.
Clinical category of TBM.
Patients with suspected TBM were categorized as definite TBM, probable TBM, possible TBM, or non-TBM, according to a recently proposed uniform case definition. In brief, they were classified as having “definite TBM” if clinical specimens were found to be positive for the AFB stain, M. tuberculosis culture, or M. tuberculosis PCR. Patients were classified as having “probable TBM” or “possible TBM” according to their scores in the uniform research case definition for TBM,38 and as having “non-TBM” if an alternative diagnosis was established. To classify the diagnostic performances of the various biomarkers, definite TBM and probable TBM were used as the reference standards for TBM, and non-TBM was used as the reference standard for without TBM.
Laboratory tests.
Microbiological and pathological specimens for diagnosis of TBM were processed by standard techniques and procedures, as described elsewhere.12 In brief, at least 2 mL of CSF sample was inoculated on liquid (BACTEC MGIT 960, BD, Franklin Lakes, NJ) and solid (Ogawa media, Korea Institute of Tuberculosis, Seoul, Korea) culture media for mycobacterial culture, and the M. tuberculosis complex was identified using a commercial DNA probe (AccuProbe Mycobacterium complex culture identification kit; Gene-Probe, San Diego, CA). M. tuberculosis PCR (COBAS® TaqMan® MTB test; Roche Diagnostics, Branchburg, NJ) was performed by using at least 100 μL of non-centrifuged CSF sample.
Mycobacterium tuberculosis–specific IFN-γ ELISPOT assay.
Mycobacterium tuberculosis–specific IFN-γ ELISPOT assays (T-SPOT.TB; Oxford Immunotec, Oxford, United Kingdom) were performed on mononuclear cells from CSF (CSF-MCs) and PBMCs. Briefly, PBMCs were separated from heparinized peripheral blood by density gradient using lymphocyte separation medium (Corning, New York, NY), and CSF-MCs were collected by centrifugation of CSF. The PBMCs and CSF-MCs were suspended in appropriate volumes of AIM-V medium, at concentrations of 2.5 × 106 cells/mL. The samples were incubated in wells precoated with antihuman IFN-γ antibody and stimulated with culture filtrate protein 10 and early secretory antigenic target 6 (ESAT6), phytohemagglutinin, and medium only. Spots were detected and counted with an automated ELISPOT plate reader (Autoimmune Diagnostika GmbH, Strasburg, Germany), and the results were expressed as spot-forming cells/2.5 × 105 cells.
Cytokine analysis.
We simultaneously analyzed levels of 18 cytokines and chemokines in CSF samples from 42 TBM patients and 45 non-TBM patients by using cytometric bead array based on microspheres for detecting cytokine/chemokine in accordance with the manufacturer’s instructions (BD Bioscience, San Jose, CA). We measured granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, interferon (IFN)-α, IFN-γ, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-12p40, IL-13, IL-17A, monocyte chemotactic protein-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated on activation and normally T-cell expressed and secreted, IFN-γ–induced protein (IP)-10, and vascular endothelial growth factor. Data were acquired using FACS CANTO II flow cytometer, FACSDiva software (BD Biosciences, San Jose, CA), and FlowJo software (FlowJo LLC, Ashland, OR).
In addition, soluble PD-1 and soluble PD-L1 levels in CSF samples were measured with commercial ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer’s protocol. Data were acquired using the SpectraMax 190 microplate reader and SoftMax Pro software (Molecular Device, San Jose, CA).
Statistical analyses.
Statistical analyses were performed using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA) and SPSS for Windows software package, version 23 (SPSS Inc., Chicago, IL). Differences between TBM and non-TBM groups were analyzed with the Mann–Whitney U test. The Spearman test was used to calculate correlation coefficients between cytokine/chemokine levels, ELISPOT results, adenosine deaminase (ADA) levels, and diagnostic scores. Optimal cutoff values for cytokine/chemokine levels, ELISPOT results, and ADA levels were assessed from receiver operating characteristic (ROC) curves, by selecting the farthest point from the diagonal line that maximized the sum of sensitivity and specificity. Diagnostic performance was expressed in terms of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR), and negative LR. All tests of significance were two-tailed, and P-values less than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics of the patients.
Eighty-seven patients with suspected TBM who agreed to sampling for PBMCs and for additional CSF were enrolled. Of these, 42 (48.3%) were classified according to a recently proposed uniform case definition as definite (n = 2), probable (n = 8), and possible (n = 32) TBM patients. We performed lumbar puncture once in six TBM patients, twice in two patients, thrice in one patient, and 4 times in one patient before the initiation of anti-TB treatment. Two definite TBM patients had positive results of M. tuberculosis culture in the first CSF sample. Finally, of these 42 patients, 10 (11.9%) corresponding to the two with definite TBM and the eight with probable TBM were classified as having TBM. The remaining 45 (51.7%) patients were classified as non-TBM. The alternative diagnoses in this non-TBM group included bacterial meningitis (n = 5), viral meningitis (n = 23), fungal meningitis (n = 5), aseptic meningitis (n = 3), autoimmune (n = 2), CNS lupus (n = 1), MGUS neuropathy (n = 1), and encephalitis with unknown origin (n = 5).
Diagnostic performances of various biomarkers.
The clinical and laboratory data for the TBM and non-TBM groups are presented in Table 1. Patients with TBM were more likely to have duration of illness more than 5 days (P = 0.009) and to yield a miliary pattern on chest radiographs (P = 0.030). With respect to CSF profile, CSF/serum glucose ratio was lower in patients with TBM than in those with non-TBM (P = 0.002), whereas ADA level was higher (P = 0.012).
Table 1.
Clinical and laboratory parameters in tuberculous meningitis (TBM) and non-TBM
| Variable | TBM (n = 10)* | Non-TBM (n = 45) |
|---|---|---|
| Age (years), mean ± SD | 58.8 ± 13.0 | 51.0 ± 19.0 |
| Female gender | 2 (20) | 15 (33) |
| Duration of illness, median days (IQR) | 15.5 (9.3–23.3) | 7.0 (3.0–11.0) |
| Duration of illness > 5 days | 10 (100) | 24 (53) |
| Underlying condition or illness | ||
| HIV | 0 (0) | 0 (0) |
| Diabetes mellitus | 4 (40) | 1 (2) |
| Solid tumor | 2 (20) | 8 (18) |
| Hematologic malignancy | 1 (10) | 0 (0) |
| Chronic renal failure | 0 (0) | 2 (4) |
| Rheumatologic disease | 0 (0) | 1 (2) |
| Transplantation | 0 (0) | 1 (2) |
| Liver cirrhosis | 3 (30) | 2 (4) |
| Immunosuppressant use | 0 (0) | 1 (2) |
| No underlying illness | 3 (30) | 33 (73) |
| Immunosuppressive condition | 3 (30) | 12 (27) |
| CXR suggestive of miliary tuberculosis | 2 (20) | 0 (0) |
| CSF profile | ||
| WBC count, median cells × 106/L (IQR) | 156.5 (64.5–386.5) | 90.0 (30.5–252.5) |
| Lymphocyte percentage, median % (IQR) | 77.0 (48.5–89.0) | 63.0 (38.0–85.5) |
| Protein level, median mg/L (IQR) | 194.2 (59.4–397.7) | 83.8 (39.15–153.0) |
| CSF/serum glucose ratio, median ratio (IQR) | 0.34 (0.25–0.43) | 0.52 (0.44–0.62) |
| CSF ADA level, median U/L (IQR) | 10.6 (3.9–16.8)† | 3.6 (2.0–6.4 ) |
| Microbiological finding | ||
| Positive AFB stain from CSF | 0/9 (0) | 0/41 (0) |
| Positive M. tuberculosis PCR from CSF | 0/8 (0) | 0/39 (0) |
| Positive M. tuberculosis culture from CSF | 2/9 (22) | 0/41 (0) |
| Positive AFB stain from other extra-neural sample | 2/6 (33) | 0/22 (0) |
| Positive M. tuberculosis PCR from extra-neural sample | 1/2 (50) | 0/4 (0) |
| Positive M. tuberculosis culture from extra-neural sample | 2/6 (33) | 0/22 (0) |
AFB = acid-fast bacilli; ADA = adenosine deaminase; CSF = cerebrospinal fluid; PCR = polymerase chain reaction.
* TBM consisted of two definite and eight probable TBM patients.
† There was no ADA data for two of the probable TBM patients.
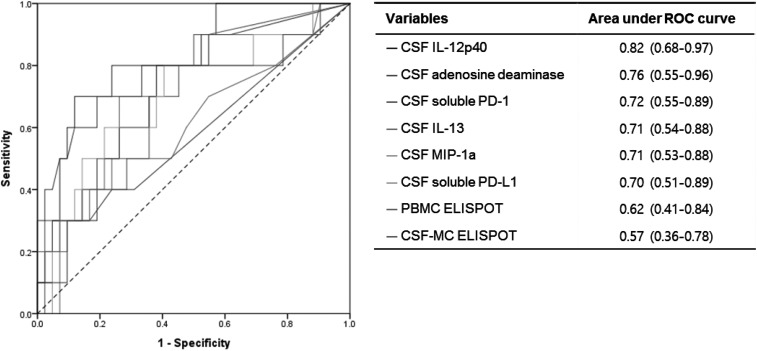
Box-and-whisker plots and the diagnostic performances of various biomarkers are shown in Figure 1, Supplemental Figure 1, and Table 2. There were no significant differences in T-cell responses to M. tuberculosis by PBMCs and CSF-MCs between patients with TBM and those patients without TBM (P > 0.05, respectively, Supplemental Figure 1). The optimal cutoff values for CSF-MC and PBMC ELISPOT assays obtained from ROC curves (Figure 2) were 13.5 spot-forming units (SFU) per 250,000 CSF-MCs and 14 SFU per 250,000 PBMCs, respectively.
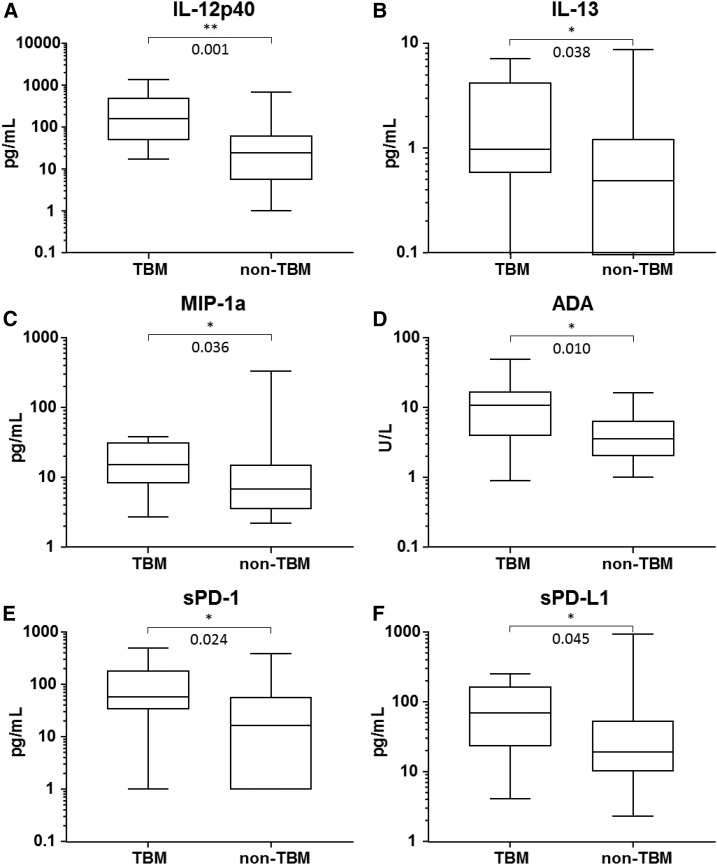
Figure 1.
Levels of expression of cerebrospinal fluid biomarkers in patients with definite or probable tuberculous meningitis (TBM) and without TBM.
Table 2.
Diagnostic performances of biomarkers in patients with definite + probable TBM vs. non-TBM
| Variable | Sensitivity | Specificity | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Proportion | Percentage (95% CI) | Proportion | Percentage (95% CI) | |||||
| CSF IL-12p40 > 52.04 pg/mL | 8/10 | 80.0 (44.4–97.5) | 33/45 | 73.3 (58.1–85.4) | 3.00 (1.69–5.33) | 0.27 (0.08–0.95) | 40.0 (27.3–54.2) | 94.3 (82.5–98.3) |
| CSF IL-13 > 0.44 pg/mL | 9/10 | 90.0 (55.5–99.8) | 21/45 | 46.7 (31.7–62.1) | 1.69 (1.20–2.38) | 0.21 (0.03–1.41) | 27.3 (21.0–34.6) | 95.5 (76.1–99.3) |
| CSF MIP-1α > 8.83 pg/mL | 8/10 | 80.0 (44.4–97.5) | 28/45 | 62.2 (46.5–76.2) | 2.12 (1.30–3.44) | 0.32 (0.09–1.13) | 32.0 (22.4–43.4) | 93.3 (79.9–98.0) |
| CSF soluble PD-1 > 35.87 pg/mL | 8/10 | 80.0 (44.4–97.5) | 27/43* | 62.8 (46.7–77.0) | 2.15 (1.31–3.53) | 0.32 (0.09–1.12) | 33.3 (23.3–45.1) | 93.1 (79.3–97.9) |
| CSF soluble PD-L1 > 24.19 pg/mL | 8/10 | 80.0 (44.4–97.5) | 26/43* | 60.5 (44.4–75.0) | 2.02 (1.25–3.28) | 0.33 (0.09–1.17) | 32.0 (22.5–43.3) | 92.9 (78.6–97.9) |
| CSF adenosine deaminase > 6.95 U/L | 7/10 | 70.0 (34.8–93.3) | 35/43* | 81.4 (66.6–91.6) | 3.76 (1.79–7.93) | 0.37 (0.14–0.96) | 46.7 (29.3–64.8) | 92.1 (81.8–96.8) |
| CSF-MC ELISPOT > 13.5 spot-forming unit/250,000 cells | 3/10 | 30.0 (6.7–65.3) | 41/45 | 91.1 (78.8–97.5) | 3.37 (0.89–12.77) | 0.77 (0.51–1.16) | 42.9 (16.5–74.0) | 85.4 (79.4-89.9) |
| PBMC ELISPOT > 14 spot-forming unit/250,000 cells | 5/10 | 50.0 (18.7–81.3) | 35/45 | 77.8 (62.9–88.8) | 2.25 (0.98–5.14) | 0.64 (0.34–1.22) | 33.3 (18.0–53.3) | 87.5 (78.7–93.0) |
CSF = cerebrospinal fluid; CSF-MC = mononuclear cells from the CSF; ELISPOT = enzyme-linked immunospot; IL = interleukin; LR = likelihood ratio; MIP-1α = macrophage inflammatory protein α; NPV = negative predictive value; PBMC = peripheral blood mononuclear cell; PD-1 = programmed death protein 1; PD-L1 = programmed death ligand 1; PPV = positive predictive value.
* P-value for comparison between 10 definite TBM + probable TBM and 43 non-TBM patients.
Figure 2.
Receiver operating characteristic curves for distinguishing definite + probable tuberculous meningitis (TBM) from non-TBM.
A total of 55 CSF specimens were available for the 18-plex cytokine bead array analysis (10 from the 10 patients with TBM and 45 from the 45 patients with non-TBM). Because of insufficient amount of CSF, CSF specimens for the soluble PD-1 and PD-L1 ELISAs were not available for 2 of the 45 non-TBM patients. The median concentrations of ADA, IL-12p40, IL-13, MIP-1α, and soluble PD-1 and PD-L1 in CSF were significantly higher in the TBM group than the non-TBM group (P < 0.05, Figure 1). From ROC curves (Figure 2), we determined optimal cutoff values for the IL-12p40, IL-13, MIP-1α, soluble PD-1, soluble PD-L1, and ADA for TBM as 52.04 pg/mL, 0.44 pg/mL, 8.83 pg/mL, 35.87 pg/mL, 24.19 pg/mL, and 6.95 U/L, respectively.
Table 2 presents the diagnostic performances of the various diagnostic tests in the definite TBM combined with probable TBM groups compared with the non-TBM group. We used the cutoff values obtained from ROC curves to calculate sensitivity, specificity, positive LR, negative LR, PPV, and NPV. The sensitivity and specificity of ADA level (> 6.95 U/L) in CSF were 70.0% (95% CI, 34.8–93.3) and 81.4% (95% CI, 66.6–91.6), respectively. The sensitivities and specificities of the cytokines were as follows: CSF IL-12p40 > 52.04 pg/mL, 80.0% (95% CI, 44.4–97.5) and 73.3% (95% CI, 58.1–85.4); CSF IL-13 > 0.44 pg/mL, 90.0% (95% CI, 55.5–99.8) and 46.7 (95% CI, 31.7–62.1); and CSF MIP-1α > 8.83 pg/mL, 80.0% (95% CI, 44.4–97.5) and 62.2% (95% CI, 46.5–76.2), respectively. When we used cutoff values for soluble PD-1 of > 35.87 pg/mL and PD-L1 of 24.19 pg/mL, their sensitivities and specificities were 80.0% (95% CI, 44.4–97.5) and 62.8% (95% CI, 46.7–77.0) and 80.0% (95% CI, 44.4–97.5) and 60.5% (95% CI, 44.4–75.0), respectively. In addition, the diagnostic performance of combination of biomarkers is shown in Supplemental Table 1. Using the cutoff values for the CSF-MC ELISPOT of 13.5 spots and for the PBMC ELISPOT of 14 spots, the sensitivities and specificities were 30% (95% CI, 6.7–65.3) and 91.1% (95% CI, 78.8–97.5) and 50% (95% CI, 18.7–81.3) and 77.8% (95% CI, 62.9–88.8), respectively. Figure 2 shows the relative discriminative accuracies of the various tests, as assessed by the areas under ROC curves.
DISCUSSION
In this study, we assessed the clinical utility of cytokines/chemokines for diagnosing TBM in patients with suspected TBM in an HIV-uninfected cohort. We found that certain cytokines and chemokine levels in the CSF had relatively high sensitivities (80–90%) with clinically acceptable specificities (60–73%) for diagnosing TBM with small samples of 10 cases of definite or probable TBM. In the previous study, we showed that the sensitivity of the ELISPOT assay for diagnosing TBM was only 47–68%,39 which is consistent with the present study. Recently, several studies have described some molecules in CSF as potential biomarkers for diagnosing TBM. Yang et al.36 analyzed the circulating and compartmentalized levels of cytokines and chemokines in plasma, pleural fluid (PF), and CSF. Among 27 molecules examined, IP-10 and MIG levels in the CSF discriminated TBM from non-TBM patients with 60% to 88% sensitivity and 90% to 95% specificity. Misra et al.37 found that the concentrations of TNFα, IL-8, IL-6, IL-1β, and IL-10 in CSF were significantly higher in TBM patients than in control patients with non-neurological diseases, and their levels declined at 3-month follow-up.
According to our present data, the concentrations of IL-12p40, IL-13, MIP-1a, sPD-1, and sPD-L1 have sensitivities more than 80% in the diagnosis of TBM. Interleukin-12 is thought to play a key role in mycobacterial infection.40 Of the two components of IL-12, the 35-kDa (p35) and 40-kDa (p40) subunits, the role of IL-12p40 seems to be more important in mycobacterial infection. In mouse models, mice lacking the p40 subunit were susceptible to mycobacteria and endogenous or exogenous IL-12p40 reduced the bacterial burden by enhancing the antigen-specific T-cell responses and cytotoxicity of effector CD8+ T cells.41,42 In humans, mycobacterial infection is common in patients with inherited IL-12p40 deficiency, and PBMCs from these patients fail to produce IFN-γ following mycobacterial stimulation.43 Therefore, elevated IL-12p40 could point to a diagnosis of TBM. Interleukin-13 and MIP-1α are elevated in patients with TBM and pleural TB.34,35 However, few studies have examined differences in the levels of these cytokines between TB and non-TB.
Programmed death protein 1 and PD-L1 are expressed on the cell surface and limit the activation and function of T cells. Soluble forms of PD-1 and PD-L1 can be detected in the blood and increased in patients with cancer, autoimmune diseases, or chronic infection.44–48 Their function and mechanism of release are unclear, but the presence of soluble PD-L1 indicates a poor prognosis and risk in several types of cancer.47 In the study of Pan et al., soluble PD-L1 and membrane-bound PD-L1 levels are elevated in the PF and monocytes of patients with tuberculous pleural effusion, and anti-PD-L1 antibody enhances T-cell proliferation.49 Also, CSF levels of both soluble PD-1 and PD-L1 in the CSF were high in patients with TBM in the present study, consistent with the idea that they are important mediators in disease progression and could be therapeutic targets.
Tumor necrosis factor-α is considered as the main proinflammatory cytokine in immune response against TB. Clinical studies12,50–52 have shown that MTB-specific TNF-α response can discriminate latent and active disease state of tuberculosis. It has been reported that dysregulation of TNF-α due to genetic variation53,54 can increase susceptibility to mycobacterial infection. Tobin et al.53 showed that even TNF-α mediates inflammatory responses to TB, excess production of TNF-α leads to necrosis of macrophage, resulting in extracellular mycobacterial growth that accounts for the worse outcome in TBM. Roca et al.55 suggested that excess TNF-α–mediated reactive oxygen species production enhances initial microbicidal activity in infected macrophage followed by increased bacterial burden. In our study, the level of TNF-α in CSF from TBM patients had a trend toward being higher than CSF from non-TBM patients (median 2.7 versus 1.2 pg/mL), but it did not reach any statistical significance (P = 0.099). Further studies are needed on the possible association of the level of TNF-α with the diagnostic or prognostic values in patients with suspected TBM.
Our study has several limitations. First, only a small number of patients had TBM, and only two of the cases were microbiologically confirmed. Second, overall sensitivity of the M. tuberculosis PCR test was about 50% in a previous meta-analysis,7 whereas it was 0% in our study. Our low detection rates of the M. tuberculosis PCR test may be due to inadequate sample size and biased recruitment of patients. It is also worth to note about the non-centrifuged sample use in clinical practice. Although there were limited data to analyze the sensitivity of the M. tuberculosis PCR test comparing centrifuged and non-centrifuged CSF samples, the previous study showed that the sensitivity of Xpert MTB/RIF from centrifuged CSF (2 mL) was significantly higher than that from non-centrifuged CSF (2 mL) (72% versus 28%, P-value = 0.008).56 Also, in previous studies, 100–200 μL of CSF was usually used for the M. tuberculosis PCR test,57,58 but we used at least 100 μL of CSF for the test. So, these factors may contribute to low sensitivity of the M. tuberculosis PCR test and limit the accuracy of estimates of the sensitivities of the various biomarkers, and the subsequent analysis of combination tests. We assume that some of the tests may be complementary to diagnose TBM and combining the tests might increase the diagnostic performance. Therefore, further studies with more TBM patients are needed. In addition, the expression of PD-1 and PD-L1 was not measured at the cellular level. Although the soluble forms of PD-1 and PD-L1 might be involved in the pathogenesis and progression of TBM, their membrane expression also needs to be investigated because PD-1 signaling mainly works on the surface of activated T cells and suppresses inflammatory responses by binding to the surface of activated T cells.
In conclusion, our data suggest that IL-12p40, IL-13, MIP-1α, and soluble PD-1 and PD-L1 levels in CSF could be useful adjuncts for diagnosing TBM. The development of rapid tests or point-of-care tests for these molecules in patients with suspected TBM could be useful for early diagnosis of TBM.
Supplemental table and figure
Note: Supplemental table and figure appear at www.ajtmh.org.
REFERENCES
- 1.WHO , 2017. Global Tuberculosis Report 2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK, 2008. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev 21: 243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thwaites GE, van Toorn R, Schoeman J, 2013. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 12: 999–1010. [DOI] [PubMed] [Google Scholar]
- 4.Torok ME, 2015. Tuberculous meningitis: advances in diagnosis and treatment. Br Med Bull 113: 117–131. [DOI] [PubMed] [Google Scholar]
- 5.Ling DI, Flores LL, Riley LW, Pai M, 2008. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS One 2: e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomons RS, van Elsland SL, Visser DH, Hoek KG, Marais BJ, Schoeman JF, van Furth AM, 2014. Commercial nucleic acid amplification tests in tuberculous meningitis—a meta-analysis. Diagn Microbiol Infect Dis 78: 398–403. [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Flores LL, Pai N, Hubbard A, Riley LW, Colford JM, Jr., 2003. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis 3: 633–643. [DOI] [PubMed] [Google Scholar]
- 8.Boehme CC, et al. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rufai SB, Singh A, Singh J, Kumar P, Sankar MM, Singh S, TB Research Team , 2017. Diagnostic usefulness of Xpert MTB/RIF assay for detection of tuberculous meningitis using cerebrospinal fluid. J Infect 75: 125–131. [DOI] [PubMed] [Google Scholar]
- 10.Nhu NT, et al. 2014. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol 52: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahr NC, et al. 2018. Diagnostic accuracy of Xpert MTB/RIF ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis 18: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JY, et al. 2018. Combined IFN-gamma and TNF-alpha release assay for differentiating active tuberculosis from latent tuberculosis infection. J Infect 77: 314–320. [DOI] [PubMed] [Google Scholar]
- 13.Porsa E, Cheng L, Graviss EA, 2007. Comparison of an ESAT-6/CFP-10 peptide-based enzyme-linked immunospot assay to a tuberculin skin test for screening of a population at moderate risk of contracting tuberculosis. Clin Vaccine Immunol 14: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T, 2005. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis 24: 529–536. [DOI] [PubMed] [Google Scholar]
- 15.Wen A, Qu XH, Zhang KN, Leng EL, Ren Y, Wu XM, 2018. Evaluation of interferon-gamma release assays in extrasanguinous body fluids for diagnosing tuberculosis: a systematic review and meta-analysis. Life Sci 197: 140–146. [DOI] [PubMed] [Google Scholar]
- 16.Huy NT, Thao NT, Diep DT, Kikuchi M, Zamora J, Hirayama K, 2010. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit Care 14: R240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, et al. 2012. The clinical diagnostic significance of cerebrospinal fluid D-lactate for bacterial meningitis. Clin Chim Acta 413: 1512–1515. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, et al. 2017. Cytokines do play a role in pathogenesis of tuberculous meningitis: a prospective study from a tertiary care center in India. J Neurol Sci 379: 131–136. [DOI] [PubMed] [Google Scholar]
- 19.Sakushima K, Hayashino Y, Kawaguchi T, Jackson JL, Fukuhara S, 2011. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J Infect 62: 255–262. [DOI] [PubMed] [Google Scholar]
- 20.Kataria J, Rukmangadachar LA, Hariprasad G, O J, Tripathi M, Srinivasan A, 2011. Two dimensional difference gel electrophoresis analysis of cerebrospinal fluid in tuberculous meningitis patients. J Proteomics 74: 2194–2203. [DOI] [PubMed] [Google Scholar]
- 21.Ruhwald M, Bodmer T, Maier C, Jepsen M, Haaland MB, Eugen-Olsen J, Ravn P, Tbnet , 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J 32: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 22.Demissie A, VACSEL Study Group et al. 2004. Healthy individuals that control a latent infection with Mycobacterium tuberculosis express high levels of Th1 cytokines and the IL-4 antagonist IL-4delta2. J Immunol 172: 6938–6943. [DOI] [PubMed] [Google Scholar]
- 23.La Manna MP, Orlando V, Li Donni P, Sireci G, Di Carlo P, Cascio A, Dieli F, Caccamo N, 2018. Identification of plasma biomarkers for discrimination between tuberculosis infection/disease and pulmonary non tuberculosis disease. PLoS One 13: e0192664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Li X, Liu W, Liu Y, Zhong Z, Wang L, Ge S, Zhang J, Xia N, 2018. IL-6 release of Rv0183 antigen-stimulated whole blood is a potential biomarker for active tuberculosis patients. J Infect 76: 376–382. [DOI] [PubMed] [Google Scholar]
- 25.Sutherland JS, de Jong BC, Jeffries DJ, Adetifa IM, Ota MO, 2010. Production of TNF-alpha, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a west African cohort. PLoS One 5: e12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ, 2014. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 40: 289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pauken KE, Wherry EJ, 2015. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R, 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682–687. [DOI] [PubMed] [Google Scholar]
- 29.Singh A, Mohan A, Dey AB, Mitra DK, 2013. Inhibiting the programmed death 1 pathway rescues Mycobacterium tuberculosis-specific interferon gamma-producing T cells from apoptosis in patients with pulmonary tuberculosis. J Infect Dis 208: 603–615. [DOI] [PubMed] [Google Scholar]
- 30.Yin W, Tong ZH, Cui A, Zhang JC, Ye ZJ, Yuan ML, Zhou Q, Shi HZ, 2014. PD-1/PD-Ls pathways between CD4(+) T cells and pleural mesothelial cells in human tuberculous pleurisy. Tuberculosis (Edinb) 94: 131–139. [DOI] [PubMed] [Google Scholar]
- 31.Jurado JO, Alvarez IB, Pasquinelli V, Martinez GJ, Quiroga MF, Abbate E, Musella RM, Chuluyan HE, Garcia VE, 2008. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 181: 116–125. [DOI] [PubMed] [Google Scholar]
- 32.McNab FW, et al. 2011. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur J Immunol 41: 1941–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Mohan A, Dey AB, Mitra DK, 2017. Programmed death-1(+) T cells inhibit effector T cells at the pathological site of miliary tuberculosis. Clin Exp Immunol 187: 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel VB, Singh R, Connolly C, Kasprowicz V, Ndung’u T, Dheda K, 2011. Comparative utility of cytokine levels and quantitative RD-1-specific T cell responses for rapid immunodiagnosis of tuberculous meningitis. J Clin Microbiol 49: 3971–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visser DH, Solomons RS, Ronacher K, van Well GT, Heymans MW, Walzl G, Chegou NN, Schoeman JF, van Furth AM, 2015. Host immune response to tuberculous meningitis. Clin Infect Dis 60: 177–187. [DOI] [PubMed] [Google Scholar]
- 36.Yang Q, et al. 2014. IP-10 and MIG are compartmentalized at the site of disease during pleural and meningeal tuberculosis and are decreased after antituberculosis treatment. Clin Vaccine Immunol 21: 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misra UK, Kalita J, Srivastava R, Nair PP, Mishra MK, Basu A, 2010. A study of cytokines in tuberculous meningitis: clinical and MRI correlation. Neurosci Lett 483: 6–10. [DOI] [PubMed] [Google Scholar]
- 38.Marais S, Thwaites G, Schoeman JF, Torok ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ, 2010. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 10: 803–812. [DOI] [PubMed] [Google Scholar]
- 39.Park KH, et al. 2016. Diagnostic usefulness of T-cell based assays for tuberculous meningitis in HIV-uninfected patients. J Infect 72: 486–497. [DOI] [PubMed] [Google Scholar]
- 40.Cooper AM, Solache A, Khader SA, 2007. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol 19: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, Orme IM, 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol 168: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 42.Holscher C, Atkinson RA, Arendse B, Brown N, Myburgh E, Alber G, Brombacher F, 2001. A protective and agonistic function of IL-12p40 in mycobacterial infection. J Immunol 167: 6957–6966. [DOI] [PubMed] [Google Scholar]
- 43.Prando C, et al. 2013. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 92: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan B, Nie H, Liu A, Feng G, He D, Xu R, Zhang Q, Dong C, Zhang JZ, 2006. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol 177: 8844–8850. [DOI] [PubMed] [Google Scholar]
- 45.Cheng HY, et al. 2014. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One 9: e95870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greisen SR, Rasmussen TK, Stengaard-Pedersen K, Hetland ML, Horslev-Petersen K, Hvid M, Deleuran B, 2014. Increased soluble programmed death-1 (sPD-1) is associated with disease activity and radiographic progression in early rheumatoid arthritis. Scand J Rheumatol 43: 101–108. [DOI] [PubMed] [Google Scholar]
- 47.Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C, 2018. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: a meta-analysis. Medicine (Baltimore) 97: e9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kruger S, et al. 2017. Serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death ligand 1 (sPD-L1) in advanced pancreatic cancer. Oncoimmunology 6: e1310358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan X, Zhong A, Xing Y, Shi M, Qian B, Zhou T, Chen Y, Zhang X, 2016. Increased soluble and membrane-bound PD-L1 contributes to immune regulation and disease progression in patients with tuberculous pleural effusion. Exp Ther Med 12: 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harari A, et al. 2011. Dominant TNF-α+ Mycobacterium tuberculosis-speficific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 17: 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jeong YH, et al. 2015. Discrimination between active and latent tuberculosis based on ratio of antigen-specific to mitogen-induced IP-10 production. J Clin Microbiol 53: 504–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Hou H, Xu L, Jane M, Peng J, Lu Y, Zhu Y, Sun Z, 2013. Mycobacterium tuberculosis-specific TNF-α is a potential biomarker for the rapid diagnosis of active tuberculosis disease in Chinese population. PLoS One 8: e79431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tobin DM, et al. 2012. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148: 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harishankar M, Selvaraj P, Bethunaickan R, 2018. Influence of genetic polymorphism towards pulmonary tuberculosis susceptibility. Front Med (Laussanne) 5: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roca FJ, Ramakrishnan L, 2013. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153: 521–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahr NC, Tugume L, Rajasingham R, Kiggundu R, Williams DA, Morawski B, Alland D, Meya DB, Rhein J, Boulware DR, 2015. Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF: a prospective study. Int J Tuberc Lung Dis 19: 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonington A, Strang JI, Klapper PE, Hood SV, Rubombora W, Penny M, Willers R, Wilkins EG, 1998. Use of Roche AMPLICOR Mycobacterium tuberculosis PCR in early diagnosis of tuberculous meningitis. J Clin Microbiol 36: 1251–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shirani K, Talaei Z, Yaran M, Ataei B, Mehrabi-Koushki A, Khorvash F, 2015. Diagnosed tuberculous meningitis using cerebrospinal fluid polymerase chain reaction in patients hospitalized with the diagnosis of meningitis in referral hospitals in Isfahan. J Res Med Sci 20: 224–227. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.