Abstract.
Rocky Mountain spotted fever (RMSF) has been reported in Colombia since 1937. Most recent outbreaks were in 2006–2008, followed by the absence of recognized cases. This report describes new clinical cases of RMSF and epidemiologic investigations. Medical records were reviewed, and fieldwork, serological and molecular diagnostic testing, and bacterial isolation were performed. Fever, hypotension, abdominal discomfort, and rash accompanied by thrombocytopenia and leukopenia were the most characteristic manifestations. Two convalescent sera from the index case and sera from two dogs revealed high anti-spotted fever group Rickettsia antibody titers. Rickettsia rickettsii was isolated from case 4. Cases 2 and 3 were identified by epidemiological connection with the index case. Thus, a new cluster of cases of RMSF was identified in Antioquia, Colombia, with the occurrence of fatal cases, which indicates the active circulation of the bacteria and a potential risk for the population.
INTRODUCTION
Rocky Mountain spotted fever (RMSF), the most lethal rickettsiosis caused by the tick-borne obligately intracellular bacterium, Rickettsia rickettsii, is reemerging in Colombia.1,2 Outbreaks have been documented in the departments of Cundinamarca (two foci, Tobia and Villeta), Antioquia (two foci, Turbo and Necoclí), and Córdoba (one focus, Los Córdobas.) The case fatality rate of 95% during 1934–1936 remains remarkably high (28% in 2008).2–6
This report of four additional cases of RMSF in Uramita, Antioquia, represents a third epidemiologic focus of the disease. The first recognition of the disease in Uramita occurred in September 2014 and led to the identification of two fatal cases that had occurred the preceding May and July in next-door neighbors. Subsequently, another fatal case was diagnosed in an inhabitant of Uramita, who was transferred to Medellin with a suspected dengue fever. The first case was documented serologically, the second and third cases by clinical manifestations and epidemiologic association with the first case, and R. rickettsii was identified in the fourth case by a molecular method and isolation of the organism in cell culture. This report describes the clinical cases and the epidemiologic investigations.
MATERIALS AND METHODS
Between the years 2014 and 2015, the research group of research on veterinary sciences–Centauro from Universidad de Antioquia and Secretaría Seccional de Salud y Protección Social de Antioquia (SSS and PSA)—investigated four clinical cases of rickettsiosis from the municipality of Uramita, Antioquia, Colombia. Two of the four cases were confirmed by specific laboratory tests and the other two by epidemiological connection with the index case after notification of the index case, followed by fieldwork in the outbreak zone.
Review of medical records.
The information about the signs and most relevant symptoms was extracted from the medical records, which were provided by the attending physicians.
Ethical considerations.
The clinical treatment of all the patients included in this study was performed according to the physicians’ standard of care in each institution where the patients were cared for. Blood samples from these patients and their neighbors were collected by SSS and PSA, who have special responsibilities to study epidemic diseases, and informed consent was obtained from volunteers bled during the fieldwork. The samples were sent to the CENTAURO laboratory for rickettsial diagnosis, and, subsequently, all results were provided to the patients and their families.
Samples collected from the fieldwork and sent from the hospitals were investigated for rickettsial diagnosis by indirect immunofluorescence antibody assay (IFA), polymerase chain reaction (PCR) detection of rickettsial DNA, and bacterial isolation, following the CENTAURO laboratory protocols and the recommendations by Oteo et al.7
Fieldwork and index case study.
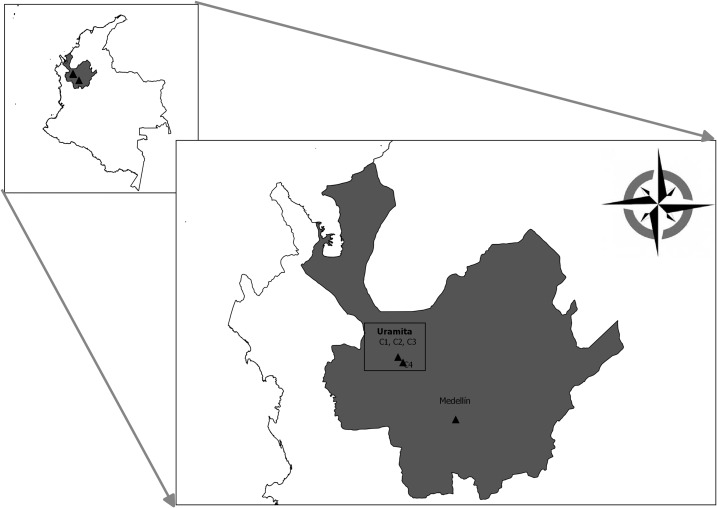
The index case study was performed in the municipality of Uramita, which is located in the Department of Antioquia, Colombia (N 6°53′55″ W 76°10′25″), at 650 m above the sea level and has an average temperature of 25°C (Figure 1). This municipality is 138 km northwest of Medellin and has a population of 8,287 inhabitants.8 The town has a high density of houses bounded by the inter-municipality road and the Uramita river. During the fieldwork, we obtained blood samples from the index case, his neighbors (residents of two adjacent houses on each side), and their dogs. Ticks were collected from the pets and inside and around the houses; these ticks were identified using dichotomous keys.9 The fieldwork was performed by the SSS and PSA, the municipality health secretariat, and researchers of the CENTAURO group.
Figure 1.
Map of Colombia showing the location of the department (gray background) and municipality (two black triangles in the square) where the outbreak occurred.
Serological tests.
We performed serological testing by IFA for both human and canine samples, using glass slides coated with cells containing R. rickettsii strain Sheila Smith donated by the University of Texas Medical Branch (UTMB). The antibody-containing samples were sent to the University of Sao Paulo, Brazil, where they were titrated by IFA with six different rickettsial antigens (each antigen in an independent test), including R. rickettsii strain Taiaçu, R. parkeri strain At24, R. amblyommatis strain Ac37, R. rhipicephali strain HJ5, R. bellii strain Mogi, and R. felis strain Pedreira. All samples were tested according to the method described by Pena et al.10 and Pinter et al.11
Molecular testing.
DNA was extracted from the acute phase blood sample (serum and blood clot) collected from case 4 on day 7 of illness and from ticks collected in the fieldwork using the DNeasy Blood and Tissue kit (QIAGEN®, Valencia, CA). We followed the manufacturer’s protocol for DNA extraction from the blood sample. Some modifications were made to the protocol for the tick samples as previously described.12 Next, we performed PCR using the CS 78 and CS 323 primers for the gltA (citrate synthase) gene, which yields a product of 401 bp.13 The positive samples were tested with the 120. M59–120.807 and CS 239–CS 1,069 primers directed against the sca5 and gltA genes, which yield products of 862 and 800 bp, respectively.13,14 All PCR products were sequenced by Macrogen (Seoul, South Korea). The sequences were assembled and edited with Seqman program from DNAstar package (Lasergene®, Madison, WI), and phylogenetic analysis was performed by the programs MEGA-6 and MrBayes 3.1.2.15,16
Bacterial isolation.
Two 25-cm2 cell culture flasks with 80% confluent VERO cells were inoculated with 200 μL of serum (from case 4 collected 7 days after the onset of illness) that was demonstrated to contain Rickettsia DNA by PCR, and a third flask was inoculated with PBS as a negative control. The cultures were incubated at 34°C in an atmosphere of 5% CO2 for an hour. One flask inoculated with the infected serum was fed with medium containing 100 units/mL of penicillin, 100 μg/mL of streptomycin, and 0.25 μg/mL of amphotericin B, and the other two flasks contained antibiotic- and antimycotic-free medium. The three flasks were incubated at 34°C in 5% CO2 and checked daily. On day three postinoculation, the medium in all flasks was changed to antibiotic- and antimycotic-free medium, and this process was repeated every 3 days for 3 weeks.
RESULTS
Clinical case descriptions.
Case 1 (index case).
A 12-year-old child, resident of the municipality of Uramita, sought medical attention in August 2014 at the local hospital after 12 days of fever, earache, emesis, diarrhea, myalgia, asthenia, lethargy, and severe abdominal pain. The patient described a tick bite on the right leg. Vital signs were as follows: blood pressure (BP) 95/69 mmHg, heart rate (HR) 120 beats per minute (bpm), and respiratory frequency (RF) 20 breaths per minute (bpm). Physical examination revealed conjunctival injection, signs of dehydration, a soft abdomen with tenderness to palpation and a maculopapular rash on the trunk, and extremities that did not blanch with digital pressure. Five hours after presentation, the patient continued to be afebrile, but was hypotensive, tachycardic, and oliguric. Laboratory studies revealed thrombocytopenia and leukopenia. Suspecting septic shock of abdominal origin, the attending physicians initiated treatment with ampicillin–sulbactam, and the patient was transferred to a referral hospital, where he was found to be dehydrated, with BP 101/69 mmHg, HR 90 bpm, RF 36 bpm, enophthalmos, dry oral mucosa, generalized pallor, diffuse abdominal pain, non-blanching maculopapular rash on the trunk and abdomen, and cold extremities. The patient was admitted to the intensive care unit (ICU) and received intravenous fluids, piperacillin–tazobactam (300 mg/kg/day divided every 6 hour), omeprazole, and acetaminophen.
Six hours after admission, the patient continued to be tachycardic, tachypneic, and hypotensive (HR 125 bpm, RF 25 bpm, and BP 89/47 mmHg) and asthenic, with anasarca, dry oral mucosa, distended abdomen with ascites, and abdominal tenderness and guarding. Laboratory studies revealed proteinuria, ketonuria, increased aspartate transaminase (AST) 162 U/L, and alanine transaminase (ALT) 78 U/L.
Empiric treatment with doxycycline 75 mg (2.2 mg/kg orally every 12 hour) was followed by clinical improvement and hospital discharge after a total of seven days of treatment with doxycycline (starting at day 13 of illness), based on clinical suspicion of rickettsiosis due to acute fever, erythematous maculopapular rash, severe capillary leakage (edema and ascites), persistent shock, and tachycardia.
Two blood cultures for aerobic bacteria, tests for dengue IgM and IgG antibodies and non-structural antigen 1 (NS1) antigen and Leptospira IgM antibodies, were negative. In the laboratory of the CENTAURO group, IFA using R. rickettsii antigen on two convalescent samples revealed titers of 8,182 on day 14 of hospitalization and 16,384 a month later (Table 1).
Table 1.
Titration results with different antigens of Rickettsia spp.
| Highest titers in immunofluorescence antibody assay | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Antigen | ||||||
| R. rickettsii | R. parkeri | R. amblyommatis | R. rhipicephali | R. bellii | R. felis | ||
| Human | Case 1, S1 | 8,192 | 4,096 | 4,096 | 2,048 | 2,048 | 256 |
| Case 1, S2 | 16,384* | 4,096 | 4,096 | 4,096 | 2,048 | 64 | |
| Dog | Pet of case 1 | 16,384* | 4,096 | 2,048 | 2,048 | 128 | 256 |
| Pet of cases 2 and 3 | 16,384 | 8,192 | 4,096 | 4,096 | 1,024 | 512 | |
S = sample.
* Probable antigen involved in homologous reaction.
Retrospectively, after the fieldwork at the house of the index case, we identified two more cases linked by an epidemiological connection. Therefore, these two cases were retrospective, and they did not receive anti-rickettsial treatment. A summary of the features and the main clinical findings for these four cases is shown in Table 2.
Table 2.
Principal clinical and diagnostic findings for these four cases
| Case | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| General information | ||||
| Age (years) | 12 | 6 | 51 | 43 |
| Gender | Male | Female | Male | Male |
| Date of onset | August 1, 2014 | May 27, 2014 | July 14, 2014 | April 30, 2015 |
| Date of admission | August 12, 2014 | May 30, 2014 | July 17, 2014 | May 6, 2015 |
| Duration of illness (days) | 22 | 5 | 5 | 7 |
| Duration of hospitalization (days) | 10 | 2 | 2 | 1 |
| Outcome | Survived | Fatal | Fatal | Fatal |
| Summary of clinical findings | ||||
| Highest body temperature (°C) | 38.4 | 40.4 | 39 | 36.5* |
| Lowest blood pressure (mmHg) | 89/47 | 35/21 | 60/40 | 90/30 |
| Heart rate first recorded (bpm) | 120 | 110 | 100 | 108 |
| Respiratory frequency first recorded (rpm) | 20 | 20 | 18 | 50 |
| Lowest platelet value (per µL) | 30,000 | 7,000 | 31,000 | 15,000 |
| Leukocytes first recorded (per µL) | 4,500 | 3,970 | 4,570 | 5,900 |
| Neutrophils first recorded (per µL) | 3,510 | 3,005 | 4,067 | 5,428 |
| Lowest leukocytes value (per µL) | 2,300 | 3,970 | 2,900 | 5,900 |
| Lowest neutrophil value (per µL) | 1,909 | 3,005 | 2,146 | 5,428 |
| Presence of signs and symptoms | ||||
| Signs of bleeding | Yes | Yes | Yes | No |
| Oliguria | Yes | No | Yes | No |
| Dehydration | Yes | Yes | Yes | Yes |
| Fever | Yes | Yes | Yes | Yes* |
| Headache | Yes | Yes | No | No |
| Chill | No | Yes | No | No |
| Abdominal pain | Yes | Yes | Yes | Yes |
| Emesis | Yes | Yes | Yes | Yes |
| Diarrhea | Yes | Yes | Yes | Yes |
| Anasarca | Yes | Yes | No | No |
| Choluria | No | No | Yes | Yes |
| Maculopapular rash | Yes | Yes | Yes | Yes |
| Myalgia | Yes | Yes | Yes | Yes |
| Arthralgia | No | Yes | No | Yes |
| Asthenia | Yes | Yes | No | No |
| Retro-orbital pain | Yes | Yes | No | No |
| Hepatomegaly | No | Yes | No | Yes |
| Jaundice | No | No | No | Yes |
| Laboratory findings | ||||
| Alterations in aminotransferases | Yes | Yes | Yes | Yes |
| Coagulopathy | Yes | Yes | Yes | Yes |
| Elevated creatinine | No | Yes | Yes | Yes |
| Diagnostic tests | ||||
| Immunofluorescence antibody assay | Yes (P) | ND | ND | Yes (N) |
| Polymerase chain reaction | ND | ND | ND | Yes (P) |
| Bacterial isolation | ND | ND | ND | Yes (P) |
N = negative; ND = no data; P = positive.
* We do not have a record of the body temperature before case 4 was referred to a hospital in Medellin.
Case 2.
Case identified by epidemiological connection with the index case after fieldwork.
A 6-year-old girl presented to the hospital of Uramita in May 2014 after 3 days of myalgia, arthralgia, retro-orbital headache, and chills. Twenty-four hours previously, she had developed fever, liquid stools, abdominal pain, and nausea. On physical examination, she was stable (BP 120/70 mmHg, HR 110 bpm, and afebrile). Dengue was suspected, and no evidence of tick bite or exposure was reported.
Four hours after admission, she was febrile (40°C). Laboratory tests revealed thrombocytopenia and leukopenia with predominance of neutrophils (76%). The patient continued to have diarrhea, diffuse abdominal pain, hepatomegaly without splenomegaly, and a disseminated violaceous erythematous macular rash. The girl appeared dehydrated with signs of hypoperfusion with speckled skin, drowsiness, anasarca, and tachycardia, and required orotracheal intubation. Dengue IgM and IgG serologies and NS1 antigen tests were negative. Physicians suspected meningococcemia or Staphylococcus aureus septic shock, and empiric treatment with vancomycin (60 mg/kg/day) and cefotaxime (150 mg/kg/day) was initiated. Laboratory testing demonstrated bandemia 25%, AST 568 U/L, ALT 135 U/L, total bilirubin 2.2 mg/dL, direct bilirubin 1.9 mg/dL, indirect bilirubin 0.3 mg/dL, creatinine 1.13 mg/dL, and prolonged partial thromboplastin time 55.9 seconds.
The patient’s condition deteriorated with refractory shock, diffuse purpuric lesions, and profuse bleeding from the mouth, nose, and nasogastric tube. Despite vasopressor therapy and hemodynamic and respiratory support, the patient died 48 hours after admission.
Case 3.
This case was epidemiologically associated with the first case. A 51-year-old man, stepfather of case 2, presented to the emergency room of the Uramita hospital in July 2014 for fever of 3 days’ duration, general discomfort, and 1 day of vomiting and diarrhea. He was hypotensive with BP 60/40 mmHg, HR 100 bpm, RF 18 bpm, afebrile (37°C), and dehydrated. No tick bite or exposure was reported. The patient was managed with intravenous fluids, ceftriaxone, and dopamine.
Eight hours later, the patient was hypotensive and tachycardic, and laboratory tests revealed leukopenia, thrombocytopenia; and elevated creatinine (1.5 mg/dL), sedimentation rate (60 mm/hour), and C-reactive protein (27 mg/dL). Diagnoses of septic shock and dengue were considered.
The patient was transferred and arrived tachypneic, dehydrated, and with abdominal pain. He received intravenous fluids, oxygen, and ciprofloxacin (2 g). A cutaneous rash was observed on the face, neck, and trunk. The patient was transferred to the ICU with hypoxemic respiratory failure requiring intubation and mechanical ventilation. He rapidly developed multiple organ failure with cardiac, pulmonary, and renal involvement. Dengue IgM and IgG antibodies and NS1 antigen were negative. Despite hemodynamic and respiratory support and broad-spectrum beta-lactam antibiotics, the patient died.
Case 4.
The patient was a 43-year-old man from the Uramita municipality, who worked in regional road maintenance. He presented to the emergency room of the local hospital in April 2015 with malaise, fever, myalgia, and liquid stools. No tick bite or exposure was reported. He received symptomatic treatment. Five days later, he returned to the hospital with jaundice, nausea, and episodes of dyspnea, and again was discharged. He returned 2 days later with emesis, hypotension (BP 85/41 mmHg), and tachypnea (RF 24 bpm). Laboratory studies demonstrated thrombocytopenia, elevated hepatic transaminases (AST 425 U/L and ALT 237 U/L), total bilirubin 1.62 mg/dL, and indirect bilirubin 1.21 mg/dL; and presence of urobilinogen (4 mg/dL), bilirubin (3 mg/dL), leukocytes (100 per high power field), and protein (25 mg/dL) in urine. He was immediately transferred to a referral hospital, where he had thrombocytopenia, alterations in hemostasis tests (prothrombin time 27.1 seconds, INR 2.17, partial thromboplastin time 66.2 seconds, and control 32.1 second), liver injury (AST 658 U/L and ALT 387 U/L), and renal failure (creatinine 7.8 mg/dL). After admission to the ICU with suspicion of dengue shock syndrome, he developed respiratory failure, generalized cyanosis, and bradycardia. The diagnosis of rickettsiosis was considered, and treatment with doxycycline was initiated; however, the patient died on the same day. Tests for HIV, hemoparasites, hepatitis B virus, hepatitis C virus, and syphilis were negative. A serum sample was sent to the CENTAURO laboratory where spotted fever group rickettsial IFA was performed, which was negative. The same serum sample tested by PCR revealed the presence of DNA of R. rickettsii.
Fieldwork.
Blood samples were collected from nine persons, including a convalescent phase sample from case 1. During the fieldwork, two fatal cases were discovered, cases 2 and 3, who lived in the neighboring house (next door) to case 1. In addition, we collected blood samples from seven dogs. One of them was a pet of case 1, another belonged to cases 2 and 3, and the other five dogs belonged to neighbors. From five of these seven dogs, we collected 27 ticks (63% arrived alive to the laboratory); all of them were identified as Rhipicephalus sanguineus sensu lato (s.l.).
In addition, we sought ticks on vegetation by flagging in the places that case 1 commonly frequented, but no ticks were collected. In addition, case 1 provided the information that his pet and home had been infested with ticks, but because of the refusal of the owners of the house, it was not possible to inspect the interior.
Serological tests.
All human samples collected in the fieldwork, except the convalescent phase sample from case 1, were negative by IFA for antibodies to spotted fever group rickettsiae. Two samples from the dogs were positive, one of them was the pet of case 1 and the other a shared pet of cases 2 and 3. The positive samples, three from the fieldwork (second sample of the index case and the two dog sera) and the first sample from the index case were sent to the University of Sao Paulo, Brazil, for a titration of antibodies with different antigens of Rickettsia spp. In all cases, the highest endpoint titers (8,192 or 16,384) were observed for R. rickettsii. In addition, one human and one canine sample had endpoint titers to R. rickettsii at least 4-fold higher than the titers to the remaining five Rickettsia species, indicating that R. rickettsii was the probable antigen involved in a homologous reaction (Table 1).
Detection of Rickettsia by PCR, phylogenetic analysis, and bacterial isolation.
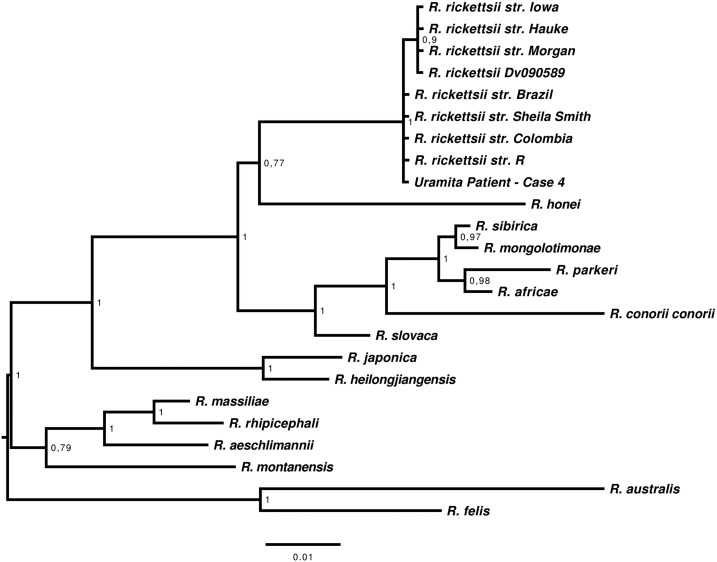
All ticks were individually processed and negative for rickettsial DNA by PCR. On the other hand, DNA extracted from serum and clot of case 4 contained rickettsial DNA detected by PCR using primers to gltA and sca5 genes. We obtained a sequence of 798 bp from the gltA gene (GenBank number MG206089) with 100% identity to R. rickettsii. Our sequence has a triplet insertion (CGC that translates to arginine) between nucleotides 608 and 610, when this is compared with the R. rickettsii Sheila Smith strain (GenBank number CP000848.1) and R. rickettsii R strain (GenBank number U59729.1). This insertion is shared with the Brazilian strain R. rickettsii Taiaçu (GenBank number DQ115890.1). Furthermore, we have a sequence of 793 bp from the sca5 gene (GenBank number MG206088) with 100% identity with R. rickettsii, as shown in the phylogenetic tree (Figure 2). A rickettsial isolate was also obtained from the serum sample; rickettsiae were detected in both cultures, with and without antibiotic–antimycotic content on day 20 postinoculation. The presence of rickettsiae was confirmed by IFA and was identified as R. rickettsii by PCR and DNA sequence.
Figure 2.
Majority-rule consensus tree generated in the Bayesian phylogenetic analysis of rickettsial sca5 gene performed with the program MrBayes v3.1.2, using the GTR + I + G substitution model. The analysis was run for 1,000,000 generations. The numbers near the nodes are posterior probabilities for the corresponding clade. The analysis involved 25 nucleotide sequences. There were a total of 778 positions in the final dataset. The tree was drawn in FigTree 1.4, and rooted with transitional group (Rickettsia felis and Rickettsia australis.)
DISCUSSION
A new focus of RMSF in Colombia was documented in this investigation. A cluster of four cases was identified by a combination of rickettsial isolation and molecular detection of R. rickettsii, serology, and clinical manifestations in two fatal cases in close proximity to a case with evidence of the presence of Rhipicephalus sanguineus s.l. ticks and strongly seropositive dogs. This cluster of severe cases is not unusual for infections caused by South American strains of R. rickettsii.17,18 The case fatality rate of Brazilian spotted fever is 40%, despite the effectiveness of doxycycline treatment if administered early enough in the infection course.19 With the recognition of the occurrence of RMSF in such locations as this, it is more likely that physicians will be alerted to the potential for future cases leading to appropriate prompt treatment and also that less severe cases will be identified.
Not all cases of RMSF present with fever, rash, and a history of tick bite. A very large proportion of patients are unaware of having been bitten by a tick. Rash usually does not appear until days 3–5 of illness or in some cases later or not at all. It should be recognized that in many cases, nausea, vomiting, diarrhea, and abdominal pain and tenderness are prominent clinical manifestations early in the illness before the appearance of the rash. During the critical stage of illness, hypotension and respiratory failure occur because of the effects of widespread endothelial infection including the pulmonary microcirculation, leading to increased vascular permeability, hypovolemia, and non-cardiogenic pulmonary edema.
It is extremely likely that cases 1–3 were transmitted by Rh. sanguineus s.l. ticks in the patients’ homes. However, infection of case 4 was potentially transmitted by a sylvatic tick such as Amblyomma patinoi, an established host of R. rickettsii in Colombia,20 during his work performing roadside maintenance. A missing epidemiological link is understanding the potential role of dogs in acquiring sylvatic infection and transmitting it to Rh. sanguineus s.l. ticks in the home. Expansion of the population of infected Rh. sanguineus s.l. ticks by further infections of dogs and transovarian transmission to subsequent generations of ticks is a logical explanation for the occurrence of outbreaks of RMSF on Navajo reservations in Arizona and in poverty-stricken neighborhoods in Sonora and Baja California, Mexico.21–23 In Arizona, a community with around 600 houses and an incidence of RMSF of 1.2 cases/1,000 inhabitants carried out a successful intervention between the years 2012 and 2013 through control of the tick population on dogs and in peridomicilary sites. This campaign was associated with a 43% reduction in the incidence of RMSF.24
Previous studies discussed by Szabó et al.25 showed the likely existence of diverse cycles where R. rickettsii circulation could be explained by the presence of Amblyomma cajennense s.l. and Rh. sanguineus s.l. ticks associated with the presence of sylvatic and urban amplifying hosts such as capybaras (Hydrochoerus hydrochaeris) and dogs, respectively. Likewise, one of our previous studies shows high Ig G against R. rickettsia in Didelphis marsupialis,12 that unlike capybaras, is a commonly found wild species in the Urabá region. Although some of these cycles have been demonstrated under both experimental and natural conditions in other countries, in Colombia, these complex interactions have yet to be elucidated. Consequently, it will only be through epidemiological research (search for infected persons and animals) and experimental transmission studies to evaluate zoonotic cycles that we may completely decipher the main components of the transmission cycles of these rickettsial agents (bridging vectors and amplifying vertebrate hosts). Then, the knowledge will exist to develop the appropriate control and preventive measures in regions of new, reemerging, and established zoonotic transmission of these bacteria.
Acknowledgments:
We would like to recognize the assistance of health workers of E.S.E. Hospital Tobias Puerta–Uramita, the Secretary of Health of the municipality of Uramita and Dr. Ana L. Correa of Hospital Pablo Tobon Uribe, Medellín—for their contributions during our fieldwork. We also express our gratitude to Colciencias for its support with the PhD scholarship for Dr. Londoño, through the official announcement 511/2010, the sustainability program of University of Antioquia 2018, and Fogarty International Center of the NIH (D43 TW010331 Research Training Program on the Impact of Zoonotic and Vector-borne Viruses, Rickettsiae, and Leptospira in Acute Undifferentiated Febrile Illnesses.)
REFERENCES
- 1.Fournier P-E, Raoult D, 2007. Bacteriology, taxonomy, and phylogeny of Rickettsia. Rickettsial Diseases. Boca Raton, FL: Taylor & Francis Group, 1–14. [Google Scholar]
- 2.Hidalgo M, et al. 2007. Rocky Mountain spotted fever, Colombia. Emerg Infect Dis 13: 1058–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patiño L, Afanador A, Paul JH, 1937. A spotted fever in Tobia, Colombia. Am J Trop Med 17: 639–653. [DOI] [PubMed] [Google Scholar]
- 4.Acosta J, et al. 2006. Brote de Rickettsia rickettsii en Necoclí, Antioquia, febrero-marzo. Inf Quinc Epidemiológico Nac 11: 177–192. [Google Scholar]
- 5.Giraldo MR, Pacheco García OE, Galeano A, Alvarez Vidal H, Echeverri EI, Echavarría Rodríguez L, Pacheco C, 2008. Estudio sindrome febril en el muncipio de Turbo, Corregimiento altos de mulatos enero 2008. Inf Quinc Epidemiol Nac 13: 145–160. [Google Scholar]
- 6.Hidalgo M, Miranda J, Heredia D, Zambrano P, Vesga JF, Lizarazo D, Mattar S, Valbuena G. Outbreak of Rocky mountain spotted fever in Cordoba, Colombia. Mem Inst Oswaldo Cruz 2011;106: 117–118. [DOI] [PubMed] [Google Scholar]
- 7.Oteo JA, Nava S, de Sousa R, Mattar S, Venzal JM, Abarca K, Labruba MB, Zavala-Castro J, 2014. Guías Latinoamericanas de la RIICER para el diagnóstico de las rickettsiosis transmitidas por garrapatas. Rev Chil Infecto 31: 54–65. [DOI] [PubMed] [Google Scholar]
- 8.Sitio oficial de Uramita en Antioquia, Colombia. Alcaldía de Uramira. Uramita, un oriyecto para todos. Avaialble at: http://www.uramita-antioquia.gov.co/presentacion.shtml. Accessed December 5, 2018.
- 9.Barros-Battesti D, Arzua M, Bechara G, 2006. Carrapatos De Importancia Médico-Veterinaria Da Regiao Neotropical: Um Guia Ilustrado Para Identificação De Espécies. Primeira. São Paulo, Brazil: Integrated Consortium on Ticks and Tick-borne Diseases-ICTTD. [Google Scholar]
- 10.Pena DCH, Mafra CL, Calic SB, Labruna MB, Milagres BS, Walker DH, Galvão MAM, 2009. Serologic survey for antibodies to Rickettsia among domestic and wild animal populations in Brazil. Clin Microbiol Infect 15 (Suppl 2): 243–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinter A, Horta MC, Pacheco RC, Moraes-Filho J, Labruna MB, 2008. Serosurvey of Rickettsia spp. in dogs and humans from an endemic area for Brazilian spotted fever in the State of São Paulo, Brazil. Cad Saude Publica 24: 247–252. [DOI] [PubMed] [Google Scholar]
- 12.Londoño AF, et al. 2017. Wild and domestic animals likely involved in rickettsial endemic zones of Northwestern Colombia. Ticks Tick Borne Dis 8: 887–894. [DOI] [PubMed] [Google Scholar]
- 13.Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH, 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42: 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux V, Raoult D, 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int J Syst Evol Microbiol 50: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronquist F, Huelsenbeck JP, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 17.Paddock CD, Fernandez S, Echenique GA, Sumner JW, Reeves WK, Zaki SR, Remondegui CE, 2008. Rocky Mountain spotted fever in Argentina. Am J Trop Med Hyg 78: 687–692. [PubMed] [Google Scholar]
- 18.Rozental T, et al. 2015. A cluster of Rickettsia rickettsii infection at an animal shelter in an urban area of Brazil. Epidemiol Infect 143: 2446–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Oliveira SV, et al. 2016. An update on the epidemiological situation of spotted fever in Brazil. J Venom Anim Toxins Incl Trop Dis 22: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faccini-Martínez ÁA, Costa FB, Hayama-Ueno TE, Ramírez-Hernández A, Cortés-Vecino JA, Labruna MB, 2015. Rickettsia rickettsii in Amblyomma patinoi ticks, Colombia. Emerg Infect Dis 21: 2010–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eremeeva ME, et al. 2011. Rickettsia rickettsii in Rhipicephalus ticks, mexicali, Mexico. J Med Entomol 48: 418–421. [DOI] [PubMed] [Google Scholar]
- 22.Álvarez-Hernández G, Roldán JFG, Milan NSH, Lash RR, Behravesh CB, Paddock CD, 2017. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect Dis 17: e189–e196. [DOI] [PubMed] [Google Scholar]
- 23.Demma LJ, et al. 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med 353: 587–594. [DOI] [PubMed] [Google Scholar]
- 24.Drexler N, et al. 2014. Community-based control of the brown dog tick in a region with high rates of Rocky Mountain spotted fever, 2012–2013. PLoS One 9: e112368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabó MPJ, Pinter A, Labruna MB, 2013. Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cel Infect Microbiol 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]