Abstract.
Strongyloides stercoralis affects 30–100 million people worldwide. The burden is underestimated because of the paucity of studies, limited geographical areas surveyed, and poor quality of diagnostic tests. This study aimed at determining the epidemiology of strongyloidiasis using sensitive microscopy testing in rural populations living at different altitudes in Cusco, Peru. Data were collected from subjects aged > 3 years living in Quellouno (elevation 2,600 ft) and Limatambo (elevation 8,379 ft) districts. Subjects provided one fresh stool sample and answer a standardized questionnaire. Fresh stool was tested on site using the Baermann’s test and agar plate culture. Formalin-preserved stool was tested by rapid sedimentation. Eighty percent (585/715) of eligible subjects consented to participate; after excluding subjects with missing data, 65% (462/715) were included. Fifty-five percentage were female; the median age was 33 years (interquartile range 13–52), and 72% had government health insurance. Half had intestinal parasites, and Strongyloides was the most common (24.5%) followed by Giardia (15.5%), Blastocystis (14.9%), and hookworm (11.5%). The agar plate culture detected more cases of Strongyloides than Baermann’s or sedimentation tests. Strongyloides infection was more common at low altitude (26.4%) than at high altitude (18.6%), but the difference was not statistically significant (P = 0.08). Older age, walking barefoot, bathing in rivers/streams, and using municipal sewage were associated with strongyloidiasis. Strongyloides was the most prevalent parasite in the areas studied and was associated with demographic, socioeconomic, and sanitary factors.
INTRODUCTION
Strongyloidiasis, caused by the nematode Strongyloides stercoralis, is estimated to affect 30–100 million people worldwide.1 However, based on burden estimates for other soil-transmitted helminths, specifically hookworm, Bisoffi et al.2 argue that a more accurate number of people infected with Strongyloides is 370 million. Although Strongyloides is a cosmopolitan parasite, the infection is more prevalent in tropical and subtropical climates. In addition, the prevalence of infection within highly endemic countries is likely to vary significantly according to the environmental conditions. In most situations, only limited areas of each country are surveyed for strongyloidiasis and a limited number of studies per country are available, thus preventing a clear understanding of the epidemiology of the infection.3
Female Strongyloides parasites live within the epithelium of the small intestine where they produce eggs that hatch and release rhabditiform larvae into the intestinal lumen. These larvae are shed in the feces; however, they may mature into infective filariform larvae in the intestine to cause autoinfection.4 Autoinfection perpetuates strongyloidiasis in the host, which can remain undiagnosed for many years.2 In people who become immunosuppressed, the parasite is able to invade multiple organs, causing life-threatening disseminated disease with a case fatality rate of up to 90%.4 Transmission of strongyloidiasis requires specific soil conditions (i.e., moisture, temperature, and acidity) to allow the free-living filariform larvae to develop.5 Host behaviors that allow direct contact of the skin with the contaminated soil favor transmission.6 Few studies compare the prevalence of strongyloidiasis among populations with different behaviors and environmental conditions.
The diagnosis of strongyloidiasis often relies on microscopic examination of stool specimens. The methods used for detection of Strongyloides larvae include direct stool microscopy, agar plate culture, and multiple concentration techniques, including sedimentation, flotation, and the Baermann’s test.7,8 The sensitivity of each method varies significantly, with the agar plate culture and Baermann’s tests being the most sensitive.9 Serologic tests for Strongyloides IgG using recombinant antigens NIE and Strongyloides stercoralis immunoreactive antigen have demonstrated high sensitivity and specificity, but the availability and performance of commercial versions of these tests are still a problem in endemic areas, limiting their use.8,10–13 Several studies have evaluated polymerase chain reaction tests for the diagnosis of Strongyloides with inconsistent results and a large variation in sensitivity.7,14,15 Thus, the low sensitivity of the diagnostic methods used in epidemiologic studies hinders the evaluation of the burden and distribution of this infection in humans.
Peru has a high prevalence of Strongyloides infection (between 10% and 20%).16 Studies in Peru have shown significant variations in prevalence, ranging from < 1% to 40% depending on the area studied and the diagnostic methods used.17 Recent reviews on the prevalence of Strongyloides in Latin America and Peru suggested a very low prevalence in the highlands of Andes Mountains (prevalence < 1.5%).16,17 The heterogeneity of the studies and diversity of diagnostic methods used limited the understanding of strongyloidiasis epidemiology at high altitude in South America. The objective of the present study was to determine the epidemiology of strongyloidiasis using a combination of sensitive microscopy tests among two populations living at different altitudes in Cusco, Peru.
METHODS
A secondary analysis of data primarily collected for another study evaluating a Strongyloides diagnostic method was performed to evaluate the prevalence and factors associated with S. stercoralis infection at different elevations. The primary cross-sectional study was conducted in the Quellouno (elevation 2,600 ft) and Limatambo (elevation 8,379 ft) districts of the Cusco region in Peru (Figure 1). The Quellouno district in the La Convencion province has a total population of 18,089 and is located in the eastern slope of the Andes Mountains, referred to as the high jungle, with an average yearly temperature of 26°C and a rainy season from September to April averaging 36 mm of monthly rainfall. The Limatambo district in the Anta province has a population of 9,801 and is located in an inter-Andean valley with an average yearly temperature of 13°C and a rainy season from November to March averaging 137 mm of monthly rainfall. The human development index for Quellouno and Limatambo was 0.2931 and 0.2947 in 2012, respectively. Most of the population is composed of subsistence farmers living in adobe houses with dirt floors. Three communities in Quellouno (Putucusi, Alto Putucusi, and San Martin) and one community in Limatambo (Sauceda) were selected based on convenience (i.e., accessibility) for data collection between August 2015 and June 2016.
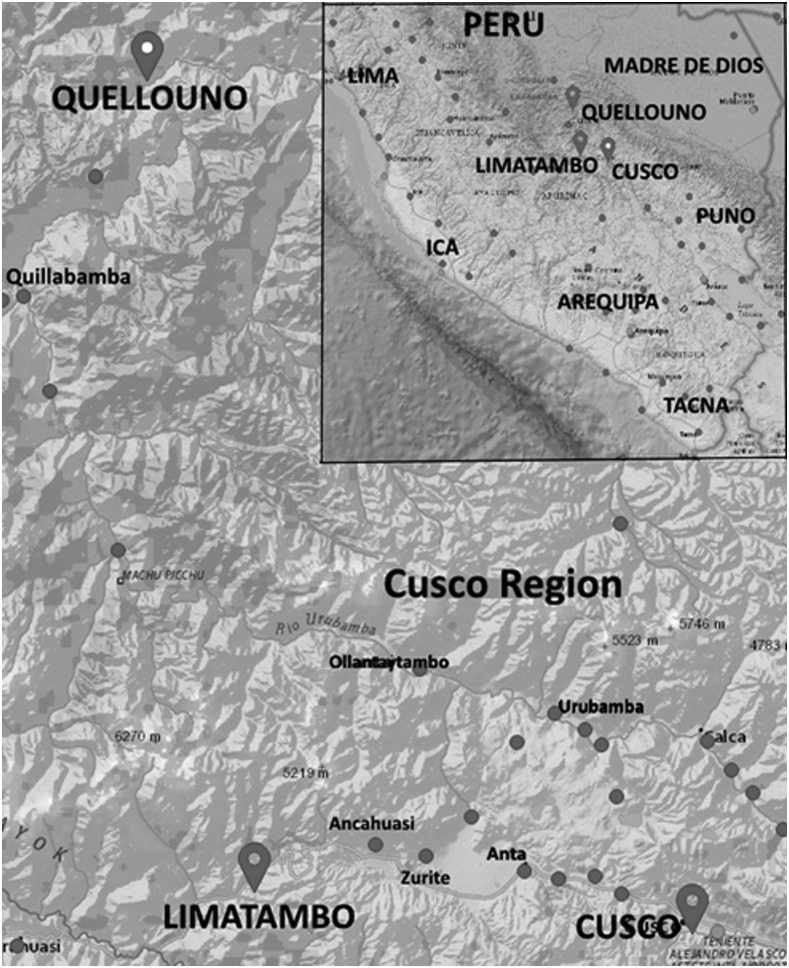
Figure 1.
Map of the Cusco region showing the geographic relationship between the study sites. The inset shows the map for the south part of Peru (adapted from www.weatherspark.com Cedar Lake Ventures, Inc.).
The study was carried out in collaboration with the local ministry of health and community authorities who were responsible for informing the population about the study. Male and female subjects aged 3 years or older residing in the selected communities were eligible to participate. Subjects were enrolled at their homes after providing informed consent. Children aged less than 18 years were required to provide assent in addition to the parent’s informed consent before enrollment. Bilingual (Spanish and Quechua) field workers conducted individual interviews with the participants and recorded their responses in standardized questionnaires. Information about demographics, socioeconomic status, and potential risk factors for Strongyloides infection was collected from participants. They were provided stool collection supplies and instructions to collect one fresh specimen in the morning of the scheduled household visit. Freshly produced specimens were collected and transported immediately to an onsite laboratory mounted for this study at the local health centers and staffed by experienced laboratory technicians. Aliquots from each stool sample were immediately tested by the Baermann’s test and agar plate culture to detect S. stercoralis larvae as previously described.18 Aliquots of each stool sample were preserved in 10% formalin to perform the Lumbreras rapid sedimentation test to detect helminth eggs at the laboratory of the Universidad Peruana Cayetano Heredia and University of Texas Medical Branch Collaborative Research Center in Cusco city.19
The data were entered and analyzed using the Statistical Package for the Social Sciences version 18.0 (SPSS Inc., Chicago, IL). Mean (±SD), medians with interquartile range (IQR), and frequencies were calculated to describe the distribution of the variables. The chi-squared test, Student’s t-test, and Mann–Whitney U-test were used to compare variables. Backward logistic regression was used to model Strongyloides and factors associated with the infection. A P < 0.05 was considered statistically significant.
The study protocol was approved by the Universidad Peruana Cayetano Heredia Institutional Review Board.
RESULTS
The census of the population for the four communities revealed 715 subjects eligible to participate in the study, and 585 (81.8%) subjects signed an informed consent form. Of these, 123 were excluded from the analysis because of the lack of complete stool test results (i.e., insufficient sample) or epidemiological information. A total of 462 (64.6%) subjects were included in the data analysis: 74.5% (344/462) from the low-altitude communities (Alto Putucusi, Putucusi, and San Martin) and 25.5% (118/462) from the high altitude-community (Sauceda) (Table 1). The median age of the participants was 33 years (IQR 13–52), and 54.9% (254/462) were female. Most participants lived in houses with dirt floors (69.9%, 305/436) and tin roofs (94.5%, 411/435). However, almost all (98%, 431/440) had drinking water tubed into their homes for at least a few hours per day. The majority (72.4%, 317/438) had health insurance provided by the government.
Table 1.
Characteristics of the study population
| Frequency (n = 462) | % | ||
|---|---|---|---|
| Community | Alto Putucusi | 106 | 22.9 |
| Putucusi | 174 | 37.7 | |
| San Martin | 64 | 13.9 | |
| Sauceda | 118 | 25.5 | |
| Median age (IQR) | 33 years (13–52) | ||
| Gender | Female | 254 | 54.9 |
| Male | 208 | 45.1 | |
| Median number of diarrhea episodes/year (IQR) | 2 (0–3) | ||
| Floor material (n = 436) | Dirt | 307 | 70.4 |
| Not dirt | 129 | 29.6 | |
| Roof material (n = 437) | Tin | 411 | 94.1 |
| Tile | 16 | 3.7 | |
| Concrete | 8 | 1.8 | |
| Source of drinking water (n = 440) | Municipal tubed | 431 | 98.0 |
| River | 3 | 0.7 | |
| Irrigation channel | 3 | 0.7 | |
| Government health insurance (n = 438) | Yes | 317 | 72.4 |
| No | 121 | 27.6 | |
IQR = interquartile range.
Feces were eliminated using the municipal sewage system (59.6%, 261/438), improved latrines (26.5%, 116/438), septic tanks (9.6%, 42/438), simple pits (3.7%, 16/438), and the open field (0.7%, 3/348). Most subjects reported inconsistent or no wearing shoes while defecating (80.7%, 356/441) and inconsistent or no hand washing after defecating (67.9%, 296/436). The most frequently wore footwear were sandals (69.8%, 308/441) and shoes (23.6%, 104/441). The majority reported not walking (44.5%, 190/427) or rarely (47.5%, 203/427) walking barefoot outside their home. Almost 60% (262/436) reported working in their farm often or every day. Almost all subjects performed personal hygiene in a shower or bathtub (98%, 433/442).
Thirty-nine percent (38.6%, 177/458) reported a past medical history; one-third of these reported malaria (29.4%, 52/177), 2.7% (12/177) anemia, and 1.8% (8/177) each typhoid, dyspepsia, and hepatitis. Almost half of these subjects (47.5%, 84/177) reported other diverse medical problems. Diarrhea in the previous 12 months was reported by 70.5% (324/459) of the subjects, and the median number of diarrheal episodes in that period was 2 (IQR 0–3). The most common symptoms reported by the participants in the 3 months before the study were cough, anorexia, and fatigue (Table 2).
Table 2.
Symptoms in the prior 3 months reported by the participants and their frequency according to the Strongyloides infection status
| Total | Strongyloides (+) | Strongyloides (−) | |||||
|---|---|---|---|---|---|---|---|
| Symptoms | % | N | % | N | % | N | P-value |
| Cough | 20.6 | 88/428 | 24.8 | 26/105 | 19.2 | 62/323 | 0.22 |
| Anorexia | 15 | 64/428 | 11.4 | 12/105 | 16.1 | 52/323 | 0.24 |
| Fatigue | 12.1 | 52/428 | 7.7 | 8/104 | 13.6 | 44/324 | 0.11 |
| Bloating | 11 | 47/429 | 4.8 | 5/105 | 13 | 42/324 | 0.02 |
| Pallor | 10.1 | 43/427 | 6.7 | 7/104 | 11.1 | 36/323 | 0.19 |
| RUQ pain | 10 | 43/428 | 10.6 | 11/104 | 9.9 | 32/324 | 0.83 |
| Weight loss | 8 | 34/427 | 6.7 | 7/104 | 8.4 | 27/323 | 0.59 |
| Diarrhea | 6.1 | 26/427 | 7.7 | 8/104 | 5.6 | 18/323 | 0.43 |
| Rash | 5.8 | 25/428 | 3.8 | 4/104 | 6.5 | 21/324 | 0.47 |
| Jaundice | 5.6 | 24/428 | 1 | 1/105 | 7.1 | 23/323 | 0.01 |
| Nausea/vomiting | 5.2 | 22/427 | 2.9 | 3/104 | 5.9 | 19/323 | 0.31 |
RUQ = right upper quadrant. N is different from total enrolled subjects because of no response to symptoms questions.
Half (50.2%, 232/462) of the participants had at least one intestinal helminth or protozoan detected by microscopy. Strongyloides stercoralis (24.5%, 113/462) was the most common intestinal helminth, and Giardia spp. (15.8%, 63/462) was the most common protozoan. Blastocystis hominis (14.9%, 69/462), hookworm (11.5%, 53/462), Trichuris trichiura (5.8%, 27/462), Hymenolepis nana (3.5%, 16/462), and Ascaris lumbricoides (1.9%, 9/462) were less commonly found. The agar plate culture test detected the most cases of Strongyloides (18.4%, 85/462), followed by the Lumbreras rapid sedimentation method (12.8%, 55/431), and the Baermann’s test (10.2%, 47/462). Of note, the agreement between the agar plate culture and the Lumbreras rapid sedimentation (Kappa = 0.36, P < 0.001) or the Baermann’s test (Kappa = 0.47, P < 0.001) was only moderate.
Except for bloating and jaundice, the symptoms reported by subjects with strongyloidiasis were not significantly different from the symptoms reported by subjects without the infection (Table 2). Strongyloides infection was significantly associated with older age, certain socioeconomic characteristics (house roof material, owning poultry, and having government health insurance), and some exposures, such as using the municipal sewage system, bathing in rivers or streams, and walking barefoot (Table 3). Strongyloides infection was more common among those living at lower altitude than those living a higher altitude (26.4% versus 18.6%) and among those working often in farms than those doing so infrequently or not at all (26.7% versus 21.8%), but the differences did not reach statistical significance. The backward logistic regression analysis showed that those who bathe in rivers and streams, used the municipal sewage system, walked barefoot often or always, and were older were significantly more likely to have Strongyloides infection. By contrast, those who raised chicken and had government-provided health insurance were less likely to have Strongyloides (Table 4).
Table 3.
Characteristics of the population according to the Strongyloides infection status
| Characteristic | Strongyloides (+) | Strongyloides (−) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | P-value | ||
| Disposal of feces | Municipal sewage | 79 | 69.9 | 182 | 52.1 | 0.001 |
| Other | 34 | 30.1 | 167 | 47.9 | ||
| Household roof | Tin | 109 | 96.5 | 302 | 86.5 | 0.003 |
| Other | 4 | 3.5 | 47 | 13.5 | ||
| Raises chicken at home | Yes | 81 | 71.7 | 292 | 83.7 | 0.005 |
| No | 32 | 28.5 | 57 | 16.3 | ||
| Age in years | Median (IQR) | 36.5 | (25–53.5) | 32 | (11.25–51) | 0.01 |
| Raises ducks at home | Yes | 22 | 19.5 | 108 | 30.9 | 0.01 |
| No | 91 | 80.5 | 241 | 69.1 | ||
| Has government insurance | Yes | 74 | 65.5 | 267 | 76.5 | 0.02 |
| No | 39 | 34.5 | 82 | 23.5 | ||
| Personal hygiene | Shower/bathtub | 103 | 95.4 | 330 | 98.8 | 0.04 |
| River/stream | 5 | 4.6 | 4 | 1.2 | ||
| Walks around barefoot | Often/always | 13 | 12.4 | 21 | 6.5 | 0.05 |
| Seldom/never | 92 | 87.6 | 301 | 93.5 | ||
| Elevation | Low | 91 | 80.5 | 253 | 72.5 | 0.08 |
| High | 22 | 19.5 | 96 | 27.5 | ||
| Works in the farm | Often/always | 70 | 64.8 | 192 | 58.5 | 0.24 |
| Seldom/never | 38 | 33.2 | 136 | 41.5 | ||
| Gender | Female | 57 | 50.4 | 197 | 56.4 | 0.26 |
| Male | 56 | 49.6 | 152 | 43.6 | ||
IQR = interquartile range.
Table 4.
Backward logistic regression analysis of variables associated with Strongyloides infection
| Characteristic | Category | Adjusted OR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Personal hygiene | River or stream | 5.91 | 1.32 | 26.38 | 0.020 |
| Disposal of feces | Municipal sewage | 2.51 | 1.47 | 4.28 | 0.001 |
| Walks around barefoot | Often/Always | 2.23 | 1.02 | 4.9 | 0.043 |
| Age | Older | 1.01 | 1 | 1.02 | 0.031 |
| Raises chicken | Yes | 0.46 | 0.26 | 0.81 | 0.007 |
| Government health insurance | Yes | 0.49 | 0.29 | 0.83 | 0.008 |
OR = odds ratio.
DISCUSSION
Strongyloidiasis is an underestimated parasitic public health problem in developing countries. In this study, one of four subjects evaluated was infected with Strongyloides and half of the subjects diagnosed with a parasite had strongyloidiasis. Strongyloides infection was associated with older age; certain socioeconomic characteristics, such as having a tin roof, owning poultry, and having government health insurance; and exposures, such as using the municipal sewage system, bathing in rivers or streams, and walking barefoot.
Importantly, the prevalence of Strongyloides at high altitude was not statistically different than the prevalence at low altitude. This finding contrasts with several reports from Peru and highlights the importance of using Strongyloides-specific microscopy methods to obtain more accurate estimates of this parasite’s burden. Similarly, a recent study from Bolivia using the same microscopy methods (sedimentation, Baermann’s, and culture) showed a Strongyloides prevalence of 6.4% at an elevation 2,500 m that was not statistically different from the prevalence (8.9%) at 400 m.20
Forrer et al.21 described a higher prevalence of Strongyloides infection in older age groups in a study involving more than 2,500 subjects from 60 communities in Cambodia. In this study, subjects older than 49 years were 60% more likely to have strongyloidiasis. Other recent studies in Angola and Lao People’s Democratic Republic (PDR) showed an association between infection and age.6,22 Similarly, in our study, strongyloidiasis was associated with increasing age. Strongyloides stercoralis has a complex life cycle, including an autoinfection phase where larvae mature within the host and start a new cycle without ever reaching the environment. Autoinfection allows Strongyloides to remain in the host for prolonged periods that may be counted in decades.23 In elderly patients, age-related malnutrition and impaired immunity can increase susceptibility to severe forms of strongyloidiasis in endemic areas.24,25 Case reports of strongyloidiasis in the elderly causing hyperinfection syndrome, invasive enteritis, and disseminated infection have resulted in high mortality.24,26,27
In the community, strongyloidiasis causes only few and unspecific symptoms which rarely prompt medical consultation or diagnostic workup.28 In our study, few subjects with strongyloidiasis reported symptoms, and in most cases, the prevalence of these was not different from the symptoms reported by subjects without the infection. This contrasts with a study by Khieu et al. among Cambodian children where almost a third of those infected reported diarrhea and 10% reported itchy skin within the previous 2 weeks. Children with strongyloidiasis were significantly more likely to report these symptoms than uninfected children.29 As with other soil-transmitted helminth infections, it is likely that subjects with the highest parasite burdens report symptoms more often. However, assessing Strongyloides burden of infection in individuals is difficult as no quantitative tests are readily available.
Strongyloides infection was associated with socioeconomic characteristics in our study population. These included living under a tin roof, owning poultry, use of the municipal sewage system, and having health insurance. More subjects with Strongyloides reported living under a tin roof than subjects without Strongyloides, and fewer subjects with Strongyloides reported owning poultry than subjects without Strongyloides. These characteristics suggest a lower socioeconomic status among subjects in these rural farming communities. Recent studies have described the association between lower socioeconomic status and Strongyloides infection.21,30 Of note, using the municipal sewage system for excreta elimination was associated with a higher prevalence of Strongyloides infection in our study. This contrasts with a study by Echazu et al.31 that showed a 2-fold increase in the risk of strongyloidiasis among subjects without access to improved sanitation. A small study in Argentina showed a decrease in incident Strongyloides infections among subjects who moved to houses with improved water and sanitation compared with those with no access to these facilities.32 Studies in South-East Asia show that the use of improved latrines compared with open defecation was associated with a lower risk of infection.29 However, earlier studies have shown inconsistent results with the use of improved sanitation. Hall et al.33 reported that the use of community latrines was associated with a higher prevalence of strongyloidiasis than owning a latrine. Yori et al.8 studied 800 subjects in a rural community from the Peruvian Amazon and failed to find an association between methods of feces disposal and strongyloidiasis. In our study, feces were observed in the floor around the toilet bowls during the household visits in Quellouno district. Although, it is unclear if this can explain our findings, it suggests that environmental contamination may have existed in the bathroom floor. Adults or children used to other types of excreta elimination may have problems adapting to improved methods like toilet bowls. This highlights the needs for education of the population when interventions to improve sanitation involve a shift in cultural norms and uses. An alternative explanation could be that defects in the integrity or treatment of the municipal sewage system caused contamination of the environment.
Our study showed that those who had government health insurance were less likely to have strongyloidiasis. Similarly, Forrer et al.21 found that another marker of health-care access, distance traveled to the health-care facility, was associated with a higher likelihood of Strongyloides and hookworm coinfection in Cambodia. Liu and Lu studied health inequalities at the community level in Rwanda and found that health insurance and travel time to the health-care facility were the two major contributors to health inequalities and medical care utilization, especially among the poorest groups.34 Thus, access to health care may also play a role in the epidemiology of Strongyloides in rural areas.
Walking barefoot and bathing in rivers/streams were associated with Strongyloides infection in our study. Other authors have reported similar results. Herrera et al.35 in a small case–control study in Peru found that participants who bathed in the river 3–4 times/week were more likely to have strongyloidiasis than those who did not bathed in the river. Senephansiri et al.6 reported that not wearing shoes when leaving the house was associated with increased frequency of strongyloidiasis in a small study involving 500 subjects in Lao PDR. Forrer et al. administered Strongyloides treatment and education on hygiene to subjects with the infection and followed them up for 2 years. Subjects who failed to wear shoes at home or when defecating despite the intervention were 2.6 times more likely to acquire strongyloidiasis in the following 12 months.36 Avoiding direct contact of bare skin with contaminated soil may be a target for prevention and control of Strongyloides. However, accomplishing this behavioral shift despite repeated educational efforts showed to be a difficult task in prior studies.36 Other studies targeting soil-transmitted helminths with packages including education, improved sanitation, and biannual albendazole had no effects over Strongyloides prevalence, highlighting the significant challenges ahead if control will be attempted.37
Our study had limitations that included a small sample size, which likely precluded finding some other associated factors such as altitude. In our study, there was a higher prevalence of infection at lower altitude communities than the high-altitude community, but this difference was not statistically significant. Some of the information collected on symptoms and past medical history relied on subject’s recollection of events happening in the previous 3 months which may have affected report. Our cross-sectional design and limited scope of testing (i.e., single stool samples and no environmental samples were collected) may have missed some factors explaining some of our findings.
In conclusion, Strongyloides is a neglected parasite with a high prevalence in some areas of the Cusco region of Peru, including the highlands. Socioeconomic and sanitary conditions were associated with the infection in our population, which calls for integrated government efforts to improve living conditions, educate the population, and create ivermectin mass deworming programs.
Acknowledgments:
We would like to acknowledge the support provided by the Red de Servicios de Salud Cusco Norte to this study that included space to mount a laboratory for stool testing.
REFERENCES
- 1.Genta RM, 1989. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev Infect Dis 11: 755–767. [DOI] [PubMed] [Google Scholar]
- 2.Bisoffi Z, et al. 2014. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beknazarova M, Whiley H, Ross K, 2016. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health 13: 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumbele IU, Ngole VM, Ekosse GI, 2014. Influence of physico-chemistry and mineralogy on the occurrence of geohelminths in geophagic soils from selected communities in the Eastern Cape, South Africa, and their possible implication on human health. Int J Environ Health Res 24: 18–30. [DOI] [PubMed] [Google Scholar]
- 6.Senephansiri P, Laummaunwai P, Laymanivong S, Boonmar T, 2017. Status and risk factors of Strongyloides stercoralis infection in rural communities of Xayaburi Province, Lao PDR. Korean J Parasitol 55: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knopp S, et al. 2014. Diagnostic accuracy of Kato–Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg 90: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yori PP, et al. 2006. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg 74: 97–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Campo Polanco L, Gutiérrez LA, Cardona Arias J, 2014. Diagnosis of Strongyloides stercoralis infection: meta-analysis on evaluation of conventional parasitological methods (1980–2013). Rev Esp Salud Publica 88: 581–600. [DOI] [PubMed] [Google Scholar]
- 10.Krolewiecki AJ, et al. 2010. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisoffi Z, et al. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fradejas I, Herrero-Martínez JM, Lizasoaín M, Rodríguez de Las Parras E, Pérez-Ayala A, 2018. Comparative study of two commercial tests for Strongyloides stercoralis serologic diagnosis. Trans R Soc Trop Med Hyg 112: 561–567. [DOI] [PubMed] [Google Scholar]
- 13.Arifin N, Hanafiah KM, Ahmad H, Noordin R, 2019. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect 52: 371–378. [DOI] [PubMed] [Google Scholar]
- 14.Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S, 2013. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop 126: 89–92. [DOI] [PubMed] [Google Scholar]
- 15.Becker SL, et al. 2015. Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d’Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Tropica 150: 210–217. [DOI] [PubMed] [Google Scholar]
- 16.Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z; COHEMI Project Study Group , 2015. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 143: 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcos LA, Cabrera R, Machicado JD, Canales M, Terashima A, 2010. Distribution of prevalence of Strongyloides stercoralis in Peru (1981–2010): an exploratory study. Rev Peru Parasitol 18: e39–e40. [Google Scholar]
- 18.Salazar SA, Gutierrez C, Berk SL, 1995. Value of the agar plate method for diagnosis of intestinal strongyloidiasis. Diagn Microbiol Infect Dis 23: 141–145. [DOI] [PubMed] [Google Scholar]
- 19.Tello R, Terashima A, Marcos LA, Machicado J, Canales M, Gotuzzo E, 2012. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis 16: e414–e416. [DOI] [PubMed] [Google Scholar]
- 20.Gétaz L, et al. 2019. Epidemiology of Strongyloides stercoralis infection in Bolivian patients at high risk of complications. PLoS Negl Trop Dis 13: e0007028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forrer A, Khieu V, Schär F, Vounatsou P, Chammartin F, Marti H, Muth S, Odermatt P, 2018. Strongyloides stercoralis and hookworm co-infection: spatial distribution and determinants in Preah Vihear province, Cambodia. Parasit Vectors 11: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dacal E, et al. 2018. Prevalence and molecular characterization of Strongyloides stercoralis, Giardia duodenalis, Cryptosporidium spp., and Blastocystis spp. isolates in school children in Cubal, Western Angola. Parasit Vectors 11: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Junior KN, Zaman R, Zaman MH, Zaman T, 2017. Twenty-five years of chronic strongyloidiasis in an immigrant. Clin Med Insights Case Rep 10: 1179547616684828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naves MM, Costa-Cruz JM, 2013. High prevalence of Strongyloides stercoralis infection among the elderly in Brazil. Rev Inst Med Trop Sao Paulo 55: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gavazzi G, Herrmann F, Krause KH, 2004. Aging and infectious diseases in the developing world. Clin Infect Dis 39: 83–91. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues MA, Fróes RC, Anefalos A, Kobayasi S, 2001. Invasive enteritis by Strongyloides stercoralis presenting as acute abdominal distress under corticosteroid therapy. Rev Hosp Clin Fac Med Sao Paulo 56: 103–106. [DOI] [PubMed] [Google Scholar]
- 27.Upadhyay D, Corbridge T, Jain M, Shah R, 2001. Pulmonary hyperinfection syndrome with Strongyloides stercoralis. Am J Med 111: 167–169. [DOI] [PubMed] [Google Scholar]
- 28.Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha JL, Seet RC, Summer AP, Nield LS, Supparatpinyo K, Chaiwarith R, Walker PF, 2007. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med 120: 545.e1–545.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khieu V, Schär F, Marti H, Sayasone S, Duong S, Muth S, Odermatt P, 2013. Diagnosis, treatment and risk factors of Strongyloides stercoralis in schoolchildren in Cambodia. PLoS Negl Trop Dis 7: e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuyizere A, Ndayambaje A, Walker TD, Bayingana C, Ntirenganya C, Dusabejambo V, Hale DC, 2018. Prevalence of Strongyloides stercoralis infection and other soil-transmitted helminths by cross-sectional survey in a rural community in Gisagara district, Southern Province, Rwanda. Trans R Soc Trop Med Hyg 112: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echazú A, et al. 2013. Effect of poor access to water and sanitation as risk factors for soil-transmitted helminth infection: selectiveness by the infective route. PLoS Negl Trop Dis 9: e0004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vargas P, Krolewiecki AJ, Echazú A, Juarez M, Cajal P, Gil JF, Caro N, Nasser J, Lammie P, Cimino RO, 2017. Serologic monitoring of public health interventions against Strongyloides stercoralis. Am J Trop Med Hyg. 97: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall A, Conway DJ, Anwar KS, Rahman ML, 1994. Strongyloides stercoralis in an urban slum community in Bangladesh: factors independently associated with infection. Trans R Soc Trop Med Hyg 88: 527–530. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Lu C, 2018. Decomposing health inequality with population-based surveys: a case study in Rwanda. Int J Equity Health 17: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera J, Marcos L, Terashima A, Alvarez H, Samalvides F, Gotuzzo E, 2006. Factors associated with Strongyloides stercoralis infection in an endemic area in Peru. Rev Gastroenterol Peru 26: 357–362. [PubMed] [Google Scholar]
- 36.Forrer A, Khieu V, Schindler C, Schär F, Marti H, Char MC, Muth S, Odermatt P, 2016. Ivermectin treatment and sanitation effectively reduce Strongyloides stercoralis infection risk in rural communities in Cambodia. PLoS Negl Trop Dis 10: e0004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinmann P, Yap P, Utzinger J, Du ZW, Jiang JY, Chen R, Wu FW, Chen JX, Zhou H, Zhou XN, 2015. Control of soil-transmitted helminthiasis in Yunnan province, People’s Republic of China: experiences and lessons from a 5-year multi-intervention trial. Acta Trop 141: 271–280. [DOI] [PubMed] [Google Scholar]