Abstract.
The conventional method of detecting Strongyloides stercoralis in fecal samples has poor diagnostic sensitivity. Detection of Strongyloides-specific antibodies increases the sensitivity; however, most tests are ELISAs that use parasite extract which may cross-react with the sera of other helminth infections. To improve the serological diagnosis of strongyloidiasis, this study aimed at developing a sensitive and specific lateral flow rapid dipstick test. Two recombinant proteins, recombinant NIE (rNIE) and recombinant Ss1a (rSs1a), were used in preparing the dipstick, with gold-conjugated antihuman IgG4 as detector reagent. In parallel, the corresponding ELISA was performed. Both assays demonstrated diagnostic sensitivity of 91.3% (21/23) when tested with serum samples of patients with Strongyloides infection, and 100% specificity with 82 sera of asymptomatic (healthy) and those with other parasitic infections. The ELISA and dipstick test results were positively correlated to each other (r = 0.6114, P = 0.0019). The developed lateral flow dipstick test may improve the serodiagnosis of strongyloidiasis and merit further validation studies.
Strongyloidiasis is a helminth disease mainly caused by the intestinal parasite Strongyloides stercoralis, which is prevalent in tropical and subtropical regions of the world. The global prevalence of the disease is about 370 million, and this may be an underestimation because of substantial variability in the disease distribution across countries, a limited number of studies and suboptimal diagnostic methods.1 It was reported that the prevalence has been increasing in Southeast Asia; Latin America; the Caribbean; Southern, Eastern, and Central Europe; and sub-Saharan Africa.2 A review by Beknazarova et al.3 suggested that strongyloidiasis should be referred to as a disease of the disadvantaged. They noted that low socioeconomic status of communities and poor sanitation system are major factors that favor the spread of the disease rather than geographic or climatic factors.
Most infected people are asymptomatic or have nonspecific symptoms and, thus, are often misdiagnosed or not diagnosed. Because of the phenomenon of autoinfection, Strongyloides can remain for many years in the body, even for life.4,5 When an infected person becomes immunosuppressed, the autoinfection may exacerbate leading to hyperinfection, and the parasite may invade various organs and tissues, resulting in disseminated infection; both of these conditions are often fatal. Because immunosuppressing conditions such as cancers, autoimmune diseases, and transplantations are increasing, infected people should be treated before the administration of immunosuppressive drugs. Antibody production in immunosuppressed patients may be affected; thus, both indirect detection (serology) and direct detection (agar plate culture or DNA amplification) should be used to detect Strongyloides infection in these individuals.
A definitive diagnosis of strongyloidiasis is usually based on the detection of larvae in fecal samples. This technique has poor sensitivity because in most cases, the larval output in stool is low and intermittent. Molecular detection by polymerase chain reaction (PCR) and real-time PCR has been reported. These advanced techniques showed improvements in the diagnostic specificity6,7; however, in terms of diagnostic sensitivity, the results from different studies are not in complete agreement. Some studies showed higher sensitivity of molecular detection than parasitological diagnosis (Baermann method and agar plate culture), whereas other studies showed equivalent sensitivity.8–11
Serodiagnosis is an important detection method for strongyloidiasis. Commercially available tests such as ELISA kits from Bordier-ELISA (Bordier Affinity Products SA, Crissier, Switzerland) and Strongyloides-ELISA (Scimedx Corporation, Dover, NJ) use native larval antigen which detect specific anti-Strongyloides IgG antibody in patients’ serum samples. The major drawbacks with this type of assay are the laborious effort in culturing the larvae12 and potential cross-reaction with other parasitic infections.13 The use of recombinant antigen may overcome these drawbacks, in which, unlike native antigen, it is easily produced on a large scale, is relatively cheaper, and can reduce or eliminate cross-reactivity with other helminth infections.14 In the present study, we used two recombinant antigens, recombinant NIE (rNIE) and rSs1a, in developing a prototype lateral flow dipstick test for IgG4 antibody detection of strongyloidiasis (Strongy Rapid) using the principle of immunochromatography.
Recombinant NIE is an established antigen for the diagnosis of Strongyloides infection. It is a 31-kDa protein derived from S. stercoralis L3 cDNA library identified by Ravi et al.,15 whereas rSs1a antigen was identified by Arifin et al.16 from immunoscreening of an S. stercoralis cDNA library derived from a mixture of L3 and adult worms. Both the rNIE and rSs1a have shown good diagnostic sensitivity and specificity when tested with different assays using either IgG or IgG4 as the detector reagent.14,16–18
In the present study, diagnostic sensitivity was determined using serum samples that had previously been shown to be positive for Strongyloides infection by at least two methods, that is, direct larvae detection via microscopy and/or real-time PCR, and indirect detection by IgG-ELISA (SciMedx Corporation, Denville, NJ). With regard to the direct method, five samples were positive by microscopy and real-time PCR, seven samples were positive by microscopy (real-time PCR not performed), one sample was positive by microscopy but negative by real-time PCR, and ten samples were positive by real-time PCR (microscopy not performed). Meanwhile, diagnostic specificity determination was performed with 82 serum samples that were seronegative by the IgG-ELISA. They came from 12 asymptomatic (healthy) individuals and 70 patients infected with other parasitic infections, that is, ascariasis (n = 4), filariasis (n = 10), giardiasis (n = 1), hookworm (n = 6), schistosomiasis (n = 11), toxocariasis (n = 20), trichuriasis (n = 4), toxoplasmosis (n = 6), echinococcosis (n = 6), and cysticercosis (n = 2). All serum samples used in this study were archived and anonymized samples from serum bank at the Institute for Research in Molecular Medicine. Ethical clearance for the use of these samples was obtained from the Universiti Sains Malaysia (USM) Human Research Ethics Committee (USM/JEPeM/17050273).
Both proteins were custom-cloned into pET expression vectors (Merck, Darmstadt, Germany): rNIE in pET32 and rSs1a in pET28. They were expressed in an Escherichia coli expression system using the shake-flask method and purified using gravity-flow purification with cOmplete™ His-Tag Purification Resin (Roche Diagnostics GmbH, Mannheim, Germany). The two recombinant proteins were mixed in a determined ratio (proprietary information), and 0.5 mg/mL was lined (as the test line) on a High-Flow Plus 90 nitrocellulose membrane card (Millipore Corporation, Bedford, MA). Goat anti-mouse IgG at 0.5 mg/mL (Invitrogen, Carlsbad, CA) was lined (as the control line) 6 mm above the test line. The dipstick strip was prepared by assembling a cellulose fiber absorbent pad on the membrane card and then cutting into 5-mm strips using a strip cutter (A-Point Technologies Inc., Gibbstown, NJ). The strips were dried at 37°C (2 hours), blocked (37°C, 2 hours), and kept in a desiccator cabinet at room temperature.
To run the Strongy Rapid test, the serum sample was first mixed (1:1) with chase buffer (Reszon Diagnostics International, Selangor, Malaysia) in the first well of a microtiter well. An amount of 25 μL of antihuman IgG4-gold–conjugated antibody at optical density (OD) 3 was pipetted into the second well, and finally, 35 μL of chase buffer was added into the third well. The dipstick was placed in the first well until all the serum sample migrated up the membrane on the dipstick before transferring the strip into the second well that contained the IgG4-gold conjugate. Once all the conjugate was fully absorbed in the dipstick, the strip was transferred to the third well to wash the excess unbound conjugate. The test result was read after 15 minutes, the dipstick that showed two red colored lines (test and control lines) was interpreted as positive, whereas the dipstick that showed only one red line (control line) was interpreted as negative. A digital camera captured the image of the dipstick and then transferred the image to a computer. The images were converted to a gray-scale format (8 bit), followed by quantifying the intensity of the test line using ImageJ 1.49v software (National Institutes of Health, Bethesda, MD).19
For comparison with the dipstick, an IgG4-ELISA using the same combined antigens and serum panel was performed following our previously reported procedure16 with some modifications: the plate was coated with 0.5 ug/mL recombinant protein, primary antibody (serum) at 1:100, and antihuman IgG4-HRP at 1:2,000. The cutoff values (COVs) of the ELISA and dipstick were determined using the assay results of 12 healthy sera, that is, their mean value plus one standard deviation. The COV of the ELISA was determined to be OD 0.1, whereas the COV of the dipstick intensity reading was at 60.
The diagnostic evaluation of Strongy Rapid showed 91.3% sensitivity and 100% specificity in detecting Strongyloides infection (Table 1). Figure 1 illustrates images of the dipstick after performing the test. Two false-negative results were obtained; the sera may have originated from patients with recent infections because anti-Strongyloides IgG4 antibody mostly detects chronic infection rather than acute infection.4,20 Meanwhile, IgG1 (the major component of IgG) was reported to be more prominent in samples from people with early infection.20,21
Table 1.
Summary of evaluation of Strongy Rapid lateral flow dipstick when tested with different groups of serum samples
| Sample | N | Reactivity | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Strongyloides infection | 23 | 21 | 2 | 91.3% | – |
| Other infections | 100% | ||||
| Ascariasis | 4 | 0 | 4 | – | – |
| Brugian filariasis | 8 | 0 | 8 | – | – |
| Bancroftian filariasis | 2 | 0 | 2 | – | – |
| Giardiasis | 1 | 0 | 1 | – | – |
| Hookworm | 6 | 0 | 6 | – | – |
| Schistosomiasis | 11 | 0 | 11 | – | – |
| Toxocariasis | 20 | 0 | 20 | – | – |
| Trichuriasis | 4 | 0 | 4 | – | – |
| Toxoplasmosis | 6 | 0 | 6 | – | – |
| Echinococcosis | 6 | 0 | 6 | – | – |
| Cysticercosis | 2 | 0 | 2 | – | – |
| Healthy individuals | 12 | 0 | 12 | – | 100% |
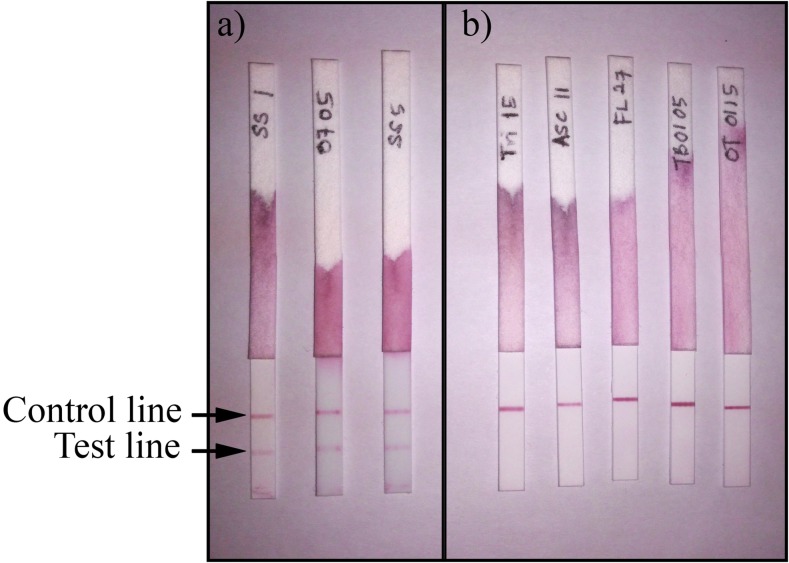
Figure 1.
The appearance of Strongy Rapid lateral flow dipstick after testing with serum samples; (A) positive results with serum samples of patients with strongyloidiasis, (B) negative results with serum samples from other diseases, that is, trichuriasis, ascariasis, filariasis, hookworm, and toxocariasis. This figure appears in color at www.ajtmh.org.
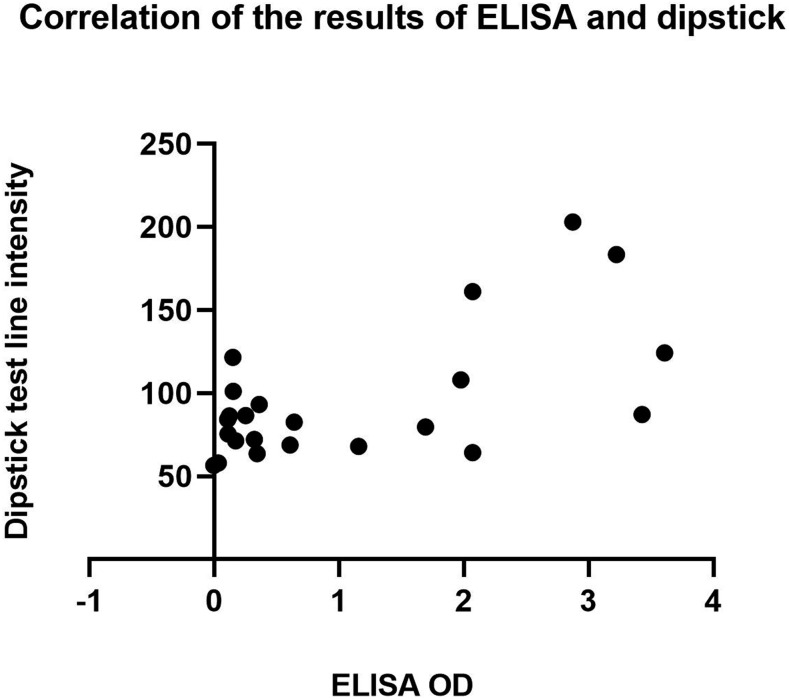
IgG4-ELISA also showed 91.3% sensitivity and 100% specificity, thus 100% concordant with the dipstick results. Pearson correlation coefficient was calculated to measure the degree of association between the results of these two tests when tested with Strongyloides-positive serum samples. There was a moderate positive correlation between the two tests (r = 0.6114, P = 0.0019), which suggests that the increase in the OD values of ELISA was correlated with the increase in test line intensities of the dipstick. Figure 2 shows a scatterplot that summarizes the results.
Figure 2.
A scatterplot showing a moderate positive linear correlation between the OD of ELISA and the test line intensity measured by ImageJ. Pearson correlation coefficient r = 0.6114, P = 0.0019.
Recombinant NIE has been used in several assays for serodiagnosis of strongyloidiasis. A luciferase immunoprecipitation system (LIPS) was developed and compared with IgG ELISA.14 The results indicated that the rNIE-LIPS showed better specificity (100%) when compared with rNIE ELISA (95%), and both assays demonstrated 97% diagnostic sensitivity. In another study, an IgG4 ELISA using rNIE antibody was developed and reported to be 93% specific and 95% sensitive.17 The same researchers also developed a fluorescent bead assay (Luminex Corporation, Austin, TX) and concluded that both IgG4-ELISA and Luminex assays have improved specificities when compared with IgG ELISA which used parasite lysate.17 An IgG ELISA prototype diagnostic kit based on rNIE (Strongy Detect™), is available from InBios International, Seattle, WA. The reported sensitivity and specificity were 83.6% and 91.3%, respectively. The second recombinant antigen in Strongy Rapid, rSs1a, has been previously tested in IgG4 assays using Western blot and ELISA and showed a sensitivity of 100% and 96%, respectively, and 93% specificity.16
The use of two or more recombinant antigens in the diagnosis of the disease has been shown to improve its diagnostic performance. Subathra et al.22 have reported that the use of combined Leptospira recombinant antigens (rLipL32 and rLipL41) increased the sensitivity and specificity of IgG ELISA and latex agglutination test for serodiagnosis of leptospirosis as compared with the use of a single recombinant antigen. In another study by our group, three Toxocara recombinant antigens were used to increase the diagnostic sensitivity of rapid dipstick assay to detect toxocariasis.23 In the present study, the use of rNIE and rSs1a allowed the development of a sensitive and specific rapid dipstick test and was more sensitive compared with using dipsticks with either single antigen (data not shown).
To date, there is still no lateral flow rapid test for strongyloidiasis. Such a test would be useful as a screening test, for patient diagnosis and seroprevalence studies because most of the infected people live in developing countries and many in areas with limited financial, infrastructure, and technical resources. Access to a rapid, affordable, and accurate test would enable more studies to be performed and provide more accurate global prevalence data of the infection.1
The present study produced a laboratory prototype and initial evaluation results of a lateral flow rapid test for strongyloidiasis. Further development of Strongy Rapid is needed to increase its diagnostic sensitivity to at least 95% and to change the format into the more physically robust cassette test that has a few simple steps and require no instrument. Multicenter validation studies using samples from various countries, including posttreatment samples, are also needed before it can be applied in the clinical setting or for epidemiological studies.
REFERENCES
- 1.Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P, 2013. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Munoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z, COHEMI Project Study Group , 2015. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 143: 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beknazarova M, Whiley H, Ross K, 2016. Strongyloidiasis: a disease of socioeconomic disadvantage. Int J Environ Res Public Health 13: E517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkins NS, Conway DJ, Lindo JF, Bailey JW, Bundy DA, 1999. L3 antigen-specific antibody isotype responses in human strongyloidiasis: correlations with larval output. Parasite Immunol 21: 517–526. [DOI] [PubMed] [Google Scholar]
- 5.Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O, 2011. Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis 17: 931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa H, Hayashida S, Ikeda Y, Sato H, 2009. Hyper-variable regions in 18S rDNA of Strongyloides spp. as markers for species-specific diagnosis. Parasitol Res 104: 869–874. [DOI] [PubMed] [Google Scholar]
- 7.Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E, 2011. Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol 6: 23–30. [PMC free article] [PubMed] [Google Scholar]
- 8.Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L, 2009. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103: 342–346. [DOI] [PubMed] [Google Scholar]
- 9.ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, van Lieshout L, Verweij JJ, 2009. Molecular diagnostics of intestinal parasites in returning travellers. Eur J Clin Microbiol Infect Dis 28: 1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saugar JM, Merino FJ, Martin-Rabadan P, Fernandez-Soto P, Ortega S, Garate T, Rodriguez E, 2015. Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop 142: 20–25. [DOI] [PubMed] [Google Scholar]
- 11.Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Munoz J, 2013. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7: e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krolewiecki AJ, et al. 2010. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol 17: 1624–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bisoffi Z, et al. 2014. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8: e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB, 2008. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis 198: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi V, Ramachandran S, Thompson RW, Andersen JF, Neva FA, 2002. Characterization of a recombinant immunodiagnostic antigen (NIE) from Strongyloides stercoralis L3-stage larvae. Mol Biochem Parasitol 125: 73–81. [DOI] [PubMed] [Google Scholar]
- 16.Arifin N, Yunus MH, Nolan TJ, Lok JB, Noordin R, 2018. Identification and preliminary evaluation of a novel recombinant protein for serodiagnosis of strongyloidiasis. Am J Trop Med Hyg 98: 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rascoe LN, Price C, Shin SH, McAuliffe I, Priest JW, Handali S, 2015. Development of Ss-NIE-1 recombinant antigen based assays for immunodiagnosis of strongyloidiasis. PLoS Negl Trop Dis 9: e0003694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pak BJ, et al. 2014. Development of a rapid serological assay for the diagnosis of strongyloidiasis using a novel diffraction-based biosensor technology. PLoS Negl Trop Dis 8: e3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW, 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arifin N, Hanafiah KM, Ahmad H, Noordin R, 2018. Serodiagnosis and early detection of Strongyloides stercoralis infection. J Microbiol Immunol Infect pii: S1684–1182(18)30426–2. Available at: 10.1016/j.jmii.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Atkins NS, Lindo JF, Lee MG, Conway DJ, Bailey JW, Robinson RD, Bundy DA, 1997. Humoral responses in human strongyloidiasis: correlations with infection chronicity. Trans R Soc Trop Med Hyg 91: 609–613. [DOI] [PubMed] [Google Scholar]
- 22.Subathra M, Senthilkumar TMA, Ramadass P, Ramaswamy V, 2011. Evaluation of the cocktail recombinant antigens, LipL32 and LipL41 for serodiagnosis of canine leptospirosis. World J Microbiol Biotechnol 27: 1077–1082. [Google Scholar]
- 23.Yunus MH, Tan Farrizam SN, Abdul Karim IZ, Noordin R, 2018. A lateral flow rapid test for human toxocariasis developed using three Toxocara canis recombinant antigens. Am J Trop Med Hyg 98: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]