Abstract.
Although the in-hospital mortality of Australian patients with melioidosis continues to decline, the ensuing clinical course of survivors is poorly described. Between January 1, 1998, and January 31, 2019, 228 patients in Cairns, tropical Australia, survived their hospitalization with melioidosis; however, 52 (23%) subsequently died. Death occurred at a median of 3.8 years after discharge, with patients dying at a mean age of 59 years. Only 1/27 (4%) without predisposing conditions for melioidosis died during follow-up, versus 51/201 (25%) with these comorbidities (P = 0.01). Death during follow-up was more likely in patients with chronic lung disease (OR [95% CI]: 4.05 (1.84–8.93, P = 0.001) and chronic kidney disease (OR [95% CI]: 2.87 [1.33–6.20], P = 0.007), and was most commonly due to infection and macrovascular disease. A significant proportion of Australians surviving hospitalization with melioidosis will die soon after discharge, usually prematurely and frequently from preventable conditions. A more holistic approach is required to their care.
Melioidosis, a disease caused by the environmental Gram-negative bacillus Burkholderia pseudomallei, is estimated to kill almost 90,000 people globally every year.1 In Southeast Asia, where the disease is endemic, the in-hospital mortality exceeds 40%.2 Melioidosis is also endemic in tropical Australia, but early recognition and access to high-quality intensive care unit (ICU) support in the country’s well-resourced health system have reduced the disease’s case fatality rate to approximately 10%.3,4
Melioidosis is an opportunistic infection, with disease developing after only one of every 4,600 exposures.5 Indeed, in tropical Australia, melioidosis is uncommon in the absence of its classically associated risk factors, which include diabetes mellitus, hazardous alcohol use, chronic kidney disease (CKD), chronic lung disease, and immunosuppression.3 As more Australians survive their acute infection, there is the opportunity to address these comorbidities more aggressively, with the goal of improving long-term outcomes.
This study examined the post-discharge course of patients who survived their hospitalization with melioidosis and aimed to determine whether advances in the acute management of the disease were matched by the patients’ long-term outcomes. It was performed at Cairns Hospital, a 531-bed, tertiary referral center located in Far North Queensland, tropical Australia. The hospital serves a population of 280,000, almost 12% of whom identify as Indigenous Australians. Its laboratory is the region’s primary public microbiology provider. All patients with a positive culture for B. pseudomallei between January 1, 1998, and January 31, 2019, were eligible for the study. Hospital records were reviewed to determine the patients’ demographics, their clinical presentation, their comorbidities, and their clinical course. Comorbidities and complications were defined as described previously.6 If the presence of a comorbidity or complication was not documented, it was presumed to be absent. The hospital’s electronic registration system was used to establish whether patients were alive or dead at follow-up. Their cause of death was confirmed, where possible, by chart review.
Data were de-identified, entered into an electronic database, and analyzed with statistical software (Stata 14, StataCorp., College Station, TX). Groups were analyzed using the Kruskal–Wallis and chi-squared tests, where appropriate. Odds ratios (ORs) were calculated using logistic regression. A Kaplan–Meier curve was constructed using survival data. The study received ethical approval from the Far North Queensland Human Research Ethics Committee (HREC/15/QCH/46-977).
There were 273 patients hospitalized with melioidosis during the study period; 38 (14%) died before discharge. The in-hospital case fatality rate declined from 13/45 (29%) in the first 5 years of the study to 14/130 (11%) in the last 5 years (P for trend = 0.006). Of the 235 patients surviving to discharge, seven (3%) were lost to follow-up, leaving 228 for analysis. The patients’ mean (95% CI) age on admission was 51 (49–53) years. Most—175/228 (77%)—were admitted during the December–April wet season; 120/228 (53%) identified as Indigenous Australians.
A traditional risk factor for melioidosis was present in 201/228 (88%): there were 123/228 (54%) with diabetes mellitus, 92/228 (40%) with hazardous alcohol use, 35/228 (15%) with immunosuppression, 34/228 (15%) with CKD, 32/228 (14%) with chronic lung disease, and 20/228 (9%) with malignancy. Bacteremia was present in 165/228 (72%), 55/228 (24%) required ICU admission, while 46/228 (22%) developed septic shock during their hospitalization.
After a median (interquartile range [IQR]) posthospital discharge follow-up period of 3.8 (1.7–8.8) years, 52/228 (23%) patients had died. These deaths occurred at a median (IQR, range) of 3.9 (1.2–7.1, 0.1–18.4) years after discharge. The cause of death was available in 40/52 (77%); this was infection in 13/40 (33%), macrovascular disease in 9/40 (17%), malignancy in 7/40 (18%), renal disease in 4/40 (10%), and lung disease in 3/40 (8%); the 4 (10%) remaining deaths were from miscellaneous causes. The 5-year survival was 76% (70/92 in those with at least 5 years of follow-up post-discharge).
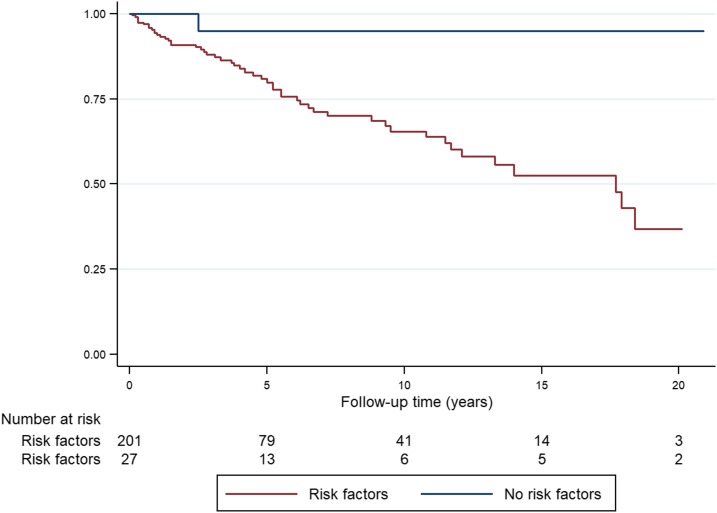
Neither the patients’ age nor their inhospital course had any impact on long-term survival (Table 1). However, the patients’ comorbidities during their initial hospitalization had more prognostic significance. Only 1/27 (4%) patients with no known risk factors for melioidosis died during follow-up compared with 51/201 (25%) of those who had a confirmed traditional risk factor (P = 0.01); OR (95% CI: 0.11 [0.01–0.85], P = 0.04) (Figure 1). The sole individual without a known risk factor for melioidosis who died during follow-up was 83 years old when he died, compared with a mean (95% CI) age of 58 (54–62) years among those with confirmed risk factors.
Table 1.
Patient characteristics and their association with death after discharge from hospitalization with melioidosis
| Patient feature | Total, n = 228 | Alive at the end of the study period, n = 176 | Died during follow-up, n = 52 | P-value * | Odds ratio (95% CI) | P-value† |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age ≥ 60 years‡ | 68 (30%) | 52 (30%) | 16 (31%) | 0.87 | 1.06 (0.54–2.08) | 0.87 |
| Male gender | 167 (73%) | 129 (73%) | 38 (73%) | 0.98 | 0.99 (0.49–1.99) | 0.98 |
| Indigenous | 120 (53%) | 88 (50%) | 32 (62%) | 0.14 | 1.60 (0.85–3.01) | 0.15 |
| Disease risk factor | ||||||
| Chronic lung disease | 31 (14%) | 16 (9%) | 15 (29%) | < 0.0001 | 4.05 (1.84–8.93) | 0.001 |
| Chronic kidney disease | 34 (15%) | 20 (11%) | 14 (27%) | 0.006 | 2.87 (1.33-6.20) | 0.007 |
| Diabetes mellitus | 123 (54%) | 91 (52%) | 32 (62%) | 0.21 | 1.49 (0.79–2.81) | 0.21 |
| Hazardous alcohol use | 92 (40%) | 68 (39%) | 24 (46%) | 0.33 | 1.36 (0.73-2.54) | 0.33 |
| Immunosuppression | 35 (15%) | 24 (14%) | 11 (21%) | 0.19 | 1.70 (0.77–3.75) | 0.19 |
| Malignancy | 20 (9%) | 13 (7%) | 7 (13%) | 0.17 | 1.95 (0.73–5.18) | 0.18 |
| Inhospital course | ||||||
| Bacteremia | 165 (72%) | 127 (72%) | 38 (73%) | 0.90 | 1.04 (0.52–2.10) | 0.90 |
| ICU admission | 55 (24%) | 42 (24%) | 13 (25%) | 0.87 | 1.06 (0.52–2.18) | 0.87 |
| Septic shock | 46 (22%) | 34 (21%) | 12 (26%) | 0.43 | 1.36 (0.64–2.90) | 0.43 |
ICU = intensive care unit.
* Calculated using chi-square or Fisher’s exact test where appropriate.
† P-value for odds ratio calculated using logistic regression.
‡ At the time of initial admission to hospital with melioidosis.
Figure 1.
Kaplan–Meier curve showing post-discharge survival of patients surviving their hospitalization with melioidosis, stratified by the presence or absence of traditional risk factors for the disease. This figure appears in color at www.ajtmh.org.
Patients with chronic lung disease were more likely to die during follow-up (OR [95% CI]: 4.05 (1.84–8.93, P = 0.001)). The commonest causes of death among the 12 patients with chronic lung disease in whom it was known were infection (4/12, 33%) or progression of their lung disease (3/12, 25%). Patients with CKD were also more likely to die during follow-up (OR [95%CI]: 2.87 [1.33–6.20], P = 0.007). The commonest causes of death among the 13 patients with CKD in whom it was known were infection (4/13, 31%), progression of their renal disease (3/13, 23%), and macrovascular events (2/13, 15%).
The difference in mortality during follow-up between diabetics and non-diabetics, between patients with and without hazardous alcohol use, and between patients with and without cancer did not reach statistical significance. This was also the case for the difference in mortality between Indigenous and non-Indigenous patients (Table 1).
These data confirm the status of melioidosis in Australia as a seasonal, opportunistic infection.3,7 Almost 90% of the patients had a documented traditional risk factor for the disease, while 77% presented during the wet season. The study also highlights the advances made in the local acute care of melioidosis, with an almost 3-fold decline in the in-hospital case fatality rate during the 21-year study period. However, patient outcomes after discharge were much worse, with the mean age of those that died over 20 years less than the national average during the study period. The 5-year survival of 77% compares with a 5-year survival of 67% for Australians diagnosed with cancer.8
There was no relationship between in-hospital course and long-term outcomes—patients admitted to the ICU were no more likely to die during follow-up than those managed on a general ward. There was even no significant relationship between the patients’ age and survival post-discharge. However, the contribution of underlying comorbidities to subsequent mortality was significant: only a single patient without risk factors died during follow-up (at the age of 83). Lung disease was particularly associated with subsequent death, with almost half of these patients dying during follow-up. Those with lung disease died at a median of only 3.3 years after discharge, suggesting more aggressive multidisciplinary efforts to support smoking cessation, encourage vaccination, promote medication adherence, optimize nutrition, and increase physical activity may be useful in these patients.9 Psychological support and aggressive macrovascular prophylaxis are also likely to be helpful.10 Meanwhile, improved macrovascular prophylaxis—particularly optimized blood pressure—while avoiding nephrotoxic medications and providing appropriate vaccinations are likely to benefit CKD patients.11–13
The fact that diabetes and hazardous alcohol use were not significantly associated with subsequent death almost certainly reflects a type 2 error in this relatively small sample, although it suggests that chronic lung or kidney disease may have greater prognostic significance. Clearly, recovery from melioidosis is also an opportunity for clinicians to take steps to improve glycemic control and reduce hazardous alcohol consumption, both of which are associated with poor long-term outcomes.14,15
The commonest cause of death during follow-up was infection. Although infection is responsible for less than 2% of Australian deaths,8 it represented 33% of the deaths among those who survived their melioidosis. The 13 deaths included two patients who died from Staphylococcus aureus bacteremia, two diabetic patients who died from polymicrobial foot infections, and two diabetic patients who died from Pseudomonas aeruginosa infection (one from mastoiditis and another with urosepsis). Another diabetic patient with lymphedema died from a Serratia marcescens soft tissue infection. Two patients died from community-acquired pneumonia, one had Mycoplasma pneumoniae infection and the other had no pathogen identified but died despite receiving meropenem, azithromycin, and noninvasive ventilation. One patient died from Streptococcus mitis bacteremia, another died from Escherichia coli biliary sepsis, and another died from urosepsis although no organism was identified. There was one HIV-positive patient who died from relapsed B. pseudomallei infection.
Although impaired neutrophil function in patients with diabetes, hazardous alcohol consumption, and CKD is believed to contribute to these patients’ increased risk of melioidosis,16 other factors including human leucocyte antigen type, genetic factors, natural killer and T-cell function, and humoral responses also appear to play a critical role in immunity to B. pseudomallei.16–18 There may be similarities in host susceptibility to melioidosis and mycobacterial infections,16,19 although the findings of this series suggest that the array of potential pathogens may be more diverse. A lower index of suspicion for infection may be appropriate in these patients, who could be advised to present for earlier review.
Meanwhile, macrovascular disease was the second most common cause of death and it is notable that 8/9 (89%) patients who died from macrovascular disease were diabetic and 7/9 (78%) were smokers. Survival from melioidosis may be an opportunity to institute more aggressive primary macrovascular prophylaxis in these patients.14
There has been enormous recent progress in the acute management of melioidosis in Australia’s well-resourced health system; however, we are presently failing our patients. There is no doubt that many of the patients have complex health needs and many—particularly Indigenous patients in remote locations—have challenges in accessing health care. However, if almost a quarter of survivors are dead within 5 years—usually prematurely and frequently from preventable causes—it is beholden on us to take a more holistic approach to their care.
Acknowledgments:
We thank the staff of Cairns Hospital who cared for the patients during their hospitalization.
REFERENCES
- 1.Limmathurotsakul D, et al. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: pii: 15008. [DOI] [PubMed] [Google Scholar]
- 2.Birnie E, et al. 2019. Thrombocytopenia impairs host defense against Burkholderia pseudomallei (melioidosis). J Infect Dis 219: 648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith S, Hanson J, Currie BJ, 2018. Melioidosis: an Australian perspective. Trop Med Infect Dis 3: pii: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens DP, Thomas JH, Ward LM, Currie BJ, 2016. Melioidosis causing critical illness: a review of 24 Years of experience from the royal Darwin Hospital ICU. Crit Care Med 44: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AC, Wuthiekanun V, Limmathurotsakul D, Chierakul W, Peacock SJ, 2008. Intensity of exposure and incidence of melioidosis in Thai children. Trans R Soc Trop Med Hyg 102 (Suppl 1): S37–S39. [DOI] [PubMed] [Google Scholar]
- 6.Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J, 2017. The epidemiology and clinical features of melioidosis in Far North Queensland: implications for patient management. PLoS Negl Trop Dis 11: e0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hempenstall AJ, Smith S, Stanton D, Hanson J, 2019. Melioidosis in the torres strait islands, Australia: exquisite interplay between pathogen, host, and environment. Am J Trop Med Hyg 100: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AIHW , 2016. Australia’s Health 2016. Australia’s Health Series No. 15. Canberra, Australia: Australian Institute of Health and Welfare. [Google Scholar]
- 9.Ambrosino N, Bertella E, 2018. Lifestyle interventions in prevention and comprehensive management of COPD. Breathe (Sheff) 14: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanfleteren L, Spruit MA, Wouters EFM, Franssen FME, 2016. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 4: 911–924. [DOI] [PubMed] [Google Scholar]
- 11.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX, 2006. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17: 2034–2047. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Coresh J, 2012. Chronic kidney disease. Lancet 379: 165–180. [DOI] [PubMed] [Google Scholar]
- 13.Kidney Health Australia , 2015. Chronic Kidney Disease (CKD) Management in General Practice, Guidance and Clinical Tips to Help Identify, Manage and Refer Patients with CKD in Your Practice. Melbourne, Victoria, Australia: Kidney Health Australia. [Google Scholar]
- 14.Baena-Diez JM, et al. 2016. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care 39: 1987–1995. [DOI] [PubMed] [Google Scholar]
- 15.GBD 2016 Alcohol Collaborators , 2018. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392: 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunachie SJ, et al. 2017. Infection with Burkholderia pseudomallei–immune correlates of survival in acute melioidosis. Sci Rep 7: 12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kronsteiner B, et al. 2019. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol 2019 Apr 29. 10.1002/eji.201848037 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh GC, Schreiber MF, Bautista R, Maude RR, Dunachie S, Limmathurotsakul D, Day NP, Dougan G, Peacock SJ, 2013. Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling. PLoS One 8: e54961. [DOI] [PMC free article] [PubMed] [Google Scholar]